Abstract
Background
The use of digital reactive hyperemia as a measure of endothelial function among children and adolescents is becoming increasingly common. However, unexpected observations of low reactive hyperemic index values in younger children in our laboratory led us to conduct a study evaluating the influence of age, sex, height, weight, blood pressure, body mass index (BMI), and finger volume on RHI values.
Methods
Endothelial function, measured by digital reactive hyperemia (reactive hyperemic index: RHI) was assessed in 113 children and adolescents (mean age 12.4±3.8 years; 64 males), with 102 also assessed for brachial artery flow-mediated dilation (FMD) using ultrasound imaging. Associations with age, sex, height, weight, systolic and diastolic blood pressure (SBP, DBP), BMI, and finger volume were evaluated.
Results
Using GLM regression, age (β=0.03, P=0.014) and SBP (β=0.015, P=0.004) were significantly associated with RHI. No measures were associated with FMD. In the subset of individuals with measured finger volume, age (β=0.025, P=0.037) was the only measure significantly associated with log RHI. Similarly, no measures were associated with FMD.
Conclusion
Younger age is associated with lower RHI but not lower FMD among children and adolescents. These findings call into question the validity and usefulness of digital reactive hyperemia as a method to quantify endothelial function among younger children.
Keywords: Endothelial Function, Reactive Hyperemic Index, Flow-Mediated Dilation, Children, Adolescents
BACKGROUND
The pathological process of cardiovascular disease begins in the first two decades of life.1–4 Therefore, identifying the initial signs of potential problems among children and adolescents may improve screening, risk stratification, and lead to earlier intervention. Endothelial activation is one of the seminal events of the atherosclerotic process, and various measures of endothelial dysfunction have been shown to predict subsequent atherosclerosis5–6 and future cardiovascular events.6–9 Because of its non-invasive nature, the most commonly-employed technique used to measure endothelial function in children is ultrasound imaging of the brachial artery during flow-mediated dilation (FMD).10 However, new methods have emerged including the measurement of digital reactive hyperemia, which quantifies small changes in finger volume at rest and during reactive hyperemia. Endothelial function, as assessed by this technique, is nitric oxide-dependent11 and has been demonstrated to be positively associated with coronary artery blood flow12 and negatively with multiple cardiovascular risk factors.13 Furthermore, lower values have been shown to independently predict future cardiovascular events in adults.14 Recently, a number of pediatric studies have utilized this technique using the EndoPAT device (EndoPAT 2000, Itamar Medical, Caesarea, Israel).15–19
As our research group began using the EndoPAT device in some of our pediatric studies, we observed that younger participants often had very low reactive hyperemic index (RHI) values. Subsequently, two separate research groups reported interesting associations between pubertal development and RHI15,20 and between age, sex, and RHI16 in children and adolescents. In an attempt to investigate these relationships further and compare them to another endothelial function technique, we conducted a study in which we simultaneously measured RHI along with FMD to evaluate their respective associations with age, sex, height, weight, blood pressure, body mass index (BMI), and finger volume. We hypothesized that age and body size measures (including finger volume) would be significantly associated with RHI but not FMD, owing to the fact that the finger probes used with the digital reactive hyperemia technique were designed for adults (i.e., the “one size fits all” nature of the finger probe may not be valid for use in children). We believed this phenomenon might explain the low RHI values often observed among younger children.
MATERIALS AND METHODS
The study protocol was approved by the University of Minnesota Institutional Review Board (IRB). The study procedures adhered to the University of Minnesota’s IRB and the Health Insurance Portability and Accountability Act (HIPAA) guidelines. All parents and participants provided written informed consent and assent, respectively.
Study Design and Participants
This cross-sectional study included 113 children and adolescents (64 males, 49 females) between 6 and 19 years of age (mean age 12.4±3.8 years) who either participated in a longitudinal community-based study investigating the early development of obesity, insulin resistance, and other cardiovascular risk factors among families, or served as healthy controls in a study assessing the vascular effects of stimulant medication use in attention deficit hyperactivity disorder. Children and adolescents from the longitudinal cohort were healthy community-dwelling youth and were included if they were offspring of parents who had participated in the initial study (many years prior). No exclusion criteria were used. Brachial artery FMD measures were obtained on all but 11 of the subjects. Inability to obtain FMD measures was the result of too much movement by the participant leading to poor image quality. Finger volume was obtained on 48 of the subjects (finger volume measurements were initiated approximately halfway through the study).
