Abstract
The endothelium plays an important role in maintaining normal vascular function. Endothelial barrier dysfunction leading to increased permeability and vascular leakage is associated with several pathological conditions such as edema and sepsis. Thus, the development of drugs that improve endothelial barrier function is an active area of research. In this chapter, the current knowledge concerning the signaling pathways regulating endothelial barrier function is discussed with a focus on cyclic nucleotide second messengers (cAMP and cGMP) and cyclic nucleotide phosphodiesterases (PDEs). Both cAMP and cGMP have been shown to have differential effects on endothelial permeability in part due to the various effector molecules, crosstalk, and compartmentalization of cyclic nucleotide signaling. PDEs, by controlling the amplitude, duration, and localization of cyclic nucleotides, have been shown to play a critical role in regulating endothelial barrier function. Thus, PDEs are attractive drug targets for the treatment of disease states involving endothelial barrier dysfunction.
Keywords: cAMP, cGMP, Endothelial barrier, Endothelial permeability, Phosphodiesterase
1 Introduction
One of the major functions of the endothelium is to act as an active barrier between the circulating blood and the underlying vessel wall and tissues (Mehta and Malik 2006; Michel and Curry 1999; van Hinsbergh 1997; van Nieuw Amerongen et al. 2001; van Nieuw Amerongen and van Hinsbergh 2002). The endothelium can respond to a variety of signals to regulate the passage of proteins, fluid, and solutes through the endothelial barrier. While oxygen, water, and solutes can pass through this barrier via diffusion, channels, and slits in the cellular junctions, macromolecules can cross the endothelial barrier via three different mechanisms. These include (1) transcellular passage through the cell via vesicles, (2) passage via pores in the cell membrane usually formed by fused vesicles at the apical and basolateral membrane, or (3) passage between endothelial cells paracellularly (Mehta and Malik 2006; van Hinsbergh 1997). Dysregulation of endothelial barrier function is characteristic of many diseases and pathological conditions including atherosclerosis, asthma, tumor growth, edema, and sepsis (van Hinsbergh 1997; van Nieuw Amerongen and van Hinsbergh 2002). Thus, understanding the mechanisms that regulate endothelial permeability may allow for the development of drugs that can enhance or decrease barrier function. In this chapter, we briefly summarize what is currently known about the various mechanisms and signaling pathways that regulate endothelial permeability by focusing on the role of cyclic nucleotide phosphodiesterases as a potential drug target for the regulation of endothelial barrier function.
2 Transcellular and Paracellular Transport of Molecules Through the Endothelial Barrier
Transport of macromolecules such as proteins, carbohydrates, and lipids across the endothelial barrier occurs transcellularly or paracellulary. Most studies of transcellular transport of macromolecules have focused on the role of caveolae and vesiculo-vacuolar organelles (VVOs) as the transport vesicles. The proteins and mechanisms involved share many similarities to those that regulate neuronal vesicle trafficking. Caveolae are hypothesized to play a role in both receptor-mediated and nonreceptor-mediated transcellular transport of macromolecules across the endothelial barrier (Cohen et al. 2004). Caveolae were first identified by electron microscopy as “flask-shaped” invaginations of the plasma membrane. It is these invaginations that are hypothesized to form the vesicles that mediate transcellular transport of macromolecules across the endothelium (Komarova and Malik 2010). For example, albumin transcytosis is initiated by binding of albumin to the albumin-binding protein gp60 found in caveolae, resulting in endocytosis and transport of the caveolae through the endothelial cell (Mehta and Malik 2006). VVOs, unlike caveolae, are uncoated interconnected vesicles and vacuoles present in endothelial cells. VVOs have been shown to be able to participate in transcellular transport of macromolecules across the endothelium, but their regulation is unknown. Roles for dynamin, SNARE complexes, actin, and microtubules in the transport of VVOs through endothelial cells have been suggested (Komarova and Malik 2010; Mehta and Malik 2006). Transcellular transport has been suggested to be regulated in part by Src kinases, certain PKC isoforms, PI3 kinases, Ca2+, and the albumin-binding protein gp60 (Komarova and Malik 2010; Mehta and Malik 2006).
Paracellular transport of macromolecules across the endothelial barrier is achieved by opening of intercellular gaps between cells. Endothelial cells are connected with each other by a variety of junctional proteins that form (a) tight, (b) adherent, and (c) gap junctions. Tight junctions consist of proteins such as claudins, occludin, and junctional adhesion molecules. These transmembrane proteins have extracellular domains that bind tightly to each other via homotypic or heterotypic bonds and are linked intracellularly to the actin cytoskeleton. They are also linked to a variety of signaling molecules such as PKC-ζ and VASP. Tight and adherent junctions form zipper-like structures between endothelial cells, while gap junction proteins such as connexons form transmembrane channels between endothelial cells.
Adherent junctions are comprised primarily by vascular endothelial (VE)-cadherin whose extracellular domains participate in transoligomeric binding between endothelial cells forming the junction. The cytoplasmic domain of VE-cadherin, like those of the proteins found in tight junctions, is also proposed to be associated with the actin cytoskeleton as well as a variety of signaling molecules such as β-catenin, RhoGTPases, VASP, casein kinase II, Src kinases, and phosphatases such as SHP-1.
Both tight and adherent junctions link endothelial cells together forming a barrier. It is thought that changes in binding of tight and adherent junction proteins function to regulate the opening and closing of gaps between endothelial cells allowing paracellular transport of macromolecules.
Gap junctions consist of transmembrane hydrophilic proteins called connexons. A connexon from each neighboring endothelial cell pair forms an intercellular pore that can be regulated by serine/threonine and tyrosine phosphorylation. The pores formed between cells by gap junctions allow for the propagation of transmembrane potentials as well as the exchange of signals between endothelial cells via second messengers such as Ca2+ and IP3 (Komarova and Malik 2010; Mehta and Malik 2006).
3 Signaling Pathways Shown to Regulate Endothelial Permeability
There are a large number of studies on endothelial permeability focused on trying to unravel the mechanisms by which permeability is both increased and decreased and how these mechanisms are regulated. The majority of the studies have addressed paracellular mechanisms of barrier function. Similar to smooth muscle, endothelial cells have a contractile system that requires actin, nonmuscle myosin, ATP, calcium, and calmodulin. Stimulation of endothelial contraction leads to the formation of small gaps between endothelial cells that, in time, lead to increased permeability. A variety of agents, including thrombin, VEGF, bradykinin, and histamine, have been found to increase endothelial permeability via increases in intracellular calcium. As with smooth muscle, increases in intracellular calcium will lead to the activation of myosin light chain kinase (MLCK). MLCK can then phosphorylate myosin light chains leading to actin-myosin-mediated endothelial cell contraction.
In addition to pathways that increase calcium, activation of other signaling pathways can also increase endothelial cell contraction. Thrombin has been shown to activate the small GTPase, RhoA. RhoA, via activation of Rho kinase, inhibits PP1M, the phosphatase that dephosphorylates myosin light chain. Thus, thrombin, in addition to increasing calcium and phosphorylation of myosin light chain via MLCK, inhibits dephosphorylation of myosin light chain via a RhoA-mediated mechanism. The cumulative effect is an increase in contraction. Other agents known to increase endothelial permeability such as TNF-α and H2O2 have also been shown to regulate RhoA and thus the contractile function of endothelial cells. All of these agents have also been shown to activate a variety of kinases that can lead to changes in the phosphorylation status of junctional proteins and barrier function and also to changes in gene expression.
