Abstract
This article reviews the relationship between dyslipidemia, chronic kidney disease, and cardiovascular diseases in patients with diabetes. Diabetes mellitus is associated with complications in the cardiovascular and renal system, and is increasing in prevalence worldwide. Modification of the multifactorial risk factors, in particular dyslipidemia, has been suggested to reduce the rates of diabetes-related complications. Dyslipidemia in diabetes is a condition that includes hypertriglyceridemia, low high-density lipoprotein levels, and increased small and dense low-density lipoprotein particles. This condition is associated with higher cardiovascular risk and mortality in diabetic patients. Current treatment guidelines focus on lowering the low-density lipoprotein cholesterol level; multiple trials have confirmed the cardiovascular benefits of treatment with statins. Chronic kidney disease also contributes to dyslipidemia, and dyslipidemia in turn is related to the occurrence and progression of diabetic nephropathy. Different patterns of dyslipidemia are associated with different stages of diabetic nephropathy. Some trials have shown that treatment with statins not only decreased the risk of cardiovascular events, but also delayed the progression of diabetic nephropathy. However, studies using statins as the sole treatment of hyperlipidemia in patients on dialysis have not shown benefits with respect to cardiovascular risk. Diabetic patients with nephropathy have a higher risk of cardiovascular events than those without nephropathy. The degree of albuminuria and the reduction in estimated glomerular filtration rate are also correlated with the risk of cardiovascular events. Treatment with angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers to reduce albuminuria in diabetic patients has been shown to decrease the risk of cardiovascular morbidity and mortality.
Keywords: type 2 diabetes, dyslipidemia, chronic kidney disease, cardiovascular disease
Abbreviations: ACCORD - Action to Control Cardiovascular Risk in Diabetes; ADVANCE - Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation; AGE - advanced glycation end-product; AIM-HIGH - Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes; ApoB - apolipoprotein B; AURORA - A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events; CARDS - Collaborative Atorvastatin Diabetes Study; CKD - chronic kidney disease; CVD - cardiovascular disease; 4D - Die Deutsche Diabetes Dialyse Studie; DAIS - Diabetes Atherosclerosis Intervention Study; DCCT - Diabetes Control and Complications Trial ; EDIC - Epidemiology of Diabetes Interventions and Complications ; eGFR - estimated glomerular filtration rate; ETDRS - Early Treatment Diabetic Retinopathy Study; KDOQI - Kidney Disease Outcomes Quality Initiative; KEEP - Kidney Early Evaluation Program; FFA - free fatty acid; FIELD - Fenofibrate Intervention and Event Lowering in Diabetes; GREACE - GREek Atorvastatin and Coronary-heart-disease Evaluation; HbA1c - hemoglobin A1c; HDL - high-density lipoprotein; HDL-C - high-density lipoprotein cholesterol; HOPE - Heart Outcome Prevention and Evaluation; HPFS - Health Professionals Follow-Up Study; HPS - Heart Protection Study; JUPITER - Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin; LDL - low-density lipoprotein; LDL-C - low-density lipoprotein cholesterol; LPL - lipoprotein lipase; MICRO-HOPE - Microalbuminuria, Cardiovascular, and Renal Outcomes in HOPE; MRFIT - Multiple Risk Factor Intervention Trial; NCEP-ATP III - National Cholesterol Education Program Adult Treatment Panel III; NHANES - National Health and Nutrition Examination Survey; PAD - peripheral artery disease; RAAS - renin-angiotensin-aldosterone system; RENAAL - Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan; SHARP - Study of Heart and Renal Protection; T2D - type 2 diabetes; TGF-beta - transforming growth factor beta; TNT - Treating to New Targets; USRDS - United States Renal Data System; VA-HIT - Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial; VLDL - very low-density lipoprotein; VLDL-C - very low-density lipoprotein cholesterol; WHO MSVDD - World Health Organization Multinational Study of Vascular Diseases in Diabetes
1. Introduction
Diabetes mellitus is a critical health issue worldwide. According to data from the International Diabetes Federation, 371 million adults were diagnosed with diabetes, and an estimated 4.8 million people died of the disease in 2012 [1]. The number of adults with diabetes may increase to 552 million in 2030 [1]. Type 2 diabetes (T2D) accounts for more than 90% of all cases of diabetes and, in the majority of patients, is diagnosed between 40 and 60 years of age. The social and economic burden of diabetes is a major global problem, especially in developing countries.
Patients with diabetes have much higher morbidity and mortality than the general population because of the complications associated with the disease. Atherosclerotic cardiovascular disease, which manifests clinically as coronary heart disease, cerebral vascular disease, and other peripheral artery diseases (PAD), is the most common macrovascular complication in diabetic patients. In the Framingham Study, the incidence of atherosclerotic disease was 2-fold to 3-fold higher in patients with diabetes compared to healthy subjects. [2]. In the Multiple Risk Factor Intervention Trial (MRFIT), the mortality rate of cardiovascular disease was 3 times higher in patients with diabetes than in normal subjects. [3]. In Taiwan, cardiovascular disease is the second leading cause of death in patients with diabetes [4]. Diabetes has been recognized as a cardiovascular disease equivalent [5-8]. The higher incidence of cardiovascular disease in diabetic patients may be related to endothelial damage caused by hyperglycemia, traditional risk factors (smoking, dyslipidemia, and hypertension), or disturbed metabolism (oxidative stress, decreased nitric oxide production, and chronic inflammation) [9].
More than one-third of diabetic patients experience microvascular complications such as retinopathy, nephropathy, and neuropathy [10, 11]. Metabolic abnormalities (hyperglycemia, accumulation of advanced glycation end products, oxidative stress) [12] and vasoactive renal factors (renin-angiotensin system and other vasoconstrictors) [13] play a role in the development of diabetic nephropathy. The classic characteristics of diabetic nephropathy include hypertension, proteinuria, and impairment of renal function. Diabetic nephropathy is the major cause of end-stage renal disease, and reports from both the National Health and Nutrition Examination Survey (NHANES) and the United States Renal Data System (USRDS) have shown a steadily increasing prevalence of diabetic nephropathy in the United States [14, 15]. A global study showed that the incidence rate of albuminuria in T2D is higher in Asian than in Caucasian patients (55% vs. 41%) [16]. Patients with T2D and chronic kidney disease (CKD) have a significantly higher mortality rate than those without nephropathy [17].
