Abstract
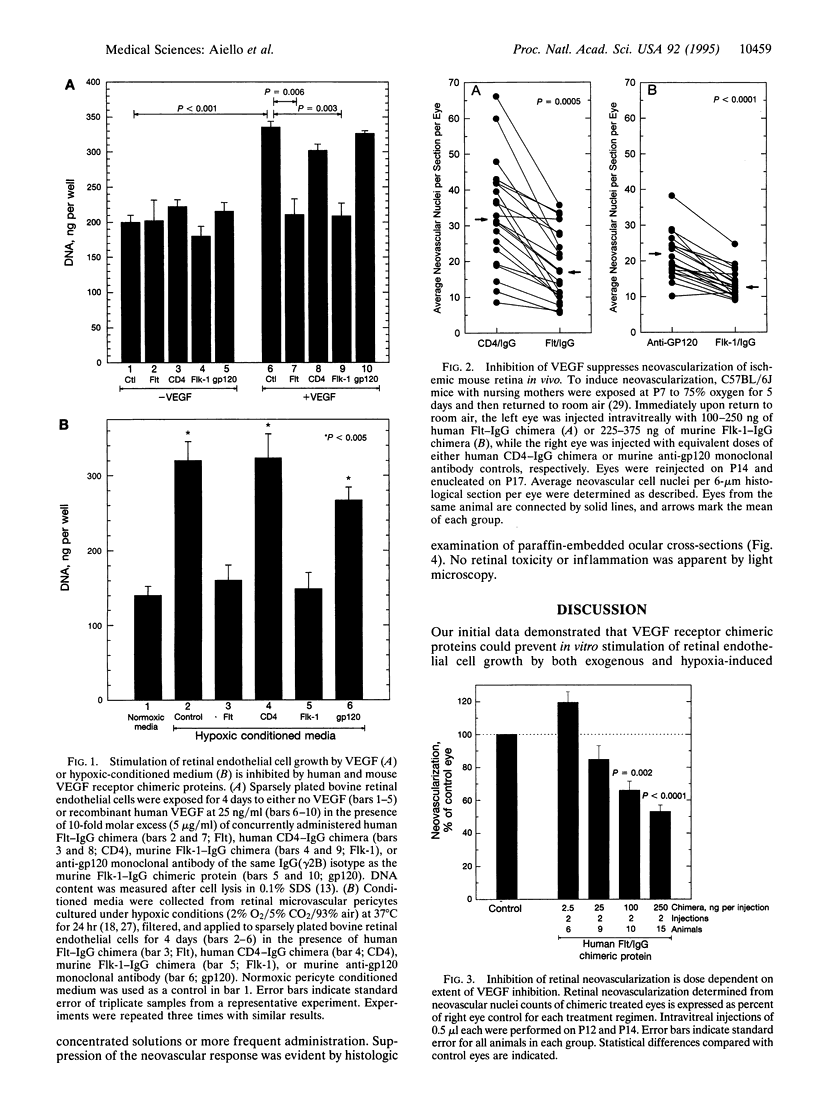
The majority of severe visual loss in the United States results from complications associated with retinal neovascularization in patients with ischemic ocular diseases such as diabetic retinopathy, retinal vein occlusion, and retinopathy of prematurity. Intraocular expression of the angiogenic protein vascular endothelial growth factor (VEGF) is closely correlated with neovascularization in these human disorders and with ischemia-induced retinal neovascularization in mice. In this study, we evaluated whether in vivo inhibition of VEGF action could suppress retinal neovascularization in a murine model of ischemic retinopathy. VEGF-neutralizing chimeric proteins were constructed by joining the extracellular domain of either human (Flt) or mouse (Flk) high-affinity VEGF receptors with IgG. Control chimeric proteins that did not bind VEGF were also used. VEGF-receptor chimeric proteins eliminated in vitro retinal endothelial cell growth stimulation by either VEGF (P < 0.006) or hypoxic conditioned medium (P < 0.005) without affecting growth under nonstimulated conditions. Control proteins had no effect. To assess in vivo response, animals with bilateral retinal ischemia received intravitreal injections of VEGF antagonist in one eye and control protein in the contralateral eye. Retinal neovascularization was quantitated histologically by a masked protocol. Retinal neovascularization in the eye injected with human Flt or murine Flk chimeric protein was reduced in 100% (25/25; P < 0.0001) and 95% (21/22; P < 0.0001) 0.0001) of animals, respectively, compared to the control treated eye. This response was evident after only a single intravitreal injection and was dose dependent with suppression of neovascularization noted after total delivery of 200 ng of protein (P < 0.002). Reduction of histologically evident neovascular nuclei per 6-microns section averaged 47% +/- 4% (P < 0.001) and 37% +/- 2% (P < 0.001) for Flt and Flk chimeric proteins with maximal inhibitory effects of 77% and 66%, respectively. No retinal toxicity was observed by light microscopy. These data demonstrate VEGF's causal role in retinal angiogenesis and prove the potential of VEGF inhibition as a specific therapy for ischemic retinal disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHTON N., WARD B., SERPELL G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954 Jul;38(7):397–432. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamis A. P., Miller J. W., Bernal M. T., D'Amico D. J., Folkman J., Yeo T. K., Yeo K. T. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994 Oct 15;118(4):445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- Adamis A. P., Shima D. T., Yeo K. T., Yeo T. K., Brown L. F., Berse B., D'Amore P. A., Folkman J. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem Biophys Res Commun. 1993 Jun 15;193(2):631–638. doi: 10.1006/bbrc.1993.1671. [DOI] [PubMed] [Google Scholar]
- Aiello L. P., Avery R. L., Arrigg P. G., Keyt B. A., Jampel H. D., Shah S. T., Pasquale L. R., Thieme H., Iwamoto M. A., Park J. E. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994 Dec 1;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Berse B., Brown L. F., Van de Water L., Dvorak H. F., Senger D. R. Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell. 1992 Feb;3(2):211–220. doi: 10.1091/mbc.3.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikfalvi A., Sauzeau C., Moukadiri H., Maclouf J., Busso N., Bryckaert M., Plouet J., Tobelem G. Interaction of vasculotropin/vascular endothelial cell growth factor with human umbilical vein endothelial cells: binding, internalization, degradation, and biological effects. J Cell Physiol. 1991 Oct;149(1):50–59. doi: 10.1002/jcp.1041490108. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Houck K., Jakeman L., Leung D. W. Molecular and biological properties of the vascular endothelial growth factor family of proteins. Endocr Rev. 1992 Feb;13(1):18–32. doi: 10.1210/edrv-13-1-18. [DOI] [PubMed] [Google Scholar]