While there are several pharmacological agonists that have been shown to decrease endothelial barrier function, there are only a few endogenous factors that have been shown to increase barrier function. These include sphingosine-1-phosphate (S1P), angiopoietin-1, and cAMP (Jho et al. 2005; Komarova et al. 2007; Moore et al. 1998; Satchell et al. 2004). S1P is a phospholipid formed by the phosphorylation of sphingosine by sphingosine kinase. S1P is found in and released from platelets. The lack of sphingosine lysase, a key regulator of S1P degradation, leads to S1P storage in platelet granules (Yatomi et al. 1995). Endothelial cells express G-coupled receptors for S1P and the endothelial differentiation gene (EDG) receptors, Edg-1, Edg-3, and Edg-5 (Ozaki et al. 2003). These receptors are Gi coupled and lead to activation of the small GTPase Rac and adherent junction assembly (Takuwa 2002).
Finally, angiopoietin-1 is a ligand for the endothelial-specific tyrosine kinase receptor, Tie-2 (Tsigkos et al. 2003). Angiopoietin-1 has been shown to inhibit permeability induced by thrombin, bradykinin, histamine, and VEGF (Pizurki et al. 2003). In studies with VEGF, angiopoietin-1 was shown to prevent Ca2+ influx and contraction (Jho et al. 2005). However, the detailed mechanisms of angiopoietin-1 action on barrier function remain to be elucidated.
4 The Role of cAMP in the Regulation of Endothelial Barrier Function
While the study of S1P and angiopoietin-1 as agonists of endothelial barrier enhancement is just beginning, the role of cAMP and agonists that increase this second messenger, such as β-adrenergic agonists and serotonin, has been studied extensively (Mehta and Malik 2006; van Hinsbergh 1997). In general, cAMP has been shown to improve barrier function and decrease permeability under both basal and stimulated conditions in vitro and in vivo. Until recently, it had been accepted that cAMP improves endothelial barrier function only via activation of PKA (Mehta and Malik 2006). PKA has been shown to inhibit thrombin-induced RhoA activation (Qiao et al. 2003). RhoA is a member of the superfamily of Rho GTPases whose activity is determined by the binding of GTP. PKA has been shown to directly phosphorylate and inhibit RhoA (Lang et al. 1996). Another possible mechanism by which PKA inhibits RhoA is via the phosphorylation of GTP dissociation inhibitor (GDI) (Qiao et al. 2008). Since RhoA has been implicated in inhibiting MLC phosphatase, which can result in increased MLC phosphorylation and contraction, PKA activation would lead to increased phosphatase activity, decreased MLC phosphorylation, and relaxation. This should improve barrier function. PKA has also been shown to phosphorylate and inhibit MLCK (Verin et al. 1998). By preventing MLC phosphorylation by MLCK, PKA can reduce endothelial cell contraction. These decreases in contraction caused by PKA presumably lead to improved tight and adherent junction stabilization, decreased gap formation, and improved barrier function.
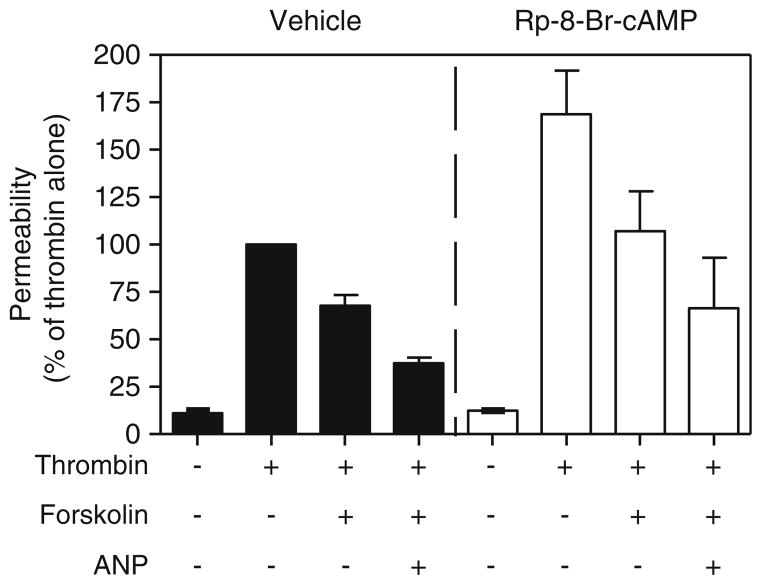
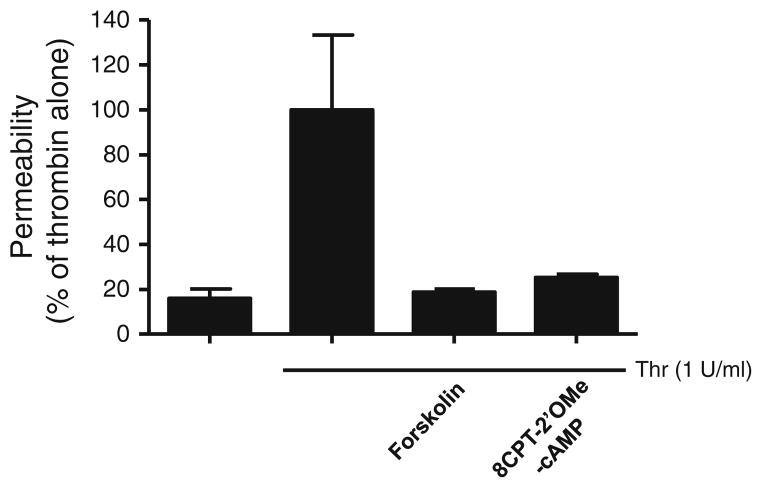
More recently, another cAMP effector molecule, Epac, was also shown to mediate at least part of the barrier-enhancing actions of cAMP (Fukuhara et al. 2005; Kooistra et al. 2005; Wittchen et al. 2005). Epac1 and Epac2 are guanine nucleotide exchange factors for the small G protein Rap (Gloerich and Bos 2010). The discovery of Epac as a target of cAMP signaling explained the various effects of cAMP that could not be attributed to PKA and cyclic nucleotide-gated ion channels. It was demonstrated that Epac can also inhibit thrombin-induced RhoA activation (Cullere et al. 2005). Similar to PKA, Epac-mediated inhibition of RhoA activity presumably leads to decreased MLC phosphorylation, endothelial relaxation, and increased barrier function. The relative contributions of PKA and Epac in mediating the barrier-enhancing effects of cAMP are just now beginning to be determined. Of the early studies describing the role of Epac in mediating the barrier-enhancing effects of cAMP, only one attempted to rule out the involvement of PKA via the use of H89, a PKA inhibitor (Fukuhara et al. 2005). However, H89 has also been shown to inhibit other kinases including Rho kinase (Leemhuis et al. 2002; Murray 2008). Thus, H89 inhibition of Rho kinase, which is downstream of RhoA and PKA (Qiao et al. 2008), may lead to decreased permeability and may not rule out the possible role of PKA in decreasing permeability. Kooistra et al. demonstrated the role of Epac in regulating endothelial cell permeability via the use of RNAi (Kooistra et al. 2005). Kooistra et al. also used Epac-specific analogues in combination with RNAi knockdown of Epac to demonstrate the role of Epac in regulating endothelial cell permeability. RhoA seems to be regulated by both PKA and Epac. The use of PKI, a specific inhibitor of PKA, was shown to partially reverse the inhibitory effect of cAMP on RhoA (Qiao et al. 2003). On the other hand, it was shown that an Epac-specific cAMP analogue, 8-pCPT-2′O-Me-cAMP, could mimic completely the inhibitory effects of forskolin- and rolipram (PDE4-specific inhibitor)-induced cAMP on RhoA, suggesting that the effects of cAMP are via Epac (Cullere et al. 2005). Further studies are needed to clarify the relative roles of PKA and Epac in mediating the inhibitory effects of cAMP on RhoA. Unpublished data from our laboratory suggest that the barrier-enhancing effects of cAMP may be due to both PKA and Epac. We found that inhibition of PKA by Rp-8-Br-cAMP increased thrombin-induced permeability, suggesting a role for PKA in preventing decreases in barrier function (Fig. 1). However, forskolin was still able to attenuate thrombin-induced permeability, and low doses of ANP, via inhibition of PDE3, were able to potentiate this effect, suggesting that another effector molecule also mediates the barrier-enhancing effect of cAMP (Surapisitchat et al. 2007). We also found that the activation of Epac was able to decrease thrombin-induced permeability using the Epac-specific activator, 8-CPT-2-O-ME-cAMP (Fig. 2). These results suggest that there may be different pools of cAMP mediating the effects of PKA and Epac on permeability. A recent report has demonstrated that cAMP can act via both PKA and Epac to effect independent and complementary pathways to regulate endothelial barrier function (Lorenowicz et al. 2008).