Dyslipidemia is common in diabetic patients. Typical manifestations of diabetes-related dyslipidemia include fasting and postprandial hypertriglyceridemia, low high-density lipoprotein cholesterol (HDL-C) levels, increased small and dense low-density lipoprotein (LDL) particles, and elevated apolipoprotein B (ApoB) levels [18-20]. All of these lipid abnormalities are highly atherogenic and determinants of cardiovascular disease. A higher prevalence of dyslipidemia is noted in patients with CKD caused by abnormalities of lipid metabolism. Impaired renal function is also an independent predictor of cardiovascular events and risk of death [21]. In the World Health Organization Multinational Study of Vascular Disease in Diabetes, both serum cholesterol level and proteinuria were strong predictors of cardiovascular disease mortality, fatal and non-fatal myocardial infarction, and stroke [22]. Greater cardiovascular risk in patients with diabetic nephropathy may partly be due to the atherogenic lipoprotein changes associated with renal failure. In addition to adverse effects on the cardiovascular system, dyslipidemia may also correlate with progression of diabetic nephropathy.
Because of the higher incidence of CKD and cardiovascular disease in patients with diabetes, we review the relationship between dyslipidemia, CKD, and cardiovascular diseases in patients with diabetes.
2. Diabetes and dyslipidemia
The pathogenesis of diabetic dyslipidemia is complex. Insulin could mediate the uptake of free fatty acids (FFAs) by striated muscle and adipose tissue. Therefore, increased insulin resistance would result in increased levels of FFAs delivered to the liver, giving rise to overproduction of very low-density lipoprotein (VLDL) and to increased very low-density lipoprotein cholesterol (VLDL-C) concentration, with the clinical manifestation of hypertriglyceridemia. Decreased lipoprotein lipase (LPL) activity also leads to an accumulation of triglyceride-rich lipoproteins in the plasma. VLDL-C could stimulate the exchange of triglycerides to cholesterol ester from HDL and LDL, which causes a higher catabolic rate of HDL and conversion of LDL to small and dense LDL [23]. The small and dense LDL could preferentially penetrate the arterial wall and is highly susceptible to glycation and oxidation, which may lead to atherosclerosis. Insulin resistance can also cause overproduction of ApoB-containing lipoprotein from the liver, manifesting as hepatic VLDL overproduction [24, 25].
Because of the increased risk of and higher mortality rate from cardiovascular disease in diabetic patients, interventions to decrease macrovascular events are important [2, 26]. Atherogenic dyslipidemia is a precipitating factor for higher risk of cardiovascular disease in patients with T2D. In the Steno-2 study, a multifactorial intervention that included lowering hemoglobin A1c (HbA1c) to less than 6.5%, blood pressure control, treatment of dyslipidemia, and anti-platelet therapy improved cardiovascular outcomes compared with standard therapy [27]. In 2012, the American Diabetes Association recommended statin therapy in addition to lifestyle modification for those at high risk for cardiovascular disease regardless of baseline lipid levels [28]. The guidelines of the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) emphasized that dyslipidemia is one of the major causes of higher cardiovascular risk in patients with diabetes [29] and suggested that LDL particles are highly atherogenic. This means that cholesterol-lowering therapy should be primarily focused on decreasing LDL-cholesterol (LDL-C) levels. Multiple landmark trials have shown that lowering LDL-C levels could lead to major clinical benefits in reducing cardiovascular events in patients with T2D. The Strong Heart Study showed that when LDL-C level increased by 10 mg/dl, the risk of cardiovascular disease increased by 12% in American Indian patients with diabetes [30]. In the Treating to New Targets (TNT) study, lowering LDL-C levels to 77 mg/dl led to a 25% reduction in major cardiovascular events in patients with diabetes and coronary heart disease compared with the use of a target LDL-C level of 100 mg/dl [31]. A meta-analysis of 18,686 patients with diabetes in 14 randomized trials of treatment with statins showed that there was a reduction of 9% in all-cause mortality, of 21% in major vascular events, and of 22% in myocardial infarction or coronary death per mmol/l reduction in LDL-C level [32]. However, diabetic patients treated with statins still have a higher residual risk of cardiovascular events than non-diabetic patients. Reduction of LDL-C level alone cannot eliminate all of the excess cardiovascular risk in patients with diabetes.
Many prospective epidemiologic studies have reported a positive relationship between serum triglyceride levels and incidence of coronary heart disease, especially in patients with diabetes [33, 34]. Lipoproteins remnants are triglyceride-rich and atherogenic, and higher levels of atherogenic remnant lipoproteins could increase the risk of coronary heart disease when triglyceride levels are ≥200 mg/dl [35]. The most accessible measure of lipoprotein remnants is VLDL-C. As a result, VLDL-C could be combined with LDL-C to increase prediction of cardiovascular risk, especially in patients with a high triglyceride level. The NCEP-ATP III guidelines suggested the use of non–HDL-C (sum of VLDL-C and LDL-C) as a secondary target for subjects with a triglyceride level >200 mg/dl [36]. Fibrates are the most effective medication to lower triglyceride levels; both the Helsinki Heart Study and the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT) showed cardiovascular benefit in non-diabetic subjects treated with fibrates compared with placebo. However, randomized controlled trials failed to show additional benefit for cardiovascular events with fibrate therapy in patients with T2D. The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study of patients with T2D showed that treatment with fenofibrate as additive therapy with a statin did not reduce the risk of major coronary events. However, fewer non-fatal myocardial infarctions and revascularizations were noted, leading to a decreased number of total cardiovascular events [37]. Most participants in the FIELD study, including both the fenofibrate treatment group and the placebo group, were also treated with a statin.
