Abstract
Background:
Evidence has shown an association of Helicobacter pylori infection with liver dysfunction and damage. We investigated if H. pylori eradication affects liver enzymes in patients referring with unexplained hypertransaminasemia.
Materials and Methods:
Patients with mild unexplained hypertransaminasemia accompanied with dyspepsia and confirmed H. pylori infection were studied. Viral, metabolic, autoimmune, and drug/toxin induced hepatitis as well as fatty liver were all ruled-out by appropriate tests. Patients received bismuth-containing quadruple-therapy for 2 weeks. Serum levels of liver enzymes (alanine transaminase (ALT) and aspartate transaminase (AST)) and successful eradication (with stool antigen test) were evaluated 4 weeks after the medication.
Results:
A total number of 107 patients (55 males, mean age = 35.0 ± 8.4 years) were studied. Eradication was successful in 93 patients (86.9%). Serum levels of AST (6.3 ± 19.6 IU/L, P = 0.002) and ALT (7.8 ± 24.9 IU/L, P = 0.001) were significantly decreased after eradication. Levels of AST and ALT decreased to normal range respectively in 46.6% and 45.7% of the cases who had baseline levels above the normal range.
Conclusion:
This study showed a decrease in liver enzymes after receiving eradication regimen of H. pylori, suggesting a role for H. pylori infection in at least some of patients with mild unexplained hypertransaminasemia. Further studies are warranted to find the underlying mechanisms by which H. pylori infection affects the liver and clinical importance of such effects.
Keywords: Helicobacter pylori, liver diseases, transaminases
INTRODUCTION
Helicobacter pylori is a pathogenic bacterium colonizing in the mucosal surface of the human stomach. Infection with H. pylori is highly prevalent worldwide; up to 90% of the adult populations in developing countries are affected by the bacteria.[1] Consequences of the infection range from a mild chronic gastritis to peptic ulcer and gastric cancers.[1] Besides gastric problems, association of H. pylori infection with other diseases such as cardiovascular, pulmonary, hematologic, ophthalmologic, dermatologic, neurologic, and hepatobiliary diseases are shown by several recent studies.[2]
Liver is one of the organs that, according to some evidences, may be affected by H. pylori infection; however, the exact effects of the infection on the liver and the underlying mechanisms are still unclear.[2] Some studies have suggested that H. pylori infection could be a risk factor for chronic diseases of the liver and biliary tract, such as chronic cholecystitis, primary sclerosing cholangitis, primary biliary cirrhosis, and even hepatocellular carcinoma.[3,4,5,6,7,8] Animal studies have shown that H. pylori can exist in the liver and gallbladder cells.[9,10] and can cause mild to moderate the multifocal hepatitis and hepatic fibrosis.[11] Regarding human studies, an association between H. pylori presence in the liver and disease progression in those with viral chronic hepatitis and cirrhosis is reported.[7,12] Furthermore, associations between H. pylori infection and cirrhosis in patients with hepatitis C virus,[13] and between hypertransaminasemia and infection with Cag-A positive H. pylori in patients with peptic ulcer disease are presented.[14]
Due to the uncertainty about the exact impacts of H. pylori on the liver and lack of studies in this regard, we investigated if liver enzymes are changed by H. pylori eradication in patients referring with hypertransaminasemia of unexplained origin and concomitant H. pylori infection.
MATERIALS AND METHODS
Patients and settings
This prospective observational study was conducted on patients with mild unexplained hypertransaminasemia who were referred for liver work-ups to the out-patient clinic of gastroenterology in Alzahra University Hospital, in Isfahan city (Central Iran), between 2011 and 2012. Patients who fulfilled the following inclusion criteria were consecutively included; (1) adult patients with double-checked (with about 2 weeks interval) alanine transaminase (ALT) or aspartate transaminase (AST) of more than 40 IU/L, but not three times above the normal; (2) no presence of fatty liver disease (investigated by ultrasonography) or viral, metabolic, autoimmune, and drug or toxin induced hepatitis as the causes of hypertransaminasemia; and (3) having dyspepsia and H. pylori infection approved by rapid urease test or stool antigen test. Patients who had recent antibiotic treatment for H. pylori eradication and pregnant and lactating women were not included into the study. With regard to the type I error (alpha) = 0.05, study power = 80%, and an expected decrease of at least 5 IU/L in liver enzymes after eradication, sample size was calculated as 100 patients. The study was approved by the Ethics Committee of the Isfahan University of Medical Sciences and all patients signed a written inform consent before entering the study.