Fig. 1.
PKA mediates some of the effects of cAMP on endothelial permeability. Permeability assays with thrombin, forskolin, and 0.3 nM ANP ± 100 μM Rp-8-Br-cAMP were performed as described previously (Surapisitchat et al. 2007). Rp-8-Br-cAMP was added 15 min prior to stimulation with thrombin, forskolin, and ANP. Data represent mean (mean±SEM) from two independent experiments using HUVEC isolated from two different umbilical cords
Fig. 2.
Epac mediates some of the effects of cAMP on endothelial permeability. HUVEC were stimulated with thrombin, thrombin + 10 μM forskolin, or thrombin + 100 μM 8-CPT-2′OMe-cAMP, and permeability assays were performed as described previously (Surapisitchat et al. 2007). Data represent mean (mean±SEM) from two independent experiments using HUVEC isolated from two different umbilical cords
While cAMP has been shown to inhibit increases in endothelial cell permeability and various mechanisms have been proposed and demonstrated, recent reports from the Steven’s group demonstrate that the effects of cAMP on permeability are more complex. Sayner et al. and Prasain et al. describe results that show a barrier-disruptive effect of cAMP can be produced in the cytosol by activation of the soluble adenylyl cyclases (Prasain et al. 2009; Sayner et al. 2004, 2006). These studies suggest that cytosolic-produced cAMP decreases barrier function, but cAMP made by membrane adenylyl cyclases improves barrier function via PKA and/or Epac. The concept of compartmentalization of cyclic nucleotide signaling has been an area of increasing study of late (Fischmeister et al. 2006; Houslay 2010). It is possible that there are several cAMP “pools” that are responsible for the regulation of endothelial permeability. While the role of cAMP in regulating endothelial permeability has received substantial study, more work is needed to understand the relative contribution of the cAMP effector molecules PKA and Epac in mediating the effects of cAMP as well as the roles of compartmentalization of cAMP and its regulation in endothelial barrier function.
5 The Role of cGMP in the Regulation of Endothelial Barrier Function
The role of cGMP on endothelial barrier function is even more controversial than that of cAMP. Both barrier-enhancing and impairing effects of cGMP have been reported (Draijer et al. 1995a; van Nieuw Amerongen and van Hinsbergh 2002; Zimmerman et al. 1990). cGMP is produced by soluble and particulate guanylyl cyclases (sGC and pGC), which are stimulated by nitric oxide or natriuretic peptides, respectively. In vitro studies have shown that NO can improve barrier function, presumably via activation of sGC and production of cGMP. Several groups demonstrated this effect via the use of exogenous NO donors such as sodium nitroprusside and DETA NONOate (Hempel et al. 1996; Suttorp et al. 1996b; Westendorp et al. 1994; Wong et al. 2004). Others have used nitric oxide synthase (NOS) inhibitors such as L-NAME to show that NO is required for maintaining endothelial barrier function (Draijer et al. 1995a; He et al. 1997b; Liu and Sundqvist 1997). In vivo, loss of NO via either genetic ablation of eNOS or treatment with L-NAME resulted in increased endothelial permeability (Predescu et al. 2005). On the other hand, several groups have shown that NO can increase endothelial permeability. For example, it was demonstrated that while NO donors and cGMP can decrease permeability in resting endothelial cells, cells stimulated with ionomycin to increase permeability showed a further increase in permeability upon addition of NO donors in vitro (Holschermann et al. 1997). Furthermore, in an ex vivo model using frog mesenteric venular microvessels, it was further demonstrated that the ability of ionomycin to increase permeability is attenuated by NOS inhibitors, suggesting a role of NO in increasing permeability (He et al. 1997a). Finally, in vivo models, including the same eNOS −/− mice, were used to show that NO is involved in mediating increases of permeability under inflammatory conditions (Bucci et al. 2005; Hatakeyama et al. 2006).
Like NO, the role of natriuretic peptides in regulating endothelial permeability is also controversial. Five different groups studying endothelial permeability found that atrial natriuretic peptide (ANP) decreases endothelial permeability (Baron et al. 1989; Hempel et al. 1996; Klinger et al. 2006; Suttorp et al. 1996b; Westendorp et al. 1994). On the other hand, ANP has also been shown to increase endothelial permeability in vitro by one group (Holschermann et al. 1997). In vivo, ANP has also been shown to increase endothelial permeability (Tucker et al. 1992; Zimmerman et al. 1990). This was further supported by the finding that endothelial-specific ablation of the ANP receptor, guanylyl cyclase-A (GC-A), resulted in decreased permeability in vivo (Sabrane et al. 2005). Further complicating this issue is the questionable involvement of PKG. For example, it was reported that the barrier-enhancing effects of cGMP is PKG dependent (Moldobaeva et al. 2006). Others also found that PKG mediated the barrier regulating effects, but instead of decreasing permeability, they found that PKG increased permeability (Holschermann et al. 1997). Additionally, the barrier-enhancing effects of cGMP were reported to be PKG independent (Gupta et al. 2001). Further complicating the issue, it has been reported that the effects of cGMP are PKG dependent in endothelial cells that express PKG, while they are independent in endothelial cells that do not express PKG (Draijer et al. 1995a, b). Thus, while it is fairly clear that increased cAMP from endogenous membrane cyclases improves endothelial barrier function, the role of cGMP, whether from stimulation of sGC by NO or from pGC activated by ANP, has until recently remained unresolved.