There is no consensus regarding combination treatment with lipid-lowering agents in diabetic patients. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, combination therapy with a fibrate plus a statin was compared with treatment with a statin alone in patients with T2D. The results showed no significant difference between the 2 groups in cardiovascular death, non-fatal myocardial infarction, or stroke. However, subgroup analysis of the ACCORD study showed that fibrate therapy in addition to a statin may decrease the risk of cardiovascular disease in patients with triglyceride levels >240 mg/dl and HDL-C levels <34 mg/dl [38].
Niacin is effective for raising HDL-C levels. In the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) study, although the addition of niacin to treatment with a statin could significantly increase HDL-C levels and decrease triglyceride levels, there was no cardiovascular benefit in patients treated with niacin [39]. The Heart Protection Study 2 THRIVE trial was a large randomized study that included patients with a total cholesterol level <135 mg/dl (on simvastatin therapy with or without ezetimibe) before randomization. Addition of extended-release niacin plus laropiprant in the treatment group reduced LDL-C levels and increased HDL-C levels compared with the placebo group. However, the primary outcome of major vascular events (non-fatal myocardial infarction, coronary heart disease, stroke, arterial revascularization) was not different between the 2 groups [40].
The association between treatment with statins and new-onset diabetes is of concern. The Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER), which was published in 2008, showed a higher incidence of diabetes in the rosuvastatin treatment group [41]. Such an association has also been confirmed by some cohort studies and meta-analysis [42-44]. In February 2012, the Food and Drug Administration changed the safety label of statins to indicate the risk of increased HbA1c and fasting serum glucose levels [45]. However, the benefit of a statin in decreasing the risk of cardiovascular disease may outweigh the risk of elevated blood glucose levels in diabetic patients.
3. Correlation between dyslipidemia and diabetic nephropathy
As a result of dysregulation of lipid metabolism, CKD alone could lead to dyslipidemia [46]. In diabetic patients with CKD, dyslipidemia could be aggravated by hyperglycemia and insulin resistance [47]. Reduced endothelial cell LPL expression and activity are also noted in patients with CKD [48]. These abnormalities can lead to delayed catabolism of ApoB containing triglyceride-rich lipoproteins. Increased levels of Apo-CIII, which are commonly found in patients with microalbuminuria, would inhibit LPL activity and suppress the removal of ApoB [46]. Hepatic lipase can hydrolyze triglycerides and phospholipid in chylomicron remnants and HDL [48]. Decreased activity and expression of hepatic lipase in CKD have been confirmed in animal models [49]. LDL receptor-related protein and VLDL-C receptor messenger RNA are downregulated in animals with CKD [50, 51]. All of these abnormalities can lead to accumulation of atherogenic chylomicrons and VLDL-C remnants. In patients with CKD, Apo-AI concentrations are decreased as a result of reduced hepatic synthesis and increased catabolism. Low Apo-AI concentration, decreased binding capacity with adenosine triphosphate-binding cassette transporter 1, and decreased lecithin-cholesterol acyltransferase activity all result in decreased synthesis of HDL [46, 48, 52]. Total cholesterol and LDL-C levels are usually within normal limits.
Many epidemiologic studies examine the effect of albuminuria and renal function on dyslipidemia in diabetic patients. A study of 200 Japanese patients with T2D investigated the lipoprotein changes in diabetic nephropathy, and the results showed that VLDL-C level did not differ at different stages of nephropathy. In patients with increased serum creatinine levels, higher intermediate-density lipoprotein cholesterol and lower HDL-C levels were noted. There were no differences in LDL-C levels in patients with diabetes and those without diabetes [53]. A large prospective study using the Hong Kong Diabetes Registry showed that increased macroalbuminuria was a risk factor for developing elevated total cholesterol and LDL-C levels. A decreased estimated glomerular filtration rate (eGFR), commencing from 110 ml/min/1.73 m2, could predict reduced HDL-C levels [54]. A small Japanese study confirmed the increased ApoB48 and small dense LDL levels in patients with diabetic nephropathy; the increase in ApoB48 level was higher in patients with macroalbuminuria than in patients with microalbuminuria [55].
There is also increasing evidence for the association of dyslipidemia and the occurrence and progression of renal disease in both diabetic and non-diabetic patients [56-59]. There are several mechanisms by which dyslipidemia could lead to diabetic nephropathy. For example, triglyceride-rich lipoprotein could stimulate the activation of the transforming growth factor-beta (TGF-β) pathway. TGF-β subsequently promotes the production of reactive oxygen species, leading to glomerular damage. Activation of TGF-β also increases matrix deposition in the tubulointerstitium and mesangium [60]. In addition to the TGF-β pathway, triglyceride-rich lipoprotein can activate monocytes and disrupt cellular glycocalyx, causing increased permeability in the glomerulus [61]. Oxidized lipoprotein can inhibit nitric oxide-mediated vasodilation, modulate mesangial cell proliferation, and increase monocyte chemoattractant expression, which all contribute to glomerular injury [62, 63]. These adverse effects can be reversed by HDL. However, a reduced HDL-C level in patients with CKD may precipitate kidney injury.