H. pylori eradication regimen
All patients received bismuth-containing quadruple-therapy for 2 weeks contained omeprazole 20 mg/bid, bismuth subcitrate 240 mg/bid, amoxicillin 1000 mg/bid, and clarithromycin 500 mg/bid. This regimen is used in our center based on the recent Iranian Association of Gastroenterology guideline[15] and a systematic review on the treatment of H pylori infection in Iran,[16] and expected to have eradication rate as about 85-90%.
Assessments
A gastroenterologist visited all patients and viral, metabolic, autoimmune, and drug/toxin induced hepatitis as well as fatty liver disease were investigated by appropriate tests. One month after the end of the eradication, patients were visited again and the eradication of H. pylori was assessed by stool antigen test (rapid immunoassay method, generic assays GmbH, Germany) with sensitivity, specificity, positive predictive value, negative predictive value of 96%, 83%, 98%, and 96% respectively.[17] Furthermore, a blood sample was taken and serum levels of the liver enzymes were re-checked. Almost all of the patients had tested in the same laboratory.
Statistical analysis
Data were analyzed using the SPSS software for windows (version 16.0). Quantitative and qualitative variables are presented as mean ± SD and number (%), respectively. Wilcoxon Test was used for comparing liver enzyme levels before and after eradication (considering not-normal distribution of liver enzymes’ levels after eradication). Furthermore, Mann-Whitney Test was used for comparing changes in liver enzymes between those with successful and unsuccessful eradication. A P < 0.05 was considered significant in all analyses.
RESULTS
During the study period, a total number of 107 patients including 55 males and 52 females with a mean age of 35.0 ± 8.4 (18-56) years were included into the study. Baseline ALT and AST levels ranged from 44 IU/L to 79 IU/L (61.2 ± 9.6) and from 27 IU/L to 70 IU/L (47.8 ± 10.5), respectively. None of the patients has AST/ALT > 2 before or after the study. Among the studied population, 93 patients (86.9%) had successful H. pylori eradication. Liver enzymes were decreased significantly by 7.8 ± 24.9 IU/L for ALT (P = 0.001) and by 6.3 ± 19.6 IU/L for AST (P = 0.002). However, there was no difference between those with successful eradication and those with failed eradication in changes in liver enzymes’ levels (P = 0.657 and 0.821 respectively for ALT and AST), Table 1.
Table 1.
Levels and changes of liver transaminases before and after Helicobacter pylori eradication
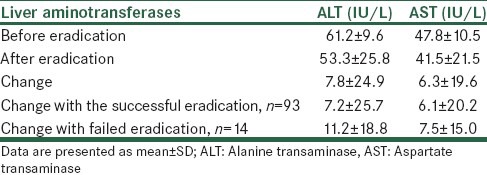
Regarding ALT, all patients were above the normal range before eradication. After eradication, ALT levels decreased to normal range in 45.7% (49/107) of the cases by 30.7 ± 10.2 IU/L. In those who has no decrease in ALT levels after eradication (48 cases), ALT levels changed by 16.4 ± 11.3 IU/L. Additional analyses also showed that in patients with baseline AST levels above the normal range (75 cases), AST levels decreased to normal range in 46.6% (35/75) of the cases by 28.5 ± 8.3 IU/L. However, in patients who had been in the normal range before eradication (32 cases), AST levels changed to above the normal in 25% (8/35) of the cases by 13.3 ± 8.6 IU/L.
DISCUSSION
Mild unexplained hypertransaminasemia is an especial concern when is found accidently in routine laboratory tests or in tests with other indications in individuals without specific liver disease or complaints. For example, a population-based study in the United States estimated an abnormal liver function test (LFT) in up to 9% of the healthy population who had a single screening test.[18] Another study showed that, if retested with about 2 weeks interval, more than 30% of abnormal cases would be reclassified as normal and only 5% of normal cases at the first test would have elevated enzymes at the second test.[19] Considering high prevalence of H. pylori infection in the general population and some evidence indicating an association between H. pylori infection with some degrees of liver damage, we evaluated if H. pylori eradication has any effects on liver enzymes in those with unexplained hypertransaminasemia. The results showed that the mean level of liver enzymes significantly reduced after receiving eradication regimen. Liver enzyme levels were also decreased in those with unsuccessful eradication, which could be due to reduction in bacterial load by eradication regimen. However, liver enzymes decreased to normal range only in about half of the patients and even increased in about 25% of the cases after eradication. These results suggest a role for H. pylori infection in the pathophysiology of unexplained hypertransaminasemia, of course not in all of the cases.