6 Endothelial Cell Cyclic Nucleotide Phosphodiesterases
While PKA, Epac, PKG, and cyclic nucleotide-gated channels (CNG) play critical roles in mediating the effects of cAMP and cGMP in endothelial cells, the role of cyclic nucleotide phosphodiesterases (PDEs) is emerging as an equally important player in endothelial function. In addition to regulating the amplitude, duration, and compartmentalization of cyclic nucleotides, PDEs are also effector molecules of cyclic nucleotides and calcium, making them critical mediators of crosstalk between various second messengers. Endothelial cells have been shown to primarily express cGMP-stimulated PDE2, cGMP-inhibited PDE3, cAMP-specific PDE4, and cGMP-specific PDE5 (Netherton and Maurice 2005). The expression of Ca2+/CaM-regulated PDE1 and the cAMP-specific PDE7A has also been described (Keravis et al. 2000; Miro et al. 2000). While these PDEs have been shown to be expressed in the endothelium, significant differences in their relative expression levels have been found in endothelial cells of different origin. The relative expression and PDE activity levels in bovine aortic endothelial cells (BAEC), human aortic endothelial cells (HAEC), human umbilical vein endothelial cells (HUVEC), and human microvascular endothelial cells (HMVEC) were determined, and large differences in the expression levels of PDE2, PDE3, PDE4, and PDE5 were found among these cells (Netherton and Maurice 2005). For example, measuring PDE activity at a substrate concentration of 1 μM cAMP, it was shown that PDE3 accounted for 15 and 36% of the cAMP activity in BAEC and HAEC, respectively, while only 7 and 6% of the cAMP activity in HUVEC and HMVEC, respectively. Differences were also seen at the protein level by immunoblot analysis. In addition to differences in PDE expression between endothelial cells of different origins, there have been differences reported in endothelial cells over time in culture. For example, early passage BAEC (4–6) expressed PDE2 and PDE5 cGMP hydrolytic activity, which were lost with passage (> 10) (Ashikaga et al. 1997). As for cAMP, PDE2 and PDE4 cAMP hydrolytic activity was found in early passage BAEC that later become predominantly PDE4 at later passages. Furthermore, there are reported differences in PDE expression in endothelial cells of different phenotypes. Confluent and resting endothelial cells take on a cobblestone-shaped morphology, while proliferating, noncontact-inhibited endothelial cells appear elongated and spindle-like. The PDE profile of BAEC has been characterized in these two different states (Keravis et al. 2000). While resting cobblestone BAEC expressed PDE2 and PDE4 cAMP hydrolytic activity, measured at a substrate concentration of 1 μM cAMP, spindle-shaped proliferating BAEC expressed increased cAMP hydrolytic activity due to increased PDE2 and PDE4 activity as well as PDE1 and PDE3 activity. cGMP-PDE activity was primarily due to PDE2 in resting cobblestone BAEC, while spindle-shaped proliferating BAEC expressed both PDE2 and PDE5. Finally, various stimuli can alter PDE expression in endothelial cells. The inflammatory cytokine, TNF-α, shown to increase endothelial expression of various adhesion molecules and decrease barrier function has also been shown to upregulate the expression of PDE2, PDE4, and PDE7 in endothelial cells (Koga et al. 1995; Miro et al. 2000; Seybold et al. 2005). The reasons for the differences in expression of PDEs in endothelial cells of different origins and in response to different stimuli are not well understood. Many of these presumed differences may relate to differences in passage number of the cells being compared.
7 The Role of Phosphodiesterases in the Regulation of Endothelial Permeability
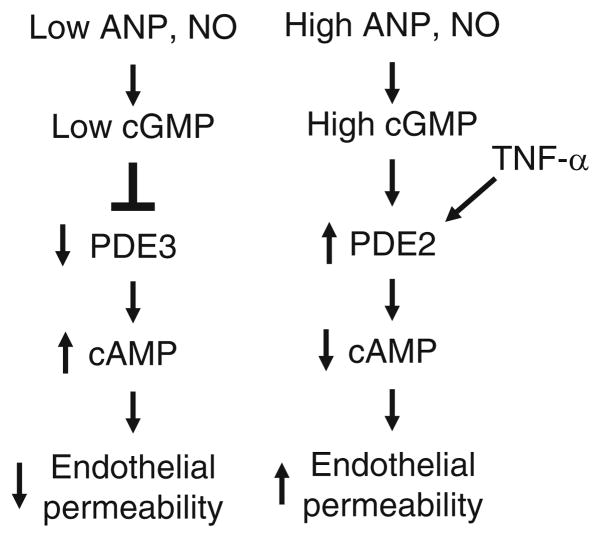
Since cAMP and cGMP play significant and sometimes conflicting roles in endothelial barrier function, endothelial cell PDEs may play critical roles in the regulation of endothelial permeability. The expression of cGMP-activated PDE2, cGMP-inhibited PDE3, cAMP-specific PDE4, and cGMP-specific PDE5 by endothelial cells may allow for differential regulation of barrier function by different PDEs. For example, we have recently demonstrated that cGMP elevating agents such as ANP and NO have biphasic effects on cAMP inhibition of thrombin-induced endothelial permeability (Surapisitchat et al. 2007). The reason for the biphasic actions of cGMP on cAMP inhibition of thrombin-induced endothelial permeability is due to differential regulation of PDE2 and PDE3 by cGMP. Furthermore, altering the expression levels of PDE2 and PDE3 in endothelial cells can alter the effect of cGMP on endothelial permeability as is seen upon stimulation of the cells by TNF-α (Fig. 3). These results likely explain many of the conflicting results reported in the literature concerning the actions of cGMP on endothelial permeability and suggest important roles for PDE2 and PDE3 in regulating endothelial function. Until recently, PDE4 inhibition was thought to improve endothelial barrier function via increased cAMP/PKA levels in the cell (Suttorp et al. 1993). However, other data have demonstrated that in some circumstances increased cAMP in the cytosol can cause increased endothelial permeability (Sayner et al. 2004, 2006). Recently, it was demonstrated that PDE4D4 is responsible for containing cAMP to a membrane pool, restricting cAMP access to the cytosol where it can lead to increased permeability (Creighton et al. 2008). These findings demonstrate the complexity of cyclic nucleotide signaling in the control of endothelial permeability and the critical role of PDEs in regulating this important function.
Fig. 3.
Proposed model of the effect of cGMP on endothelial cell permeability. cGMP generated by NO or ANP stimulation can effect endothelial permeability in several ways. cGMP can inhibit PDE3 leading to increased cAMP in the endothelial cell and decreased permeability. cGMP can also activate PDE2 leading to decreased cAMP and increased permeability. The concentration of cGMP within the cell plays a central role in whether the dominant effect is due to PDE3 inhibition (low cGMP) or PDE2 activation (high cGMP). TNF-α can increase the amount of PDE2 expressed in the endothelial cell and thus alter the response of the cell to cGMP and permeability (from Surapisitchat et al. 2007)
8 Crosstalk Between cGMP and cAMP: The Role of PDEs
The findings that cGMP can regulate cAMP hydrolysis by both PDE2 and PDE3 to alter cAMP levels are in agreement with previous work demonstrating a barrier-enhancing effect of cAMP and further our understanding of the regulation of cAMP in endothelial cells (Surapisitchat et al. 2007). At low concentrations, cGMP can regulate cAMP-mediated enhancement of barrier function via inhibition of PDE3, but, at higher cGMP concentrations, via activation of PDE2, it can mediate breakdown of barrier function. These results are some of the few in which the same pool of cGMP has been shown to both inhibit PDE3 and activate PDE2 to regulate the same cellular function. For example, in human atrial myocytes, cGMP can regulate L-type Ca2+ channel current by modulating cAMP and PKA via PDE2 and PDE3 (Vandecasteele et al. 2001). The functional effect of cGMP is concentration-dependent since the IC50 of cGMP for PDE3A is nearly 20-fold less than the EC50 for activation of PDE2 (Leroy et al. 1996; Surapisitchat et al. 2007; Yamamoto et al. 1983). This difference correlates well with normal versus pathological levels of guanylyl cyclase agonists such as ANP and NO. In healthy individuals, these agonists are relatively low, resulting in low levels of cGMP leading to inhibition of PDE3A, increased cAMP, and enhanced barrier function. Under several pathological conditions such as heart failure and inflammation, ANP and NO increase significantly leading to greatly increased cGMP levels, activation of PDE2A, decreased cAMP levels, and thus decreased barrier function. In addition under some pathological conditions, the relative expression of PDEs can be altered. For example, TNF-α, an inflammatory cytokine, induces PDE2 and decreases PDE3 expression, thereby altering the cellular responses to cGMP signaling (Seybold et al. 2005; Surapisitchat et al. 2007). Thus, PDE2 and PDE3 in the endothelium may act as sensors or switches to detect normal versus pathological concentrations of cGMP and thus regulate endothelial permeability accordingly. Furthermore, the relative expression of these PDEs controls the sensitivity of this switch to cGMP.
9 Cyclic Nucleotide Pools in Endothelial Barrier Function
Studies suggest that there may be multiple pools of cAMP and cGMP in endothelial cells regulated by PDEs (Creighton et al. 2008; Sayner and Stevens 2006; Sayner et al. 2006). This is not unexpected since it is becoming increasingly clear that cellular signaling is a highly compartmentalized phenomenon in which there are many microdomains that are differentially regulated by various proteins and stimuli (Houslay et al. 2007; Houslay 2010).