The Health Professionals Follow-Up Study (HPFS) and Atherosclerosis Risk in Communities Study showed that hypertriglyceridemia and a decreased HDL-C level, which were characteristic of dyslipidemia in patients with diabetes, were associated with a decreased glomerular filtration rate in individuals with T2D [56, 64]. In some prospective studies, hypercholesterolemia predicts the development of diabetic nephropathy [65-67]. In the Early Treatment Diabetic Retinopathy Study (ETDRS), hypertriglyceridemia was a risk factor for end-stage renal disease in patients with T2D [67]. The World Health Organization Multinational Study of Vascular Diseases in Diabetes (WHO MSVDD) also showed that triglyceride level was a risk factor for renal failure in patients with T2D [68]. Hypertriglyceridemia and hyper-ApoB level may play a role in the onset of albuminuria in patients with T2D [69, 70]. In a cross-sectional study involving Chinese patients with T2D without renal dysfunction, closer correlation between non-HDL-C and urinary albumin excretion rates was noted than with other lipid parameters [71]. There are diverse results in the association between the pattern of dyslipidemia and diabetic nephropathy, possibly because the different types of dyslipidemia are associated with different stages of diabetic nephropathy. In a study of 549 Taiwanese patients with T2D, differential dyslipidemia was observed in different stages of microalbuminuria and macroalbuminuria [72]. ApoB levels increased in the microalbuminuria stage, whereas lipoprotein(a) levels increased in the macroalbuminuria stage [72]. However, only levels of triglycerides progressively increased with albuminuria, from normoalbuminuria to microalbuminuria and to macroalbuminuria [72]. In the Kidney Early Evaluation Program (KEEP) study of diabetic patients with CKD stage 3 to 5, only an elevated HDL-C level was associated with decreased odds of microalbuminuria [73]. There was no statistical significance between the albumin excretion rate and other lipid profiles in patients with T2D [73].
Because dyslipidemia may play a role in the progression of diabetic nephropathy, treatment with lipid-lowering agents may be beneficial for renal outcomes besides their beneficial effects on CVD in patients with T2D [74]. In animal models of diabetes, treatment with a statin decreases lipid peroxidation, increases antioxidant enzyme levels, reduces accumulation of advanced glycation end-products, and reverses podocyte injury [75-77]. A meta-analysis of diabetic and non-diabetic patients with renal disease demonstrated that control of dyslipidemia with a statin has a beneficial effect on renal outcomes in terms of improved glomerular filtration rate (GFR) and proteinuria [78]. In the MRC/BHF Heart Protection Study (HPS), a smaller decrease in eGFR during follow-up was noted in the simvastatin treatment group than in the placebo group, especially in diabetic patients [79]. In the Collaborative Atorvastatin Diabetes Study (CARDS), treatment with atorvastatin improved renal function deterioration in T2D, especially in patients with albuminuria [80]. A prospective analysis of patients with T2D in Hong Kong also confirmed the benefit of treatment with a statin to reduce diabetic nephropathy in T2D [81]. In the GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study, treatment with atorvastatin increased eGFR in both diabetic and non-diabetic patients [82].
Beside statins, fibrates may also produce renal benefits in diabetic patients. Results from the Diabetes Atherosclerosis Intervention Study (DAIS) showed that treatment with fenofibrate decreased the risk of developing microalbuminuria [83]. In the FIELD study, subjects in the fenofibrate group showed a slower progression of albuminuria than those in the placebo group [37]. However, statin therapy in diabetic patients undergoing dialysis is not recommended in the Kidney Disease Outcomes Quality Initiative (KDOQI) Clinical Practice Guideline [84]. The results of Die Deutsche Diabetes Dialyse Studie (4D), A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events (AURORA), and a subgroup analysis of the Study of Heart and Renal Protection (SHARP) all failed to show a significant benefit of statin treatment on primary cardiovascular outcomes in patients on dialysis [85-87]. On post-hoc analysis of diabetic participants in the AURORA study, a greater risk of hemorrhagic stroke was noted in the rosuvastatin treatment group, although the risk of cardiac death or non-fatal myocardial infarction was decreased [88]. However, in SHARP, combination therapy with simvastatin 20 mg plus ezetimibe 10 mg daily reduced major atherosclerotic events in patients with or without dialysis intervention [89].
4. Correlation between renal disease and cardiovascular disease
In the 2007 European Society of Cardiology and the European Atherosclerosis Society Guidelines, CKD is acknowledged as a CVD risk equivalent [90]. Both abnormal urinary albumin excretion and decreased eGFR are strong predictors of CVD [91], and the correlation would be more prominent in diabetic patients [92]. In diabetic patients with microalbuminuria, increased subclinical atherosclerosis is confirmed by higher values for brachial-ankle pulse wave velocity and intima-media thickness [93]. There are many pathological mechanisms linking a higher risk of CVD and mortality in diabetic patients with kidney disease. Firstly, hypertension and dyslipidemia, which are traditional risk factors for CVD, are common in diabetic patients with albuminuria. Other non-traditional risk factors, such as chronic inflammation, insulin resistance, endothelial dysfunction, and atherogenic lipoprotein changes, also play a role in pathogenesis. Multiple biochemical parameters such as von Willebrand factor, plasminogen activator inhibitor type 1, soluble vascular cell adhesion molecule 1, intercellular adhesion molecule 1, plasma endothelin 1, C-reactive protein, and fibrinogen are associated with microalbuminuria [64, 94-98]. In the Hoorn study, both lower eGFR and greater urinary albumin excretion rate were independently associated with greater arterial stiffness [99]. Microalbuminuria had a linear association with impaired endothelium-dependent, flow-mediated vasodilatation of the brachial artery and increased circumferential wall tension and wall stress in the carotid artery in individuals with and without diabetes in the same study [100, 101]. All of these abnormalities explain the association between microalbuminuria and atherosclerosis through mechanisms of chronic inflammation, endothelial dysfunction, and diffuse vascular damage. Compared with diabetic patients with normoalbuminuria, more severe insulin resistance with higher levels of FFAs was noted in diabetic patients with albuminuria. Yu et al. confirmed the association between impaired flow-mediated vasodilatation and peripheral insulin resistance [94].