To the best of our knowledge, this is the first study that has investigated if H. pylori eradication affects liver enzymes in patients with hypertransaminasemia. One other study by Karahalil et al. found no change in alpha-glutathione S-transferase (a marker of hepatocellular damage better than transaminases in toxic and autoimmune hepatitis)[20] or other LFTs after eradicating H. pylori; however, included patients in this study had normal LFTs at enrolment.[21] In another study, Graham et al. evaluated LFTs of patients with peptic ulcer and H. pylori before and 3 months after H. pylori eradication. Authors found higher AST levels with Cag-A positive H. pylori infection, but found no change in liver enzymes after eradication. Again, included patients in the mentioned study had normal LFTs at enrolment.[14] Considering no association of H. pylori with ALT level, which is more specific than AST, and no change in liver enzymes after eradication, these authors introduced two hypothesizes; that there is an extra-hepatic source for increased AST level; and/or there is a host genetic susceptibility to both H. pylori infection and increased levels of liver enzymes.[14] However, our results are against these studies and suggest that H. pylori infection plays a role in at least some patients with hypertransaminasemia, and liver enzymes would decrease after eradication.
The underlying mechanisms of liver damage by H. pylori infection are still unclear. In an in-vitro study, Le Roux-Goglin et al. showed that H. pylori can stimulate podosome formation and collagen accumulation in cultured mouse hepatocytes that can lead to liver fibrosis and possibly cancer in-vivo.[22] Another animal study by Goo et al. showed that inoculation of H. pylori in hepatic fibrosis-induced mice accelerates the fibrosis. This finding suggests a role for H. pylori infection in the development/progression of liver cirrhosis.[11] A similar study by Ki et al. indicated pro-inflammatory signaling pathways as the underlying mechanisms of accelerated hepatic fibrosis by H. pylori infection.[23] Human studies also have shown an association between H. pylori presence in the liver (genetically similar to those isolated from the stomach) and disease progression in patients with viral chronic hepatitis and cirrhosis.[7,12] Furthermore, associations between H. pylori infection and cirrhosis in patients with hepatitis C virus,[13] and between hypertransaminasemia and infection with Cag-A positive H. pylori in patients with peptic ulcer disease are presented.[14] It is suggested that H. pylori can reach to and colonized in the liver via the blood or the biliary system as a result of structural changes in the hepatobiliary system.[7,10] Furthermore, concomitant infection with H. pylori and other helicobacter species such as H. hepaticus are possible. Studies showed that H. hepaticus could progress the liver disease.[24] It must be noted that infection with such spices also responds to current H. pylori eradication regimens.[25,26]
There are some limitations to our study. First of all, it was better to conduct a controlled trial with an arm for which no eradication was carried out. Then, we could evaluate the exact effects of eradication on liver enzymes changes. Furthermore, if we had more workups to discover the exact reasons of enzymes elevation (e.g., by liver biopsy), we could have more judicable data to conclude an association between H. pylori and liver damage. However, it is not possible and not recommended to do a liver biopsy in all patients with mild hypertransaminasemia. We did not investigate celiac disease in our study population and according to some reports from our society about 10% of patients presenting with hypertransaminasemia are celiac disease cases.[27] Furthermore, evaluation of Cag-A positive strain of H. pylori would have provided information on the possible association of specific H. pylori strains and liver damage. We focused our study on patients with less than threefold abnormal enzyme elevation, and thus we cannot generalize our results to more severe cases. Furthermore, the sample size of our study was not large enough for comparison of those with and without successful eradication, and the follow-up was not long enough to monitor the long-term effects of H. pylori eradication on liver enzymes.