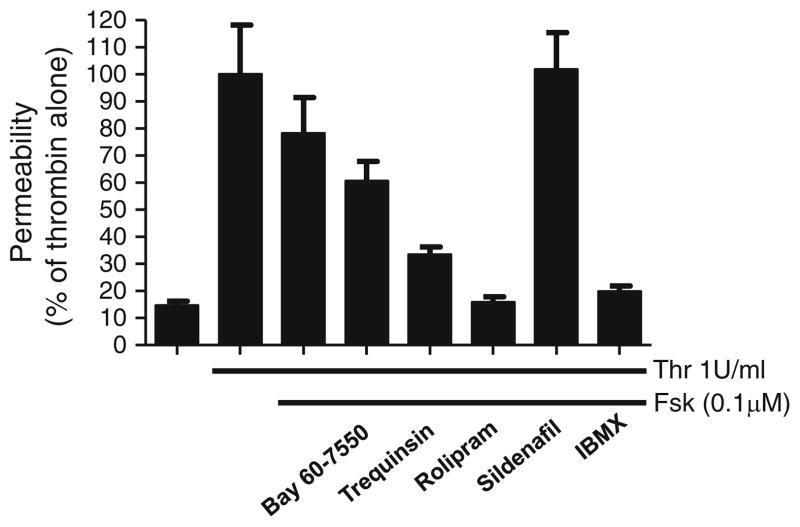
Even with our recent realization that different amounts of PDE2 and PDE3 may account for much of the variability in responses reported for cAMP and cGMP on barrier function, substantial controversy still exists. For example, in contrast to our results (Surapisitchat et al. 2007) and that of others (Adamson et al. 1998; Langeler and van Hinsbergh 1991; van Hinsbergh 1997) indicating that cAMP produced by transmembrane adenylyl cyclases decreases permeability, Sayner et al.’s (2004, 2006) work suggests that increases in a cytosolic pool of cAMP increase permeability. These authors go on to propose a model in which cAMP made by transmembrane adenylyl cyclases is maintained in a microdomain near the plasma membrane by PDE4. The idea is that if PDE4 is inhibited, cAMP can diffuse into the cytosolic compartment (Sayner et al. 2006). Recently, it has been proposed that PDE4D4 is the PDE4 isoform responsible for maintaining cAMP at the membrane (Creighton et al. 2008). This contrast, to previous results by several other groups and our own data demonstrating a barrier-enhancing effect by forskolin and PDE4 inhibition (Suttorp et al. 1996a). Our data indicate that forskolin (10 μM) can almost completely abolish thrombin-induced permeability and that rolipram can potentiate the barrier-enhancing effects of lower forskolin concentrations (Fig. 4). This discrepancy might, however, be explained by differences in the types of endothelial cells studied, microvascular versus macrovascular (Creighton et al. 2008). In particular, microvascular cells are almost always more greatly expanded and studied at a later passage number. Nevertheless, these findings strongly suggest that there are different “pools” of cAMP regulating different aspects of barrier function.
Fig. 4.
Effects of PDE inhibitors on forskolin-inhibited thrombin-induced permeability. HUVEC were stimulated with vehicle, 1 U/ml thrombin (Thr), 1 U/ml thrombin and 0.1 μM forskolin (Fsk), or thrombin, forskolin, and PDE inhibitors. Bay 60-7550 (PDE2) was used at 100 nM. Trequinsin (PDE3) was used at 30 nM. Rolipram (PDE4) was used at 10 μM. Sildenafil (PDE5) was used at 100 nM. IBMX (nonspecific) was used at 100 μM. All inhibitors were added 15 min prior to thrombin + forskolin stimulation. Permeability was analyzed as described previously (Surapisitchat et al. 2007). Data represent means (mean±SEM) from three independent experiments using HUVEC isolated from three different umbilical cords
As with cAMP, pools of cGMP regulating various distinct cellular functions have been described. This was expected with the existence of soluble and particulate guanylyl cyclases. In our studies, we found that cGMP generated from either soluble or particulate guanylyl cyclases had the same biphasic effect on permeability. It is possible though that there are still distinct pools of cGMP regulating barrier function via both PDE2 and PDE3. One possibility is that PDE2 and PDE3 are regulating the same pool of cAMP that controls permeability and two pools of cGMP regulate these PDEs, but they cannot be detected by measuring changes in permeability. For example, cGMP made by soluble guanylyl cyclases may be more effective than that of cGMP made from particulate cyclases, but this difference, a shift in the dose–response curves for the agonists, cannot be detected by measuring permeability. Complicating this further is the time variable in which levels of either cAMP or cGMP may change within a certain pool and with time. Again, many combinations of PDEs, cyclases, effector molecules, and pools are possible. Until more sensitive techniques with increased resolution become available, an accurate description of cyclic nucleotide signaling pools in time and space will have to wait.
The findings that the inflammatory cytokine, TNF-α, can increase PDE2 and decrease PDE3A expression demonstrates that endothelial cells can be altered by various stimuli to respond to cGMP signaling under different conditions. The alteration in PDE expression in response to various stimuli is a common mechanism that many cells and tissues use to alter cyclic nucleotide signaling. For example, nitroglycerine (NTG) is used in the treatment of hypertension for its vasorelaxing effects, but its therapeutic use is limited due to the development of nitrate tolerance. In rats treated with NTG, PDE1A expression and activity is upregulated (Kim et al. 2001). Inhibition of PDE1A leads to partial restoration of smooth muscle responsiveness to nitrates. PDE1C is found to be upregulated in proliferating smooth muscle, suggesting a potential target in the treatment of atherosclerosis or restenosis after angioplasty (Rybalkin et al. 1997). PDE7 upregulation is required for T-cell activation by CD3 and CD28 (Li et al. 1999). Other examples of PDE upregulation include the upregulation of PDE1B in monocyte to macrophage differentiation and PDE5 in vascular smooth muscle in response to angiotensin II stimulation (Bender et al. 2005; Kim et al. 2005). Few examples of downregulation of PDEs have been reported to our knowledge. PDE3A expression and activity has been found to be decreased in patients with heart failure (Ding et al. 2005). In 3T3-L1 adipocytes, TNF-α decreased PDE3B expression, implicating a mechanism by which TNF-α regulates lipolysis (Rahn Landstrom et al. 2000). The findings that TNF-α can also decrease PDE3A in addition to increasing PDE2 to alter endothelial response to cGMP signaling add to this growing list of examples in which alteration of PDE expression and activity in response to physiological stimuli can lead to altered cellular responses. Changes in PDE expression may change the “size and shape” of the normal cyclic nucleotide pool to one that is pathologic. Understanding how changes in PDE expression by pathological stimuli alter endothelial barrier function will be important for the development of treatments of disease states involving endothelial barrier dysfunction.
10 Conclusions
The last several years have been filled with new and exciting findings concerning the role of cyclic nucleotides and PDEs in the regulation of endothelial barrier function. Whereas less than ten years ago the dogma had been that cAMP improved endothelial barrier function while the role of cGMP was debatable, today the role of these two second messengers in endothelial permeability has been shown to be highly dependent on the concentration and localization of these two second messengers within the endothelial cell. PDEs have been shown to play a critical role in regulating the amplitude, duration, and localization of cAMP and cGMP in endothelial cells and thus regulate endothelial barrier function. The concept of different subcellular pools of cAMP and cGMP, controlled by PDEs, regulating different cellular functions is becoming increasingly important. Altered PDE expression in pathological states may alter these pools and thus the effects of these cyclic nucleotides. Thus, understanding the role of PDEs in regulating cyclic nucleotide signaling in normal and pathological endothelial functions may be important for the development of drugs targeting specific PDEs in order to treat diseases that are caused by endothelial dysfunction.