Many epidemiologic studies have demonstrated the relationship between CKD and CVD in diabetic patients [92]. A study in Hong Kong showed that among various components of metabolic syndrome, albuminuria is the strongest predictor of CVD mortality in Chinese patients with T2D [102]. A review of studies of patients with T2D showed a 2-fold increase in CVD morbidity or mortality in patients with microalbuminuria compared with those with normoalbuminuria [103]. The Heart Outcome Prevention and Evaluation (HOPE) study showed that any degree of albuminuria is a strong predictor of CVD outcomes in both diabetic and non-diabetic patients. The major CVD risk (myocardial infarction, stroke, cardiovascular death) increases by 5.9% for every 0.4 mg/mmol increase in urinary albumin excretion rate. In diabetic individuals, the adjusted relative risk (patients with microalbuminuria vs. those without microalbuminuria) for major CVD risk and hospitalization for congestive heart failure was 1.97 and 3.70, respectively [104]. In a study of 914 patients who underwent coronary angiography, there was a significant correlation of albuminuria and the severity of angiographically determined coronary atherosclerosis in both diabetic and non-diabetic patients [105]. In Chinese patients with T2D, deteriorated glomerular filtration rate could predict a higher risk of cardiovascular end points and all-cause mortality after adjustment for albuminuria [106]. In the Action in Diabetes and Vascular disease: preterAx and diamicroN-MR Controlled Evaluation (ADVANCE) study, which included 10,640 patients with T2D, a 10-fold elevation of baseline urinary albumin/creatinine ratio resulted in a 2.48-fold increased risk of cardiovascular events compared with baseline. Every 50% reduction in baseline eGFR value increased the risk of cardiovascular events 2.20-fold. There was no interaction between albuminuria and decreased eGFR in this study. In patients with both macroalbuminuria and stage 3-5 CKD, the risk of cardiovascular events increased 3.2-fold [107]. Albuminuria is also associated with ankle-brachial index in an inverse pattern [93] and with peripheral artery disease [108, 109].
Angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers are proposed in the treatment of diabetes with microalbuminuria or macroalbuminuria [28]. Because albuminuria and CKD are closely correlated with cardiovascular disease risk, medications that could lower the urinary albumin excretion rate are expected to have cardiovascular benefits. In the HOPE and Microalbuminuria, Cardiovascular, and Renal Outcomes in HOPE (MICRO-HOPE) studies, treatment of diabetic patients with ramipril reduced the risk of diabetic nephropathy, stroke, cardiovascular death, revascularization, and total mortality. In a subgroup analysis, the effect was more prominent in patients with microalbuminuria and the primary outcome effect persisted after adjustment for changes in blood pressure [110, 111]. The Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) study, a double-blind randomized trial of 1513 patients with T2D, showed a positive correlation between baseline albuminuria and the risk of the CVD end point or heart failure. Treatment with losartan could reduce proteinuria and risk reduction for end-stage renal disease [112]. Further analysis showed that every 50% reduction in albuminuria resulted in an 18% reduction in cardiovascular risk and a 27% reduction in heart failure risk [113].
In the Losartan Intervention for Endpoint reduction in hypertension (LIFE) study, losartan was compared with atenolol in relation to its blood pressure lowering effect and reduction in cardiovascular morbidity and mortality in diabetic patients with hypertension and left ventricular hypertrophy. Even with the same anti-hypertensive effect, greater risk reduction in cardiovascular mortality, stroke, and myocardial infarction was noted in the losartan treatment group [114, 115]. A post-hoc analysis combining the RENAAL study and the Irbesartan in Diabetic Nephropathy Trial showed that each decrement in log albuminuria would lead to an approximately 15% risk reduction in cardiovascular events [116]. The association between urinary albumin/creatinine ratio and peripheral artery disease also became insignificant in patients treated with angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, implying that the use of angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers might lower the risk of peripheral artery disease [109].
5. Correlation between glucose control with lipid levels, diabetic nephropathy, and cardiovascular complications
Glycemic control is important for all diabetic patients, with better blood glucose control affecting microvascular and macrovascular outcomes in patients with diabetes. The United Kingdom Prospective Study, which included 5102 patients with newly diagnosed T2D, showed that intensive blood glucose control (HbA1c of 7.0% versus 7.9%) decreased the risk of microvascular disease (relative risk reduction: 25%) and microalbuminuria (relative risk reduction: 33%) over a median follow-up of 10 years [117]. There was a trend toward decreased cardiovascular events with intensive blood glucose control, but this did not reach statistical significance (relative risk reduction: 16%; p = 0.052). However, a continued reduction in microvascular risk (relative risk reduction: 24%) and a significant reduction in myocardial infarction (relative risk reduction: 15%, p = 0.01) were noted during 10 years of post-trial follow-up, despite an early loss of glycemic differences [118].
In the Diabetes Control and Complications Trial (DCCT, 1982-1993) and the Epidemiology of Diabetes Interventions and Complications (EDIC, 1994-2006) follow-up study in type 1 diabetes, intensive glucose therapy decreased the risk of progression to microalbuminuria and overt nephropathy [119]. There was no statistically significant difference in the DCCT between the 2 groups (HbA1c of 7.2% versus 9.1%) with regard to cardiovascular disease. However, the benefit of intensive blood glucose control on cardiovascular outcomes was noted with 17 years of follow-up and observation in the EDIC study; the risk of cardiovascular disease (non-fatal myocardial infarction, stroke, death from cardiovascular disease, confirmed angina, and need for coronary artery revascularization) was reduced by 42% and the risk of non-fatal heart attack, stroke, or death from cardiovascular causes was reduced by 57% [117].
In the ADVANCE study, further reduction of HbA1c to 6.5% compared with 7.3% led to a 10% relative risk reduction in the combined outcome of major macrovascular and microvascular events, especially nephropathy [120]. These studies suggested that intensive glucose control is most effective when implemented early in the course of diabetes. In the ACCORD study, which included patients with T2D and established CVD or multiple risk factors, the risk of major cardiovascular events was not reduced in the group that received more intensive therapy (HbA1c of 6.4%) over 3.5 years of follow-up [121].
These results suggested that intensive blood glucose control reduces the risk of diabetic nephropathy and probably reduces CVD events as well during long-term follow-up. Better blood glucose control would improve dyslipidemia because of the mechanism of diabetic dyslipidemia. A trial of 1150 patients with T2D showed a close relationship between triglyceride level and fasting plasma glucose level and HbA1c [33].