In summary, the results of the present study suggest a role for H. pylori infection in some of the patients referring with mild unexplained hypertransaminasemia, and reduction in liver enzyme levels after receiving eradication regimen in these patients. According to limitations, the results of our study should be interpreted cautiously. We recommend conducting a controlled trial with enough sample size and follow-up period to investigate the exact effects of H. pylori eradication on liver enzymes changes in patients referring with unexplained hypertransaminasemia. Further, studies are also required regarding the association of specific H. pylori strains with liver dysfunction and the underlying mechanisms, and long-term clinical consequences of such association.
Footnotes
Source of Support: Isfahan University of Medical Sciences
Conflict of Interest: None declared.
REFERENCES
- 1.Brown LM. Helicobacter pylori: Epidemiology and routes of transmission. Epidemiol Rev. 2000;22:283–97. doi: 10.1093/oxfordjournals.epirev.a018040. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki H, Franceschi F, Nishizawa T, Gasbarrini A. Extragastric manifestations of Helicobacter pylori infection. Helicobacter. 2011;16:65–9. doi: 10.1111/j.1523-5378.2011.00883.x. [DOI] [PubMed] [Google Scholar]
- 3.Avenaud P, Marais A, Monteiro L, Le Bail B, Bioulac Sage P, Balabaud C, et al. Detection of Helicobacter species in the liver of patients with and without primary liver carcinoma. Cancer. 2000;89:1431–9. [PubMed] [Google Scholar]
- 4.Dore MP, Realdi G, Mura D, Graham DY, Sepulveda AR. Helicobacter infection in patients with HCV-related chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Dig Dis Sci. 2002;47:1638–43. doi: 10.1023/a:1015848009444. [DOI] [PubMed] [Google Scholar]
- 5.Verhoef C, Pot RG, de Man RA, Zondervan PE, Kuipers EJ, IJzermans JN, et al. Detection of identical Helicobacter DNA in the stomach and in the non-cirrhotic liver of patients with hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2003;15:1171–4. doi: 10.1097/00042737-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Ito K, Nakamura M, Toda G, Negishi M, Torii A, Ohno T. Potential role of Helicobacter pylori in hepatocarcinogenesis. Int J Mol Med. 2004;13:221–7. [PubMed] [Google Scholar]
- 7.Rocha M, Avenaud P, Ménard A, Le Bail B, Balabaud C, Bioulac-Sage P, et al. Association of Helicobacter species with hepatitis C cirrhosis with or without hepatocellular carcinoma. Gut. 2005;54:396–401. doi: 10.1136/gut.2004.042168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulajic M, Panic N, Stimec B, Isaksson B, Jesenofsky R, Schneider-Brachert W, et al. PCR in Helicobacter spp. diagnostic in extragastric malignancies of digestive system. Eur J Gastroenterol Hepatol. 2012;24:117–25. doi: 10.1097/MEG.0b013e32834dfde1. [DOI] [PubMed] [Google Scholar]
- 9.Wadström T, Ljungh A, Willén R. Primary biliary cirrhosis and primary sclerosing cholangitis are of infectious origin! Gut. 2001;49:454. doi: 10.1136/gut.49.3.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Tian XF, Fan XG, Fu CY, Zhu C. The pathological effect of Helicobacter pylori infection on liver tissues in mice. Clin Microbiol Infect. 2009;15:843–9. doi: 10.1111/j.1469-0691.2009.02719.x. [DOI] [PubMed] [Google Scholar]
- 11.Goo MJ, Ki MR, Lee HR, Yang HJ, Yuan DW, Hong IH, et al. Helicobacter pylori promotes hepatic fibrosis in the animal model. Lab Invest. 2009;89:1291–303. doi: 10.1038/labinvest.2009.90. [DOI] [PubMed] [Google Scholar]
- 12.Esmat G, El-Bendary M, Zakarya S, Ela MA, Zalata K. Role of Helicobacter pylori in patients with HCV-related chronic hepatitis and cirrhosis with or without hepatocellular carcinoma: Possible association with disease progression. J Viral Hepat. 2012;19:473–9. doi: 10.1111/j.1365-2893.2011.01567.x. [DOI] [PubMed] [Google Scholar]