Assessment of Anthropometrics, Pubertal Development, and Other Clinical Variables
Height and weight were obtained using a standard stadiometer and electronic scale, respectively. BMI was calculated as weight in kilograms divided by height in meters-squared, and BMI percentile was determined based on population data from the United States. Left finger volume was measured by water displacement and reported to the nearest 0.25 mL. Tanner stage was determined by trained pediatricians. A single blood pressure measurement was obtained on the right arm with an appropriate cuff size using an automatic sphygmomanometer after 5 minutes of quiet rest in the supine position. Fasting (≥8 hours) blood samples were analyzed for glucose, insulin, and lipids using standard procedures.
Measurement of Endothelial Function
The vascular testing was performed in the morning after the participants had been fasting for at least 8 hours. Following 15 minutes of quiet rest in the supine position, RHI (EndoPAT 2000, Itamar Medical, Caesarea, Israel) and brachial artery FMD using a conventional ultrasound scanner (Siemens, Sequoia 512, New York, NY, USA) were assessed simultaneously. A 15-8 MHz linear array probe held at a constant distance from the skin with a stereotactic arm and at a fixed point over the imaged brachial artery was utilized during FMD assessment. Following baseline measurements in the index fingers and brachial artery, a blood pressure cuff was placed on the upper forearm (immediately distal to the elbow) and inflated to a supra-systolic level for 5 minutes using techniques previously described.21–22 RHI was calculated with an automated algorithm using the EndoPAT device. The algorithm derives RHI values by calculating the ratio of pulse amplitude for 60 seconds beginning one minute after cuff release (an average of the 60- to 90-second and 90- to 120-second intervals) to the baseline pulse amplitude divided by the corresponding ratio in the control finger. Brachial artery FMD images were digitized and stored on a personal computer for later off-line analysis with an electronic wall-tracking software program (Medical Imaging Applications, Coralville, IA, USA). FMD was calculated as the maximal percent change diameter in relation to the baseline diameter, regardless of when the maximal diameter change occurred after cuff release (i.e., no set time point post-cuff release was used). Reproducibility of FMD23 and RHI24 has been previously reported.
Statistical Analysis
Statistical analyses were performed with Stata/SE 12.0 (StataCorp, College Station, TX, USA). Kolmogorov-Smirnov tests were used to test measures for normal distribution. Independent measurements included age, sex, height, weight, systolic blood pressure (SBP), diastolic blood pressure (DBP), BMI, and BMI percentile. Dependent measures included RHI and FMD; RHI was distributed non-normally and FMD was distributed normally. Demographic results are expressed as mean ± standard deviation (SD) for normally distributed data, and median ± interquartile range (IQR) for non-normal data. An alpha value of 0.05 was used to signify statistical significance.
Due to RHI non-normality within the main analysis, a generalized linear model (GLM) with a statistically determined ‘family-link’ was applied. A Modified Park Test was used to identify the appropriate ‘family’ as ‘inverse-Gaussian (i.e. Wald),’ and a Pregibon Link Test was used to identify the ‘identity’ link for the model. Moreover, an inverse-Gaussian GLM with an identity link was used to evaluate possible associations between RHI and the other measures. Conversely, given the normal distribution of FMD within the main analysis, an ordinary least squares (OLS) regression was used to evaluate possible associations between FMD and these measures as well.
Within a smaller subset of individuals with available finger volume data, RHI data were logarithmically transformed to generate a close-to-normal distribution. FMD data were normally distributed. Therefore, an OLS regression was used to evaluate possible associations between both RHI and FMD with the other measures. A cubic transformation was applied to DBP within the smaller subset due to its non-normal distribution.
Within all analyses, height, weight, BMI percentile, and right finger volume were not included within the regression analysis due to strong correlations (ρ>0.70, multi-collinearity) concerns between age-height and weight, height-weight, BMI-weight, BMI-BMI percentile, and right-left finger volume. Additionally, Spearman’s rank-order correlation analyses were used to assess potential associations between RHI and FMD with body size measures (including finger volume).