Acknowledgments
The authors would like to thank the members of the Beavo lab for their support over the course of these experiments. This work was funded by grants GM083926, AR056221, and Foundation Leducq to J.A.B.
Contributor Information
James Surapisitchat, Department of Pharmacology, University of Washington School of Medicine, 1959 NE Pacific St., Box 357280, Seattle, WA 98195-7280, USA and McEwen Centre for Regenerative Medicine, University Health Network, 101 College Street, Room 8-601, Toronto, ON, Canada, M5G 1L7.
Joseph A. Beavo, Email: beavo@u.washington.edu, Department of Pharmacology, University of Washington School of Medicine, 1959 NE Pacific St., Box 357280, Seattle, WA 98195-7280, USA
References
- Adamson RH, Liu B, Fry GN, Rubin LL, Curry FE. Microvascular permeability and number of tight junctions are modulated by cAMP. Am J Physiol. 1998;274:H1885–H1894. doi: 10.1152/ajpheart.1998.274.6.H1885. [DOI] [PubMed] [Google Scholar]
- Ashikaga T, Strada SJ, Thompson WJ. Altered expression of cyclic nucleotide phosphodi-esterase isozymes during culture of aortic endothelial cells. Biochem Pharmacol. 1997;54:1071–1079. doi: 10.1016/s0006-2952(97)00287-6. [DOI] [PubMed] [Google Scholar]
- Baron DA, Lofton CE, Newman WH, Currie MG. Atriopeptin inhibition of thrombin-mediated changes in the morphology and permeability of endothelial monolayers. Proc Natl Acad Sci USA. 1989;86:3394–3398. doi: 10.1073/pnas.86.9.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender AT, Ostenson CL, Wang EH, Beavo JA. Selective up-regulation of PDE1B2 upon monocyte-to-macrophage differentiation. Proc Natl Acad Sci USA. 2005;102:497–502. doi: 10.1073/pnas.0408535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci M, Roviezzo F, Posadas I, Yu J, Parente L, Sessa WC, Ignarro LJ, Cirino G. Endothelial nitric oxide synthase activation is critical for vascular leakage during acute inflammation in vivo. Proc Natl Acad Sci USA. 2005;102:904–908. doi: 10.1073/pnas.0408906102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AW, Hnasko R, Schubert W, Lisanti MP. Role of caveolae and caveolins in health and disease. Physiol Rev. 2004;84:1341–1379. doi: 10.1152/physrev.00046.2003. [DOI] [PubMed] [Google Scholar]
- Creighton J, Zhu B, Alexeyev M, Stevens T. Spectrin-anchored phosphodiesterase 4D4 restricts cAMP from disrupting microtubules and inducing endothelial cell gap formation. J Cell Sci. 2008;121:110–119. doi: 10.1242/jcs.011692. [DOI] [PubMed] [Google Scholar]
- Cullere X, Shaw SK, Andersson L, Hirahashi J, Luscinskas FW, Mayadas TN. Regulation of vascular endothelial barrier function by Epac, a cAMP-activated exchange factor for Rap GTPase. Blood. 2005;105:1950–1955. doi: 10.1182/blood-2004-05-1987. [DOI] [PubMed] [Google Scholar]
- Ding B, Abe J, Wei H, Huang Q, Walsh RA, Molina CA, Zhao A, Sadoshima J, Blaxall BC, Berk BC, Yan C. Functional role of phosphodiesterase 3 in cardiomyocyte apoptosis: implication in heart failure. Circulation. 2005;111:2469–2476. doi: 10.1161/01.CIR.0000165128.39715.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draijer R, Atsma DE, van der Laarse A, van Hinsbergh VW. cGMP and nitric oxide modulate thrombin-induced endothelial permeability. Regulation via different pathways in human aortic and umbilical vein endothelial cells. Circ Res. 1995a;76:199–208. doi: 10.1161/01.res.76.2.199. [DOI] [PubMed] [Google Scholar]
- Draijer R, Vaandrager AB, Nolte C, de Jonge HR, Walter U, van Hinsbergh VW. Expression of cGMP-dependent protein kinase I and phosphorylation of its substrate, vasodilator-stimulated phosphoprotein, in human endothelial cells of different origin. Circ Res. 1995b;77:897–905. doi: 10.1161/01.res.77.5.897. [DOI] [PubMed] [Google Scholar]
- Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, Vandecasteele G. Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res. 2006;99:816–828. doi: 10.1161/01.RES.0000246118.98832.04. [DOI] [PubMed] [Google Scholar]
- Fukuhara S, Sakurai A, Sano H, Yamagishi A, Somekawa S, Takakura N, Saito Y, Kangawa K, Mochizuki N. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol. 2010;50:355–375. doi: 10.1146/annurev.pharmtox.010909.105714. [DOI] [PubMed] [Google Scholar]
- Gupta MP, Ober MD, Patterson C, Al-Hassani M, Natarajan V, Hart CM. Nitric oxide attenuates H(2)O(2)-induced endothelial barrier dysfunction: mechanisms of protection. Am J Physiol Lung Cell Mol Physiol. 2001;280:L116–L126. doi: 10.1152/ajplung.2001.280.1.L116. [DOI] [PubMed] [Google Scholar]
- Hatakeyama T, Pappas PJ, Hobson RW, 2nd, Boric MP, Sessa WC, Duran WN. Endothelial nitric oxide synthase regulates microvascular hyperpermeability in vivo. J Physiol. 2006;574:275–281. doi: 10.1113/jphysiol.2006.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P, Liu B, Curry FE. Effect of nitric oxide synthase inhibitors on endothelial [Ca2+]i and microvessel permeability. Am J Physiol. 1997a;272:H176–H185. doi: 10.1152/ajpheart.1997.272.1.H176. [DOI] [PubMed] [Google Scholar]
- He P, Zeng M, Curry FE. Effect of nitric oxide synthase inhibitors on basal microvessel permeability and endothelial cell [Ca2+]i. Am J Physiol. 1997b;273:H747–H755. doi: 10.1152/ajpheart.1997.273.2.H747. [DOI] [PubMed] [Google Scholar]
- Hempel A, Noll T, Muhs A, Piper HM. Functional antagonism between cAMP and cGMP on permeability of coronary endothelial monolayers. Am J Physiol. 1996;270:H1264–H1271. doi: 10.1152/ajpheart.1996.270.4.H1264. [DOI] [PubMed] [Google Scholar]
- Holschermann H, Noll T, Hempel A, Piper HM. Dual role of cGMP in modulation of macromolecule permeability of aortic endothelial cells. Am J Physiol. 1997;272:H91–H98. doi: 10.1152/ajpheart.1997.272.1.H91. [DOI] [PubMed] [Google Scholar]
- Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci. 2010;35:91–100. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Baillie GS, Maurice DH. cAMP-Specific phosphodiesterase-4 enzymes in the cardiovascular system: a molecular toolbox for generating compartmentalized cAMP signaling. Circ Res. 2007;100:950–966. doi: 10.1161/01.RES.0000261934.56938.38. [DOI] [PubMed] [Google Scholar]
- Jho D, Mehta D, Ahmmed G, Gao XP, Tiruppathi C, Broman M, Malik AB. Angiopoietin-1 opposes VEGF-induced increase in endothelial permeability by inhibiting TRPC1-dependent Ca2 influx. Circ Res. 2005;96:1282–1290. doi: 10.1161/01.RES.0000171894.03801.03. [DOI] [PubMed] [Google Scholar]
- Keravis T, Komas N, Lugnier C. Cyclic nucleotide hydrolysis in bovine aortic endothelial cells in culture: differential regulation in cobblestone and spindle phenotypes. J Vasc Res. 2000;37:235–249. doi: 10.1159/000025738. [DOI] [PubMed] [Google Scholar]