6. Conclusions
In summary, there is a close correlation between dyslipidemia, CKD, and CVD in patients with T2D. Diabetes mellitus is a strong risk factor for CKD and CVD. Dyslipidemia is common in diabetic patients and diabetic dyslipidemia is strongly correlated with diabetic nephropathy and CVD. The relationship between dyslipidemia, diabetic nephropathy, and CVD in patients with T2D is summarized in Figure 1. Because treatment of dyslipidemia with a statin may delay the progression of diabetic nephropathy and reduce cardiovascular risk, early identification and aggressive treatment, if needed, is important in all diabetic patients without contraindications or end-stage renal disease.
Figure 1. Relationship between dyslipidemia, chronic kidney disease, and cardiovascular disease in diabetes mellitus.
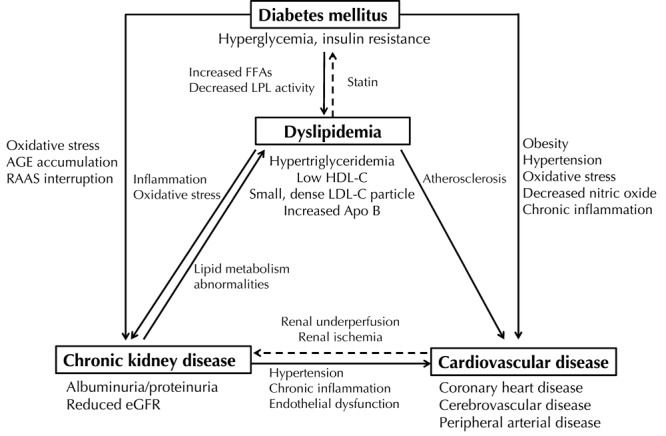
AGE - advanced glycation end-product, Apo B - apolipoprotein B, eGFR - estimated glomerular filtration rate, FFA - free fatty acid, HDL-C - high-density lipoprotein cholesterol, LDL-C - low-density lipoprotein cholesterol, LPL - lipoprotein lipase, RAAS - renin-angiotensin-aldosterone system.
Disclosures: The authors report no conflict of interests.
Acknowledgments
The authors thank the Department of Health (DOH89-TD-1035; DOH97-TD-D-113-97009) and the National Science Council (NSC101-2314-B-002-117, NSC102-2314-B-002-067) of Taiwan for their continuous support of epidemiologic studies in diabetes.
References
- 1.International Diabetes Federation Diabetes Atlas Update 2012. International Diabetes Federation; http://www.idf.org/diabetesatlas/5e/Update2012. [Google Scholar]
- 2.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241(19):2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 3.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes care. 1993;16(2):434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 4.Tseng CH. Mortality and causes of death in a national sample of diabetic patients in Taiwan. Diabetes Care. 2004;27(7):1605–1609. doi: 10.2337/diacare.27.7.1605. [DOI] [PubMed] [Google Scholar]
- 5.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 6.Hadaegh F, Fahimfar N, Khalili D, Sheikholeslami F, Azizi F. New and known type 2 diabetes as coronary heart disease equivalent: results from 7.6 year follow up in a Middle East population. Cardiovasc Diabetol. 2010;9:84. doi: 10.1186/1475-2840-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schramm TK, Gislason GH, Kober L, Rasmussen S, Rasmussen JN, Abildstrom SZ, Hansen ML, Folke F, Buch P, Madsen M. et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation. 2008;117(15):1945–1954. doi: 10.1161/CIRCULATIONAHA.107.720847. [DOI] [PubMed] [Google Scholar]
- 8.Chuang SY, Hsu PF, Sung SH, Chou P, Chen CH. Diabetes and 15-year cardiovascular mortality in a Chinese population: Differential impact of hypertension and metabolic syndrome. J Chin Med Assoc. 2010;73(5):234–240. doi: 10.1016/S1726-4901(10)70051-4. [DOI] [PubMed] [Google Scholar]
- 9.Deedwania PC. Diabetes is a vascular disease: the role of endothelial dysfunction in pathophysiology of cardiovascular disease in diabetes. Cardiol Clin. 2004;22(4):505–509. doi: 10.1016/j.ccl.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Battisti WP, Palmisano J, Keane WE. Dyslipidemia in patients with type 2 diabetes. relationships between lipids, kidney disease and cardiovascular disease. Clin Chem Lab Med. 2003;41(9):1174–1181. doi: 10.1515/CCLM.2003.181. [DOI] [PubMed] [Google Scholar]
- 11.Tai TY, Tseng CH, Sung SM, Huang RF, Chen CZ, Tsai SH. Retinopathy, neuropathy and nephropathy in non-insulin-dependent diabetic patients. J Formos Med Assoc. 1991;90(10):936–940. [PubMed] [Google Scholar]
- 12.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 13.Wassef L, Langham RG, Kelly DJ. Vasoactive renal factors and the progression of diabetic nephropathy. Curr Pharm Des. 2004;10(27):3373–3384. doi: 10.2174/1381612043383052. [DOI] [PubMed] [Google Scholar]
- 14.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W. et al. United States Renal Data System 2011 Annual Data Report: Atlas of chronic kidney disease and end-stage renal disease in the United States. Am J Kidney Dis. 2012;59(1 Suppl 1):A7. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 16.Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG. Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int. 2006;69(11):2057–2063. doi: 10.1038/sj.ki.5000377. [DOI] [PubMed] [Google Scholar]
- 17.Barkoudah E, Skali H, Uno H, Solomon SD, Pfeffer MA. Mortality rates in trials of subjects with type 2 diabetes. J Am Heart Assoc. 2012;1(1):8–15. doi: 10.1161/JAHA.111.000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chahil TJ, Ginsberg HN. Diabetic dyslipidemia. Endocrinol Metab Clin North Am. 2006;35(3):491–510. doi: 10.1016/j.ecl.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Packard CJ, Saito Y. Non-HDL cholesterol as a measure of atherosclerotic risk. J Atheroscler Thromb. 2004;11(1):6–14. doi: 10.5551/jat.11.6. [DOI] [PubMed] [Google Scholar]
- 20.Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2009;5(3):150–159. doi: 10.1038/ncpendmet1066. [DOI] [PubMed] [Google Scholar]