Age groups 6–9 years, 10–14 years, and 15–19 years were further assessed for differences in RHI and FMD. A Kruskal-Wallis one-way analysis of variance (ANOVA) and Mann-Whitney rank sum tests were used to assess potential age group differences in RHI, and a one-way ANOVA with a Bonferroni correction was performed to assess differences in FMD and peak shear stress between age groups. A separate analysis of possible associations between RHI and FMD and the other measures was also performed for all subjects who had Tanner stage assessed. The measures for this separate analysis included sex, Tanner stage, SBP, DBP, and BMI. Age was excluded from the analysis due to multi-collinearity concerns with Tanner stage.
RESULTS
The study included 113 children and adolescents (mean age 12.4±3.8 years; 64 males). Descriptive, clinical, and vascular characteristics are displayed in Table 1. Median RHI and mean FMD for the entire study population were 1.26±0.45 and 7.56±3.69%, respectively.
Table 1.
Demographic, Clinical, and Vascular Characteristics
| Age (years) | 12.4±3.8 |
|
| |
| Sex (n, %) | |
| Male | 64 (57%) |
| Female | 49 (43%) |
|
| |
| Tanner Stage (n, %) † | |
| Tanner I | 10 (9%) |
| Tanner II | 5 (4%) |
| Tanner III | 7 (6%) |
| Tanner IV | 12 (11%) |
| Tanner V | 9 (8%) |
|
| |
| Race (n, %) | |
| White | 79 (70%) |
| Black | 27 (24%) |
| American Indian | 4 (4%) |
| Asian/Pacific Islander | 1 (<1%) |
| Other/More than one race | 2 (2%) |
|
| |
| Height (cm) | 150.7±18.8 |
|
| |
| Weight (kg) | 49.7±22.7 |
|
| |
| BMI (kg/m2) | 20.6±5.6 |
|
| |
| Systolic BP (mmHg) | 109.0±10.4 |
|
| |
| Diastolic BP (mmHg) | 55.7±7.3 |
|
| |
| Pulse Pressure (mmHg) | 53.2±8.8 |
|
| |
| Glucose (mg/dL) * | 74.7±14.8 |
|
| |
| Insulin (mU/L) * | 7.1±7.1 |
|
| |
| Total Cholesterol (mg/dL) * | 150.1±25.6 |
|
| |
| LDL-Cholesterol (mg/dL) * | 85.5±20.7 |
|
| |
| HDL-Cholesterol (mg/dL) * | 50.4±13.3 |
|
| |
| Triglycerides (mg/dL) * | 70.8±27.5 |
|
| |
| Finger Volume (mL) † | 6.58±1.28 |
|
| |
| Peak Shear Stress (sec −1) † | 326.89±79.28 |
|
| |
| RHI, median±IQR | 1.26±0.45 |
|
| |
| Peak FMD (%) † | 7.56±3.69 |
|
| |
| Baseline Diameter (mm) † | 3.10±0.51 |
All measures (N=113) listed as Mean (±SD), unless otherwise noted. BP, blood pressure; BMI, body mass index; Mlbm, insulin resistance; LDL, low-density lipoproteins; HDL, high-density lipoproteins; RHI, reactive hyperemic index; FMD, flow-mediated dilation.
Tanner stage, Finger Volume, Peak Shear Stress, Peak FMD, and Baseline Diameter were assessed in n=43, 48, 71, 102, and 102 subjects, respectively.
Total Cholesterol, LDL-Cholesterol, Triglycerides, and Fasting Insulin were assessed in n=62, Fasting Glucose in n=61
Main Analysis
Table 2 displays variables and their associations with RHI and FMD within the main analysis. Specifically, age and SBP were significantly (positively) associated with RHI, while FMD was not significantly associated with any of the variables. A significant correlation was observed between RHI and age (ρ=0.444, P<0.0001), as well as weight (ρ=0.415, P<0.0001). Additionally, RHI and FMD were not statistically significantly correlated (ρ=−0.0046, P=0.9630).
Table 2.