- Kim D, Aizawa T, Wei H, Pi X, Rybalkin SD, Berk BC, Yan C. Angiotensin II increases phosphodiesterase 5A expression in vascular smooth muscle cells: a mechanism by which angiotensin II antagonizes cGMP signaling. J Mol Cell Cardiol. 2005;38:175–184. doi: 10.1016/j.yjmcc.2004.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Rybalkin SD, Pi X, Wang Y, Zhang C, Munzel T, Beavo JA, Berk BC, Yan C. Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation. 2001;104:2338–2343. doi: 10.1161/hc4401.098432. [DOI] [PubMed] [Google Scholar]
- Klinger JR, Warburton R, Carino GP, Murray J, Murphy C, Napier M, Harrington EO. Natriuretic peptides differentially attenuate thrombin-induced barrier dysfunction in pulmonary microvascular endothelial cells. Exp Cell Res. 2006;312:401–410. doi: 10.1016/j.yexcr.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Koga S, Morris S, Ogawa S, Liao H, Bilezikian JP, Chen G, Thompson WJ, Ashikaga T, Brett J, Stern DM, et al. TNF modulates endothelial properties by decreasing cAMP. Am J Physiol. 1995;268:C1104–C1113. doi: 10.1152/ajpcell.1995.268.5.C1104. [DOI] [PubMed] [Google Scholar]
- Komarova Y, Malik AB. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu Rev Physiol. 2010;72:463–493. doi: 10.1146/annurev-physiol-021909-135833. [DOI] [PubMed] [Google Scholar]
- Komarova YA, Mehta D, Malik AB. Dual regulation of endothelial junctional permeability. Sci STKE. 2007;2007:re8. doi: 10.1126/stke.4122007re8. [DOI] [PubMed] [Google Scholar]
- Kooistra MR, Corada M, Dejana E, Bos JL. Epac1 regulates integrity of endothelial cell junctions through VE-cadherin. FEBS Lett. 2005;579:4966–4972. doi: 10.1016/j.febslet.2005.07.080. [DOI] [PubMed] [Google Scholar]
- Lang P, Gesbert F, Delespine-Carmagnat M, Stancou R, Pouchelet M, Bertoglio J. Protein kinase A phosphorylation of RhoA mediates the morphological and functional effects of cyclic AMP in cytotoxic lymphocytes. EMBO J. 1996;15:510–519. [PMC free article] [PubMed] [Google Scholar]
- Langeler EG, van Hinsbergh VW. Norepinephrine and iloprost improve barrier function of human endothelial cell monolayers: role of cAMP. Am J Physiol. 1991;260:C1052–C1059. doi: 10.1152/ajpcell.1991.260.5.C1052. [DOI] [PubMed] [Google Scholar]
- Leemhuis J, Boutillier S, Schmidt G, Meyer DK. The protein kinase A inhibitor H89 acts on cell morphology by inhibiting Rho kinase. J Pharmacol Exp Ther. 2002;300:1000–1007. doi: 10.1124/jpet.300.3.1000. [DOI] [PubMed] [Google Scholar]
- Leroy MJ, Degerman E, Taira M, Murata T, Wang LH, Movsesian MA, Meacci E, Manganiello VC. Characterization of two recombinant PDE3 (cGMP-inhibited cyclic nucleotide phosphodiesterase) isoforms, RcGIP1 and HcGIP2, expressed in NIH 3006 murine fibroblasts and Sf9 insect cells. Biochemistry. 1996;35:10194–10202. doi: 10.1021/bi952711t. [DOI] [PubMed] [Google Scholar]
- Li L, Yee C, Beavo JA. CD3- and CD28-dependent induction of PDE7 required for T cell activation. Science. 1999;283:848–851. doi: 10.1126/science.283.5403.848. [DOI] [PubMed] [Google Scholar]
- Liu SM, Sundqvist T. Nitric oxide and cGMP regulate endothelial permeability and F-actin distribution in hydrogen peroxide-treated endothelial cells. Exp Cell Res. 1997;235:238–244. doi: 10.1006/excr.1997.3675. [DOI] [PubMed] [Google Scholar]
- Lorenowicz MJ, Fernandez-Borja M, Kooistra MR, Bos JL, Hordijk PL. PKA and Epac1 regulate endothelial integrity and migration through parallel and independent pathways. Eur J Cell Biol. 2008;87:779–792. doi: 10.1016/j.ejcb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- Michel CC, Curry FE. Microvascular permeability. Physiol Rev. 1999;79:703–761. doi: 10.1152/physrev.1999.79.3.703. [DOI] [PubMed] [Google Scholar]
- Miro X, Casacuberta JM, Gutierrez-Lopez MD, de Landazuri MO, Puigdomenech P. Phosphodiesterases 4D and 7A splice variants in the response of HUVEC cells to TNF-alpha(1) Biochem Biophys Res Commun. 2000;274:415–421. doi: 10.1006/bbrc.2000.3146. [DOI] [PubMed] [Google Scholar]
- Moldobaeva A, Welsh-Servinsky LE, Shimoda LA, Stephens RS, Verin AD, Tuder RM, Pearse DB. Role of protein kinase G in barrier-protective effects of cGMP in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L919–L930. doi: 10.1152/ajplung.00434.2005. [DOI] [PubMed] [Google Scholar]
- Moore TM, Chetham PM, Kelly JJ, Stevens T. Signal transduction and regulation of lung endothelial cell permeability. Interaction between calcium and cAMP. Am J Physiol. 1998;275:L203–L222. doi: 10.1152/ajplung.1998.275.2.L203. [DOI] [PubMed] [Google Scholar]
- Murray AJ. Pharmacological PKA inhibition: all may not be what it seems. Sci Signal. 2008;1:re4. doi: 10.1126/scisignal.122re4. [DOI] [PubMed] [Google Scholar]
- Netherton SJ, Maurice DH. Vascular endothelial cell cyclic nucleotide phosphodiesterases and regulated cell migration: implications in angiogenesis. Mol Pharmacol. 2005;67:263–272. doi: 10.1124/mol.104.004853. [DOI] [PubMed] [Google Scholar]
- Ozaki H, Hla T, Lee MJ. Sphingosine-1-phosphate signaling in endothelial activation. J Atheroscler Thromb. 2003;10:125–131. doi: 10.5551/jat.10.125. [DOI] [PubMed] [Google Scholar]
- Pizurki L, Zhou Z, Glynos K, Roussos C, Papapetropoulos A. Angiopoietin-1 inhibits endothelial permeability, neutrophil adherence and IL-8 production. Br J Pharmacol. 2003;139:329–336. doi: 10.1038/sj.bjp.0705259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasain N, Alexeyev M, Balczon R, Stevens T. Soluble adenylyl cyclase-dependent microtubule disassembly reveals a novel mechanism of endothelial cell retraction. Am J Physiol Lung Cell Mol Physiol. 2009;297:L73–L83. doi: 10.1152/ajplung.90577.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predescu D, Predescu S, Shimizu J, Miyawaki-Shimizu K, Malik AB. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am J Physiol Lung Cell Mol Physiol. 2005;289:L371–L381. doi: 10.1152/ajplung.00175.2004. [DOI] [PubMed] [Google Scholar]
- Qiao J, Holian O, Lee BS, Huang F, Zhang J, Lum H. Phosphorylation of GTP dissociation inhibitor by PKA negatively regulates RhoA. Am J Physiol Cell Physiol. 2008;295:C1161–C1168. doi: 10.1152/ajpcell.00139.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao J, Huang F, Lum H. PKA inhibits RhoA activation: a protection mechanism against endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2003;284:L972–L980. doi: 10.1152/ajplung.00429.2002. [DOI] [PubMed] [Google Scholar]