- 21.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 22.Fuller JH, Stevens LK, Wang SL. Risk factors for cardiovascular mortality and morbidity: the WHO Mutinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S54–S64. doi: 10.1007/pl00002940. [DOI] [PubMed] [Google Scholar]
- 23.Ginsberg HN. Insulin resistance and cardiovascular disease. J Clin Invest. 2000;106(4):453–458. doi: 10.1172/JCI10762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avramoglu RK, Qiu W, Adeli K. Mechanisms of metabolic dyslipidemia in insulin resistant states: deregulation of hepatic and intestinal lipoprotein secretion. Front Biosci. 2003;8:464–476. doi: 10.2741/1022. [DOI] [PubMed] [Google Scholar]
- 25.Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem. 2009;42(13-14):1331–1346. doi: 10.1016/j.clinbiochem.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 26.Miettinen H, Lehto S, Salomaa V, Mahonen M, Niemela M, Haffner SM, Pyorala K, Tuomilehto J. Impact of diabetes on mortality after the first myocardial infarction. The FINMONICA Myocardial Infarction Register Study Group. Diabetes Care. 1998;21(1):69–75. doi: 10.2337/diacare.21.1.69. [DOI] [PubMed] [Google Scholar]
- 27.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–393. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 28.Executive summary: Standards of medical care in diabetes 2012. Diabetes Care. 2012;35(Suppl 1):S4–S10. doi: 10.2337/dc12-s004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 30.Howard BV, Robbins DC, Sievers ML, Lee ET, Rhoades D, Devereux RB, Cowan LD, Gray RS, Welty TK, Go OT. et al. LDL cholesterol as a strong predictor of coronary heart disease in diabetic individuals with insulin resistance and low LDL: The Strong Heart Study. Arterioscler Thromb Vasc Biol. 2000;20(3):830–835. doi: 10.1161/01.atv.20.3.830. [DOI] [PubMed] [Google Scholar]
- 31.Shepherd J, Barter P, Carmena R, Deedwania P, Fruchart JC, Haffner S, Hsia J, Breazna A, LaRosa J, Grundy S. et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006;29(6):1220–1226. doi: 10.2337/dc05-2465. [DOI] [PubMed] [Google Scholar]
- 32.Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371(9607):117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 33.Tseng CH, Tseng CP, Chong CK, Cheng JC, Tai TY. Independent association between triglycerides and coronary artery disease in Taiwanese type 2 diabetic patients. Int J Cardiol. 2006;111(1):80–85. doi: 10.1016/j.ijcard.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 34.Bos G, Dekker JM, Nijpels G, de Vegt F, Diamant M, Stehouwer CD, Bouter LM, Heine RJ. A combination of high concentrations of serum triglyceride and non-high-density-lipoprotein-cholesterol is a risk factor for cardiovascular disease in subjects with abnormal glucose metabolism - The Hoorn Study. Diabetologia. 2003;46(7):910–916. doi: 10.1007/s00125-003-1141-5. [DOI] [PubMed] [Google Scholar]
- 35.Havel RJ. Remnant lipoproteins as therapeutic targets. Curr Opin Lipidol. 2000;11(6):615–620. doi: 10.1097/00041433-200012000-00008. [DOI] [PubMed] [Google Scholar]
- 36.Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44(3):720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P. et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 38.Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J. et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 40.Rosenson RS, Gotto AM Jr. When clinical trials fail to address treatment gaps: the failure of niacin-laropiprant to reduce cardiovascular events. Curr Atheroscler Rep. 2013;15(6):332. doi: 10.1007/s11883-013-0332-x. [DOI] [PubMed] [Google Scholar]
- 41.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG. et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 42.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW. et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 43.Wang KL, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW. et al. Statins, risk of diabetes, and implications on outcomes in the general population. J Am Coll Cardiol. 2012;60(14):1231–1238. doi: 10.1016/j.jacc.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 44.Waters DD, Ho JE, DeMicco DA, Breazna A, Arsenault BJ, Wun CC, Kastelein JJ, Colhoun H, Barter P. Predictors of new-onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. J Am Coll Cardiol. 2011;57(14):1535–1545. doi: 10.1016/j.jacc.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 45.FDA drug safety communication: important safety label changes to cholesterol lowering statin drugs. US Food and Drug Administration; www.fda.gov/Drugs/DrugSafety/ucm293101.htm. [Google Scholar]
- 46.Keane WF, Tomassini JE, Neff DR. Lipid abnormalities in patients with chronic kidney disease. Contrib Nephro. 2011;171:135–142. doi: 10.1159/000327317. [DOI] [PubMed] [Google Scholar]
- 47.Kimoto E, Shoji T, Emoto M, Miki T, Tabata T, Okuno Y, Ishimura E, Inaba M, Nishizawa Y. Effect of diabetes on uremic dyslipidemia. J Atheroscler Thromb. 2002;9(6):305–313. doi: 10.5551/jat.9.305. [DOI] [PubMed] [Google Scholar]
- 48.Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol Renal Physiol. 2006;290(2):F262–F272. doi: 10.1152/ajprenal.00099.2005. [DOI] [PubMed] [Google Scholar]