Main Analysis: Model Associations between RHI, FMD, and Childhood Measures
| RHI β (P-value) |
Peak FMD β (P-value) |
|
|---|---|---|
| Age (yrs) | 0.030 (0.014) * | 0.013 (0.916) |
| Sex (Female) | 0.119 (0.096) | 0.903 (0.223) |
| BMI (kg/m2) | −0.006 (0.539) | 0.149 (0.083) |
| Systolic BP (mmHg) | 0.015 (0.004) * | 0.002 (0.967) |
| Diastolic BP (mmHg) | −0.008 (0.196) | −0.019 (0.760) |
The main analysis included subjects with RHI (n=113) and FMD (n=102) measures, and was assessed for associations among childhood measure. BMI, body mass index; BP, blood pressure.
Denotes clinical significance (P-value <0.05).
Finger Volume (Subset) Analysis
Table 3 displays variables and their associations with RHI and FMD among the subset with finger volume measurements. Age (β=0.025, P=0.037) was the only measure significantly associated with log transformed RHI. Conversely, FMD was not associated with any measures. Spearman’s correlations between RHI, FMD, and the other variables are displayed in Table 3. Most variables were significantly correlated with RHI, while FMD was not significantly associated with any of the variables. Additionally, a significant correlation was observed between age and finger volume (ρ=0.559, P<0.0001), weight (ρ=0.824, P<0.0001), and BMI (ρ=0.565, P<0.0001).
Table 3.
Finger Volume Analysis: Spearman’s Correlations between RHI, Peak FMD, and Childhood Measures
| RHI ρ (P-value) |
Peak FMD ρ (P-value) |
|
|---|---|---|
| Age (yrs) | 0.595 (<0.0001) * | 0.088 (0.586) |
| Height (cm) | 0.542 (0.0001) * | 0.134 (0.404) |
| Weight (kg) | 0.571 (<0.0001) * | 0.201 (0.207) |
| BMI (kg/m2) | 0.548 (0.0001) * | 0.264 (0.095) |
| Systolic BP (mmHg) | 0.354 (0.014) * | 0.196 (0.220) |
| Diastolic BP (mmHg) | 0.158 (0.284) | 0.037 (0.816) |
| Finger Volume (mL) | 0.508 (0.0002) * | 0.086 (0.592) |
A finger volume analysis included subjects with RHI (n=48) and FMD (n=41) measures, and was assessed for childhood measure correlations. BMI, body mass index; BP, blood pressure.
Denotes clinical significance (P-value <0.05).
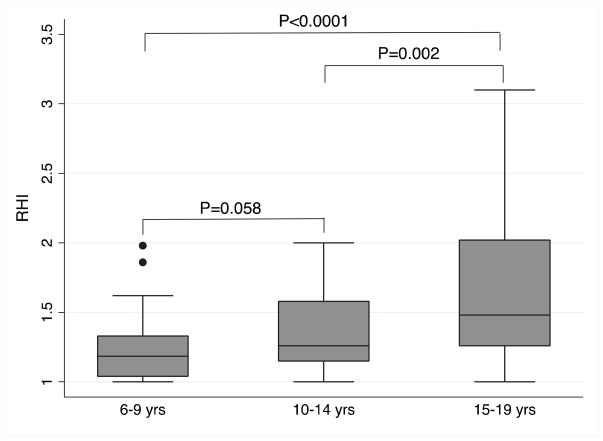
Median RHI values, by age group, are displayed in Figure 1. Analysis between age groups 6–9 years, 10–14 years, and 15–19 years revealed median RHI values of 1.19±0.29, 1.26±0.43, and 1.48±0.76, respectively. Mean FMD among the different age groups was 6.68±3.70, 7.69±3.20, and 8.49±3.97 %, respectively. Both median RHI data among ages 6–9 years (P<0.0001) and 10–14 years (P=0.002) were significantly different from age 15–19 years. Ages 6–9 years and 10–14 years did not quite meet the threshold for statistical significance (P=0.058). FMD was not statistically significantly different across age groups, F(2,99)=2.20, P=0.116. Tanner stage was available for 43 subjects with RHI measures and 37 subjects with FMD measures. Regression analysis including Tanner stage is displayed in Table 4. Neither Tanner stage nor any of the other measures were significantly associated with RHI or FMD following regression analysis. Finally, peak shear stress was not significantly different across age groups, F(2,68)=0.51, P=0.604.
Figure 1. RHI and Age Groups.