- 13.Queiroz DM, Rocha AM, Rocha GA, Cinque SM, Oliveira AG, Godoy A, et al. Association between Helicobacter pylori infection and cirrhosis in patients with chronic hepatitis C virus. Dig Dis Sci. 2006;51:370–3. doi: 10.1007/s10620-006-3150-y. [DOI] [PubMed] [Google Scholar]
- 14.Graham DY, Osato MS, Olson CA, Zhang J, Figura N. Effect of H. pylori infection and CagA status on leukocyte counts and liver function tests: Extra-gastric manifestations of H. pylori infection. Helicobacter. 1998;3:174–8. doi: 10.1046/j.1523-5378.1998.08018.x. [DOI] [PubMed] [Google Scholar]
- 15.Massarrat S, Saberi-Firoozi M, Ebrahimi-Daryani N, Malekzadeh R. Approach to dyspepsia according to Helicobacter pylori status in Iran. Govaresh. 2009;14:35–8. [Google Scholar]
- 16.Barazandeh F, Moradi Gh, Malekzadeh R. Evaluation of efficacy of H. pylori eradication regimens in Iran: A systematic review. Govaresh. 2012;16:215–22. [Google Scholar]
- 17.Kazemi S, Tavakkoli H, Habizadeh MR, Emami MH. Diagnostic values of Helicobacter pylori diagnostic tests: Stool antigen test, urea breath test, rapid urease test, serology and histology. J Res Med Sci. 2011;16:1097–104. [PMC free article] [PubMed] [Google Scholar]
- 18.Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999-2002. Am J Gastroenterol. 2006;101:76–82. doi: 10.1111/j.1572-0241.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 19.Lazo M, Selvin E, Clark JM. Brief communication: Clinical implications of short-term variability in liver function test results. Ann Intern Med. 2008;148:348–52. doi: 10.7326/0003-4819-148-5-200803040-00005. [DOI] [PubMed] [Google Scholar]
- 20.Vaubourdolle M, Chazouillères O, Briaud I, Legendre C, Serfaty L, Poupon R, et al. Plasma alpha-glutathione S-transferase assessed as a marker of liver damage in patients with chronic hepatitis C. Clin Chem. 1995;41:1716–9. [PubMed] [Google Scholar]
- 21.Karahalil B, Yaðar S, Ozin Y. Release of alpha-glutathione S-transferase (alpha-GST) and hepatocellular damage induced by Helicobacter pylori and eradication treatment. Curr Drug Saf. 2007;2:43–6. doi: 10.2174/157488607779315426. [DOI] [PubMed] [Google Scholar]
- 22.Le Roux-Goglin E, Varon C, Spuul P, Asencio C, Mégraud F, Génot E. Helicobacter infection induces podosome assembly in primary hepatocytes in vitro. Eur J Cell Biol. 2012;91:161–70. doi: 10.1016/j.ejcb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Ki MR, Goo MJ, Park JK, Hong IH, Ji AR, Han SY, et al. Helicobacter pylori accelerates hepatic fibrosis by sensitizing transforming growth factor-®1-induced inflammatory signaling. Lab Invest. 2010;90:1507–16. doi: 10.1038/labinvest.2010.109. [DOI] [PubMed] [Google Scholar]
- 24.Pellicano R, Ménard A, Rizzetto M, Mégraud F. Helicobacter species and liver diseases: Association or causation? Lancet Infect Dis. 2008;8:254–60. doi: 10.1016/S1473-3099(08)70066-5. [DOI] [PubMed] [Google Scholar]
- 25.Kerton A, Warden P. Review of successful treatment for Helicobacter species in laboratory mice. Lab Anim. 2006;40:115–22. doi: 10.1258/002367706776319033. [DOI] [PubMed] [Google Scholar]
- 26.Kostomitsopoulos N, Donnelly H, Kostavasili I, Paronis E, Alexakos P, Karayannacos P. Eradication of Helicobacter bilis and H. hepaticus from infected mice by using a medicated diet. Lab Anim (NY) 2007;36:37–40. doi: 10.1038/laban0507-37. [DOI] [PubMed] [Google Scholar]
- 27.Emami MH, Hashemi M, Kouhestani S, Taheri H, Karimi S. Should we look for celiac disease among all patients with liver function test abnormalities? Int J Prev Med. 2012;3:167–72. [PMC free article] [PubMed] [Google Scholar]