- Rahn Landstrom T, Mei J, Karlsson M, Manganiello V, Degerman E. Down-regulation of cyclic-nucleotide phosphodiesterase 3B in 3T3-L1 adipocytes induced by tumour necrosis factor alpha and cAMP. Biochem J. 2000;346(Pt 2):337–343. doi: 10.1042/bj3460337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybalkin SD, Bornfeldt KE, Sonnenburg WK, Rybalkina IG, Kwak KS, Hanson K, Krebs EG, Beavo JA. Calmodulin-stimulated cyclic nucleotide phosphodiesterase (PDE1C) is induced in human arterial smooth muscle cells of the synthetic, proliferative phenotype. J Clin Invest. 1997;100:2611–2621. doi: 10.1172/JCI119805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabrane K, Kruse MN, Fabritz L, Zetsche B, Mitko D, Skryabin BV, Zwiener M, Baba HA, Yanagisawa M, Kuhn M. Vascular endothelium is critically involved in the hypotensive and hypovolemic actions of atrial natriuretic peptide. J Clin Invest. 2005;115:1666–1674. doi: 10.1172/JCI23360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satchell SC, Anderson KL, Mathieson PW. Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J Am Soc Nephrol. 2004;15:566–574. doi: 10.1097/01.asn.0000115397.22519.03. [DOI] [PubMed] [Google Scholar]
- Sayner S, Stevens T. Soluble adenylate cyclase reveals the significance of compartmentalized cAMP on endothelial cell barrier function. Biochem Soc Trans. 2006;34:492–494. doi: 10.1042/BST0340492. [DOI] [PubMed] [Google Scholar]
- Sayner SL, Alexeyev M, Dessauer CW, Stevens T. Soluble adenylyl cyclase reveals the significance of cAMP compartmentation on pulmonary microvascular endothelial cell barrier. Circ Res. 2006;98:675–681. doi: 10.1161/01.RES.0000209516.84815.3e. [DOI] [PubMed] [Google Scholar]
- Sayner SL, Frank DW, King J, Chen H, VandeWaa J, Stevens T. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res. 2004;95:196–203. doi: 10.1161/01.RES.0000134922.25721.d9. [DOI] [PubMed] [Google Scholar]
- Seybold J, Thomas D, Witzenrath M, Boral S, Hocke AC, Burger A, Hatzelmann A, Tenor H, Schudt C, Krull M, Schutte H, Hippenstiel S, Suttorp N. Tumor necrosis factor-alpha-dependent expression of phosphodiesterase 2: role in endothelial hyperpermeability. Blood. 2005;105:3569–3576. doi: 10.1182/blood-2004-07-2729. [DOI] [PubMed] [Google Scholar]
- Surapisitchat J, Jeon KI, Yan C, Beavo JA. Differential regulation of endothelial cell permeability by cGMP via phosphodiesterases 2 and 3. Circ Res. 2007;101:811–818. doi: 10.1161/CIRCRESAHA.107.154229. [DOI] [PubMed] [Google Scholar]
- Suttorp N, Ehreiser P, Hippenstiel S, Fuhrmann M, Krull M, Tenor H, Schudt C. Hyperpermeability of pulmonary endothelial monolayer: protective role of phosphodiesterase isoenzymes 3 and 4. Lung. 1996a;174:181–194. doi: 10.1007/BF00173310. [DOI] [PubMed] [Google Scholar]
- Suttorp N, Hippenstiel S, Fuhrmann M, Krull M, Podzuweit T. Role of nitric oxide and phosphodiesterase isoenzyme II for reduction of endothelial hyperpermeability. Am J Physiol. 1996b;270:C778–C785. doi: 10.1152/ajpcell.1996.270.3.C778. [DOI] [PubMed] [Google Scholar]
- Suttorp N, Weber U, Welsch T, Schudt C. Role of phosphodiesterases in the regulation of endothelial permeability in vitro. J Clin Invest. 1993;91:1421–1428. doi: 10.1172/JCI116346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa Y. Subtype-specific differential regulation of Rho family G proteins and cell migration by the Edg family sphingosine-1-phosphate receptors. Biochim Biophys Acta. 2002;1582:112–120. doi: 10.1016/s1388-1981(02)00145-2. [DOI] [PubMed] [Google Scholar]
- Tsigkos S, Koutsilieris M, Papapetropoulos A. Angiopoietins in angiogenesis and beyond. Expert Opin Investig Drugs. 2003;12:933–941. doi: 10.1517/13543784.12.6.933. [DOI] [PubMed] [Google Scholar]
- Tucker VL, Simanonok KE, Renkin EM. Tissue-specific effects of physiological ANP infusion on blood-tissue albumin transport. Am J Physiol. 1992;263:R945–R953. doi: 10.1152/ajpregu.1992.263.4.R945. [DOI] [PubMed] [Google Scholar]
- van Hinsbergh WM. Endothelial permeability for macromolecules. Mechanistic aspects of pathophysiological modulation. Arterioscler Thromb Vasc Biol. 1997;17:1018–1023. doi: 10.1161/01.atv.17.6.1018. [DOI] [PubMed] [Google Scholar]
- van Nieuw Amerongen GP, Loomans DS, van Hinsbergh VW. Mechanisms involved in endothelial hyperpermeability and endothelial barrier stabilization. In: Catravas JD, editor. Vascular endothelium: source and target of inflammatory mediators. IOS Press; Amsterdam: 2001. [Google Scholar]
- van Nieuw Amerongen GP, van Hinsbergh VW. Targets for pharmacological intervention of endothelial hyperpermeability and barrier function. Vascul Pharmacol. 2002;39:257–272. doi: 10.1016/s1537-1891(03)00014-4. [DOI] [PubMed] [Google Scholar]
- Vandecasteele G, Verde I, Rucker-Martin C, Donzeau-Gouge P, Fischmeister R. Cyclic GMP regulation of the L-type Ca(2+) channel current in human atrial myocytes. J Physiol. 2001;533:329–340. doi: 10.1111/j.1469-7793.2001.0329a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verin AD, Gilbert-McClain LI, Patterson CE, Garcia JG. Biochemical regulation of the nonmuscle myosin light chain kinase isoform in bovine endothelium. Am J Respir Cell Mol Biol. 1998;19:767–776. doi: 10.1165/ajrcmb.19.5.3126. [DOI] [PubMed] [Google Scholar]
- Westendorp RG, Draijer R, Meinders AE, van Hinsbergh VW. Cyclic-GMP-mediated decrease in permeability of human umbilical and pulmonary artery endothelial cell monolayers. J Vasc Res. 1994;31:42–51. doi: 10.1159/000159030. [DOI] [PubMed] [Google Scholar]
- Wittchen ES, Worthylake RA, Kelly P, Casey PJ, Quilliam LA, Burridge K. Rap1 GTPase inhibits leukocyte transmigration by promoting endothelial barrier function. J Biol Chem. 2005;280:11675–11682. doi: 10.1074/jbc.M412595200. [DOI] [PubMed] [Google Scholar]
- Wong D, Dorovini-Zis K, Vincent SR. Cytokines, nitric oxide, and cGMP modulate the permeability of an in vitro model of the human blood–brain barrier. Exp Neurol. 2004;190:446–455. doi: 10.1016/j.expneurol.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Manganiello VC, Vaughan M. Purification and characterization of cyclic GMP-stimulated cyclic nucleotide phosphodiesterase from calf liver. Effects of divalent cations on activity. J Biol Chem. 1983;258:12526–12533. [PubMed] [Google Scholar]
- Yatomi Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine-1-phosphate: a platelet-activating sphingolipid released from agonist-stimulated human platelets. Blood. 1995;86:193–202. [PubMed] [Google Scholar]
- Zimmerman RS, Trippodo NC, MacPhee AA, Martinez AJ, Barbee RW. High-dose atrial natriuretic factor enhances albumin escape from the systemic but not the pulmonary circulation. Circ Res. 1990;67:461–468. doi: 10.1161/01.res.67.2.461. [DOI] [PubMed] [Google Scholar]