RHI data by age groups 6–9 yrs (n=41), 10–14 yrs (n=37), and 15–19 yrs (n=35) had median (interquartile range (IQR)) of 1.19(0.29), 1.26(0.43), and 1.48(0.76), respectively. P-value for Kruskal-Wallis one-way ANOVA on ranks across age categories is P=0.001. P-values between age categories are displayed using Mann-Whitney rank sum test. Ages 6–9 vs. 15–19 yrs are significant different with respect to median RHI (P<0.0001). Ages 10–14 vs. 15–19 yrs are also significant different (P=0.002). Ages 6–9 and 10–14 yrs are not significant different (P=0.058).
Table 4.
Tanner Stage Sub-Analysis of Associations between RHI, Peak FMD, and Childhood Measures
| RHI β (P-value) |
Peak FMD β (P-value) |
|
|---|---|---|
| Sex (Female) | 0.002 (0.987) | 1.608 (0.138) |
| Tanner Stage | 0.012 (0.737) | 0.729 (0.081) |
| BMI (kg/m2) | −0.002 (0.849) | 0.008 (0.942) |
| Systolic BP (mmHg) | 0.014 (0.072) | −0.140 (0.055) |
| Diastolic BP (mmHg) | −0.001 (0.911) | 0.120 (0.209) |
The analysis included subjects with RHI (n=43) and FMD (n=37) measures, and was assessed for childhood measure correlations. BMI, body mass index; BP, blood pressure.
Denotes clinical significance (P-value <0.05).
DISCUSSION
Results of the present study suggest that age is associated with, and predicts, RHI among children and adolescents. To a lesser extent, SBP, age, height, and weight are associated with RHI. Conversely, neither age nor any of these other variables were associated with FMD, the most widely-used and validated measure of endothelial function among youth. Therefore, the influence of age appears to be unique only to the digital reactive hyperemia technique and is likely related to the method itself, and not a physiological phenomenon related to pubertal development, as has been previously reported.15,16,20 The finding is in line with our original hypothesis that mechanical/structural factors associated with the finger probes (i.e., the “one size fits all” finger probe developed for adults may not be valid for children) likely explains the low RHI values often observed among younger children.
Our findings also indicate that SBP was significantly associated (positively) with RHI in children and adolescents, which is in agreement with a previous report.20 Additionally, a significant positive association between SBP and RHI has been reported among adults.25 Variables excluded due to multi-collinearity concerns may have contributed to this observation.
Among children and adolescents, RHI has been reported to increase with age and pubertal development.15,16,19,20,26 Indeed, Radtke et al.20 reported that pubertal status was associated with RHI, and that RHI significantly increased with each pubertal stage group. Two other groups15,16 have suggested that microvascular development may not be complete until late adolescence and that this phenomenon might explain the paradoxical age-RHI association. However, to our knowledge, no such evidence exists suggesting that microvascular function, measured directly or indirectly, differs by age or pubertal development. While our results support the previous findings of lower RHI among younger children and adolescents, we did not observe an association between RHI and Tanner stage. An important aspect of our study is that we simultaneously assessed brachial artery FMD, a well-established measure of endothelial function among children and adolescents. We found that none of the variables were associated with FMD. Additionally, FMD did not differ across age groups, suggesting that neither age nor Tanner stage is associated with endothelial function. Furthermore, in a separate study performed by our group, we found no association between Tanner stage and FMD.27 In the current study we also found that peak shear stress in the brachial artery, considered by some to be a measure of microvascular function, did not differ significantly across age groups. It should be noted, however, that some of the differences observed between RHI and FMD in our study may have been a reflection of the fact that different vascular beds are evaluated with these distinct techniques. Digital reactive hyperemia utilizes a volumetric method that tracks small changes in finger volume stimulated by changes in blood flow in the resistance arteries (microvasculature), whereas FMD measures diameter changes in the brachial artery, which is a conduit vessel.
Some experts have proposed that an RHI cutoff value of 1.35 should be used to identify endothelial dysfunction among adults12, yet the median RHI value in our study of healthy children and adolescents was 1.26±0.45. While no such cutoff for endothelial dysfunction has been suggested for children, it is noteworthy that 58% of our pediatric study population (n=65) would be classified as having endothelial dysfunction according to the adult cutoff value. Although this observation may be partly explained by the location of the occluding cuff placement used in our study, it is highly unlikely that most of the healthy participants in our study truly had endothelial dysfunction.