- Houck K. A., Ferrara N., Winer J., Cachianes G., Li B., Leung D. W. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991 Dec;5(12):1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- Houck K. A., Leung D. W., Rowland A. M., Winer J., Ferrara N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem. 1992 Dec 25;267(36):26031–26037. [PubMed] [Google Scholar]
- King G. L., Goodman A. D., Buzney S., Moses A., Kahn C. R. Receptors and growth-promoting effects of insulin and insulinlike growth factors on cells from bovine retinal capillaries and aorta. J Clin Invest. 1985 Mar;75(3):1028–1036. doi: 10.1172/JCI111764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D. W., Cachianes G., Kuang W. J., Goeddel D. V., Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989 Dec 8;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- McNamara J. A., Tasman W., Vander J. F., Brown G. C. Diode laser photocoagulation for retinopathy of prematurity. Preliminary results. Arch Ophthalmol. 1992 Dec;110(12):1714–1716. doi: 10.1001/archopht.1992.01080240054029. [DOI] [PubMed] [Google Scholar]
- Miller J. W., Adamis A. P., Shima D. T., D'Amore P. A., Moulton R. S., O'Reilly M. S., Folkman J., Dvorak H. F., Brown L. F., Berse B. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol. 1994 Sep;145(3):574–584. [PMC free article] [PubMed] [Google Scholar]
- Nayak R. C., Berman A. B., George K. L., Eisenbarth G. S., King G. L. A monoclonal antibody (3G5)-defined ganglioside antigen is expressed on the cell surface of microvascular pericytes. J Exp Med. 1988 Mar 1;167(3):1003–1015. doi: 10.1084/jem.167.3.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. E., Chen H. H., Winer J., Houck K. A., Ferrara N. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J Biol Chem. 1994 Oct 14;269(41):25646–25654. [PubMed] [Google Scholar]
- Pierce E. A., Avery R. L., Foley E. D., Aiello L. P., Smith L. E. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):905–909. doi: 10.1073/pnas.92.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plate K. H., Breier G., Weich H. A., Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992 Oct 29;359(6398):845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Galli S. J., Dvorak A. M., Perruzzi C. A., Harvey V. S., Dvorak H. F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983 Feb 25;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Feder J., Dvorak H. F. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986 Nov;46(11):5629–5632. [PubMed] [Google Scholar]
- Shweiki D., Itin A., Soffer D., Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Simorre-Pinatel V., Guerrin M., Chollet P., Penary M., Clamens S., Malecaze F., Plouet J. Vasculotropin-VEGF stimulates retinal capillary endothelial cells through an autocrine pathway. Invest Ophthalmol Vis Sci. 1994 Aug;35(9):3393–3400. [PubMed] [Google Scholar]
- Smith L. E., Wesolowski E., McLellan A., Kostyk S. K., D'Amato R., Sullivan R., D'Amore P. A. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994 Jan;35(1):101–111. [PubMed] [Google Scholar]
- Stefansson E. Oxygen and diabetic eye disease. Graefes Arch Clin Exp Ophthalmol. 1990;228(2):120–123. doi: 10.1007/BF00935719. [DOI] [PubMed] [Google Scholar]
- Thieme H., Aiello L. P., Takagi H., Ferrara N., King G. L. Comparative analysis of vascular endothelial growth factor receptors on retinal and aortic vascular endothelial cells. Diabetes. 1995 Jan;44(1):98–103. doi: 10.2337/diab.44.1.98. [DOI] [PubMed] [Google Scholar]
- de Vries C., Escobedo J. A., Ueno H., Houck K., Ferrara N., Williams L. T. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992 Feb 21;255(5047):989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]