Strengths of the present study include the concurrently measured RHI and brachial artery FMD, as well as the inclusion of finger volume. One limitation of the study was that Tanner stage was only available for 43 subjects and finger volume for 48 subjects. Additional data on Tanner stage and finger volume would have offered greater precision and statistical power. Another limitation was the modest sample size, which may have restricted our ability to identify other potentially important factors that might be independently associated with RHI.
In conclusion, our findings suggest that age is the primary factor explaining the low RHI values often observed in younger children. Our data call into question the validity of the “one size fits all” finger probe used with the EndoPAT device when applied to children; thus digital reactive hyperemia data from pediatric studies should be interpreted with caution. Future research should attempt to identify the age cutoff at which this technique may be valid among children and adolescents, and perhaps development of pediatric finger probes should be explored.
Highlights.
Digital reactive hyperemia has been used to measure endothelial function in youth.
Finger probes used with this technique were not designed for use in children.
We demonstrate a paradoxical relationship between age and reactive hyperemic index.
Digital reactive hyperemia may not be a valid technique in children.
Acknowledgments
Funding was provided by a grant from the Thrasher Research Fund (grant awarded to A.S.K.) and from National Institutes of Health awards - NIDDK: R01DK072124-01A3, (J.S.); GCRC: M01-RR00400, General Clinical Research Center Program; National Center for Research Resources (NCRR) Grants: 1UL1RR033183, Clinical and Translational Science Institute (CTSI). EndoPAT finger probes were generously donated by Itamar Medical.
Footnotes
Disclosures: None of the authors have relevant disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berenson GS, Srinivasan SR, Bao W, Newman WP, III, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N Engl J Med. 1998;338(23):1650–6. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- 2.McGill HC, Jr, McMahan CA, Zieske AW, Tracy RE, Malcom GT, Herderick EE, Strong JP. Association of Coronary Heart Disease Risk Factors with microscopic qualities of coronary atherosclerosis in youth. Circulation. 2000;102(4):374–9. doi: 10.1161/01.cir.102.4.374. [DOI] [PubMed] [Google Scholar]
- 3.McGill HC, Jr, McMahan CA, Zieske AW, Sloop GD, Walcott JV, Troxclair DA, Malcom GT, Tracy RE, Oalmann MC, Strong JP. Associations of coronary heart disease risk factors with the intermediate lesion of atherosclerosis in youth. The Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb Vasc Biol. 2000;20(8):1998–2004. doi: 10.1161/01.atv.20.8.1998. [DOI] [PubMed] [Google Scholar]
- 4.McGill HC, Jr, McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, Strong JP. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation. 2002;105(23):2712–8. doi: 10.1161/01.cir.0000018121.67607.ce. [DOI] [PubMed] [Google Scholar]
- 5.Halcox JP, Donald AE, Ellins E, Witte DR, Shipley MJ, Brunner EJ, Marmot MG, Deanfield JE. Endothelial function predicts progression of carotid intima-media thickness. Circulation. 2009;119(7):1005–12. doi: 10.1161/CIRCULATIONAHA.108.765701. [DOI] [PubMed] [Google Scholar]
- 6.Lekakis J, Abraham P, Balbarini A, Blann A, Boulanger CM, Cockcroft J, Cosentino F, Deanfield J, Gallino A, Ikonomidis I, Kremastinos D, Landmesser U, Protogerou A, Stefanadis C, Tousoulis D, Vassalli G, Vink H, Werner N, Wilkinson I, Vlachopoulos C. Methods for evaluating endothelial function: a position statement from the European Society of Cardiology Working Group on Peripheral Pirculation. Eur J Cardiovasc Prev Rehabil. 2011;18:775–89. doi: 10.1177/1741826711398179. [DOI] [PubMed] [Google Scholar]
- 7.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106(6):653–8. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 8.Schindler TH, Hornig B, Buser PT, Olschewski M, Magosaki N, Pfisterer M, Nitzsche EU, Solzbach U, Just H. Prognostic value of abnormal vasoreactivity of epicardial coronary arteries to sympathetic stimulation in patients with normal coronary angiograms. Arterioscler Thromb Vasc Biol. 2003;23(3):495–501. doi: 10.1161/01.ATV.0000057571.03012.F4. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida T, Kawano H, Miyamoto S, Motoyama T, Fukushima H, Hirai N, Ogawa H. Prognostic value of flow-mediated dilation of the brachial artery in patients with cardiovascular disease. Intern Med. 2006;45(9):575–9. doi: 10.2169/internalmedicine.45.1534. [DOI] [PubMed] [Google Scholar]
- 10.Urbina EM, Williams RV, Alpert BS, Collins RT, Daniels SR, Hayman L, Jacobson M, Mahoney L, Mietus-Snyder M, Rocchini A, Steinberger J, McCrindle B. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009;54(5):919–50. doi: 10.1161/HYPERTENSIONAHA.109.192639. [DOI] [PubMed] [Google Scholar]
- 11.Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006;101(2):545–8. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 12.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44(11):2137–41. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 13.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117(19):2467–74. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Lavi S, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–8. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 15.Bhangoo A, Sinha S, Rosenbaum M, Shelov S, Ten S. Endothelial function as measured by peripheral arterial tonometry increases during pubertal advancement. Horm Res Paediatr. 2011;76:226–33. doi: 10.1159/000328455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Dangardt F, Osika W, Berggren K, Gronowitz, Friberg P. Age- and sex-related differences in vascular function and vascular response to mental stress. Atherosclerosis. 2012;220:269–74. doi: 10.1016/j.atherosclerosis.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 17.Kelly AS, Metzig A, Steinberger J, Braunlin E. Endothelial function in children and adolescents with mucopolysaccharidosis. J Inherit Metab Dis. 2013;36:221–5. doi: 10.1007/s10545-011-9438-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koska J, Schwartz E, Mullin M, Schwenke D, Reaven P. Improvement of postprandial endothelial function after a single dose of exenatide in individuals with impaired glucose tolerance and recent-onset type 2 diabetes. Diabetes Care. 2010;33:1028–30. doi: 10.2337/dc09-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahmud F, Hill D, Cuerden M, Clarson C. Impaired vascular function in obese adolescents with insulin resistance. J Pediatr. 2009;155:678–82. doi: 10.1016/j.jpeds.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 20.Radtke T, Khattab K, Eser P, Kriemler S, Saner H, Wilhelm M. Puberty and microvascular function in healthy children and adolescents. J Pediatr. 2012;161:887–91. doi: 10.1016/j.jpeds.2012.04.033. [DOI] [PubMed] [Google Scholar]
- 21.Celermajer D, Sorensen K, Gooch V, Spiegelhalter D, Miller O, Sullivan D, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–5. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 22.Kelly AS, Wetzsteon R, Kaiser D, Steinberger J, Bank A, Dengel D. Inflammation, insulin, and endothelial function in overweight children and adolescents: the role of exercise. J Pediatr. 2004;145:731–6. doi: 10.1016/j.jpeds.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Kelly AS, Kaiser DR, Dengel DR, Bank AJ. Comparison of B-mode and echo tracking methods of assessing flow-mediated dilation. Ultrasound Med Biol. 2004;30:1447–9. doi: 10.1016/j.ultrasmedbio.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Selamet Tierney ES, Newburger JW, Gauvreau K, Geva J, Coogan E, Colan SD, DeFerranti SD. Endothelial pulse amplitude testing: feasibility and reproducibility in adolescents. J Pediatr. 2009;154:901–5. doi: 10.1016/j.jpeds.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 25.Hamburg N, Palmisano J, Larson M, Sullivan L, Lehman B, Vasan R, Levy D, Mitchell G, Vita J, Benjamin E. Relation of brachial and digital measures of vascular function in the community: the Framingham heart study. Hypertension. 2011;57:390–6. doi: 10.1161/HYPERTENSIONAHA.110.160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tryggestad J, Thompson D, Copeland K, Short K. Obese children have higher arterial elasticity without a difference in endothelial function: the role of body composition. Obesity. 2012;20:165–71. doi: 10.1038/oby.2011.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marlatt K, Steinberger J, Dengel D, Sinaiko A, Moran A, Chow L, Steffen L, Zhou X, Kelly AS. Impact of pubertal development on endothelial function and arterial elasticity. J Pediatr. 2013;163:1432–6. doi: 10.1016/j.jpeds.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]