- 49.Klin M, Smogorzewski M, Ni Z, Zhang G, Massry SG. Abnormalities in hepatic lipase in chronic renal failure: role of excess parathyroid hormone. J Clin Invest. 1996;97(10):2167–2173. doi: 10.1172/JCI118657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim C, Vaziri ND. Down-regulation of hepatic LDL receptor-related protein (LRP) in chronic renal failure. Kidney Int. 2005;67(3):1028–1032. doi: 10.1111/j.1523-1755.2005.00166.x. [DOI] [PubMed] [Google Scholar]
- 51.Vaziri ND, Liang K. Down-regulation of VLDL receptor expression in chronic experimental renal failure. Kidney Int. 1997;51(3):913–919. doi: 10.1038/ki.1997.129. [DOI] [PubMed] [Google Scholar]
- 52.Vaziri ND, Norris K. Lipid disorders and their relevance to outcomes in chronic kidney disease. Blood Purif. 2011;31(1-3):189–196. doi: 10.1159/000321845. [DOI] [PubMed] [Google Scholar]
- 53.Shoji T, Emoto M, Kawagishi T, Kimoto E, Yamada A, Tabata T, Ishimura E, Inaba M, Okuno Y, Nishizawa Y. Atherogenic lipoprotein changes in diabetic nephropathy. Atherosclerosis. 2001;156(2):425–433. doi: 10.1016/s0021-9150(00)00673-0. [DOI] [PubMed] [Google Scholar]
- 54.Yang X, So WY, Ma R, Ko G, Kong A, Lam C, Ho CS, Cockram C, Chow CC, Tong P. et al. Effects of albuminuria and renal dysfunction on development of dyslipidaemia in type 2 diabetes - the Hong Kong Diabetes Registry. Nephrol Dial Transplant. 2008;23(9):2834–2840. doi: 10.1093/ndt/gfn149. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto T, So WY, Ma R, Ko G, Kong A, Lam C, Ho CS, Cockram C, Chow CC, Tong P. et al. Significant increase of apolipoprotein B48 levels by a standard test meal in type 2 diabetic patients with nephropathy. J Atheroscler Thromb. 2008;15(4):199–205. doi: 10.5551/jat.e558. [DOI] [PubMed] [Google Scholar]
- 56.Muntner P, Coresh J, Smith JC, Eckfeldt J, Klag MJ. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. 2000;58(1):293–301. doi: 10.1046/j.1523-1755.2000.00165.x. [DOI] [PubMed] [Google Scholar]
- 57.Shestakova MV, Koshel LV, Vagodin VA, Dedov II. Risk factors of diabetic nephropathy progression in patients with a long history of diabetic mellitus as shown by a retrospective analysis. Ter Arkh. 2006;78(5):60–64. [PubMed] [Google Scholar]
- 58.Yokoyama H, Sone H, Oishi M, Kawai K, Fukumoto Y, Kobayashi M. Prevalence of albuminuria and renal insufficiency and associated clinical factors in type 2 diabetes: the Japan Diabetes Clinical Data Management study (JDDM15) Nephrol Dial Transplant. 2009;24(4):1212–1219. doi: 10.1093/ndt/gfn603. [DOI] [PubMed] [Google Scholar]
- 59.Shestakova MV, Chugunova LA, Shamkhalova M, Dirochka Iu A, Dedov II. Diabetic nephropathy: risk factors of rapid progression of renal failure. Ter Arkh. 1999;71(6):45–49. [PubMed] [Google Scholar]
- 60.Choi ME. Mechanism of transforming growth factor-beta1 signaling. Kidney Int Suppl. 2000;77:S53–S58. [PubMed] [Google Scholar]
- 61.Prakash J. Dyslipidemia in diabetic kidney disease. Clinical Queries Nephrology. 2012;1(2):115–118. [Google Scholar]
- 62.Wanner C, Greiber S, Kramer-Guth A, Heinloth A, Galle J. Lipids and progression of renal disease: role of modified low density lipoprotein and lipoprotein(a) Kidney Int Suppl. 1997;63:S102–S106. [PubMed] [Google Scholar]
- 63.Rovin BH, Tan LC. LDL stimulates mesangial fibronectin production and chemoattractant expression. Kidney Int. 1993;43(1):218–225. doi: 10.1038/ki.1993.35. [DOI] [PubMed] [Google Scholar]
- 64.Lin J, Hu FB, Rimm EB, Rifai N, Curhan GC. The association of serum lipids and inflammatory biomarkers with renal function in men with type II diabetes mellitus. Kidney Int. 2006;69(2):336–342. doi: 10.1038/sj.ki.5000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ravid M, Brosh D, Ravid-Safran D, Levy Z, Rachmani R. Main risk factors for nephropathy in type 2 diabetes mellitus are plasma cholesterol levels, mean blood pressure, and hyperglycemia. Arch Intern Med. 1998;158(9):998–1004. doi: 10.1001/archinte.158.9.998. [DOI] [PubMed] [Google Scholar]
- 66.Gall MA, Hougaard P, Borch-Johnsen K, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: prospective, observational study. BMJ. 1997;314(7083):783–788. doi: 10.1136/bmj.314.7083.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cusick M, Chew EY, Hoogwerf B, Agron E, Wu L, Lindley A, Ferris FL 3rd. Risk factors for renal replacement therapy in the Early Treatment Diabetic Retinopathy Study (ETDRS), Early Treatment Diabetic Retinopathy Study Report No. 26. Kidney Int. 2004;66(3):1173–1179. doi: 10.1111/j.1523-1755.2004.00869.x. [DOI] [PubMed] [Google Scholar]
- 68.Colhoun HM, Lee ET, Bennett PH, Lu M, Keen H, Wang SL, Stevens LK, Fuller JH. Risk factors for renal failure: the WHO Mulinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S46–S53. doi: 10.1007/pl00002939. [DOI] [PubMed] [Google Scholar]
- 69.Kazumi T, Hozumi T, Ishida Y, Ikeda Y, Kishi K, Hayakawa M, Yoshino G. Increased urinary transferrin excretion predicts microalbuminuria in patients with type 2 diabetes. Diabetes Care. 1999;22(7):1176–1180. doi: 10.2337/diacare.22.7.1176. [DOI] [PubMed] [Google Scholar]
- 70.Tseng CH. Lipid abnormalities associated with urinary albumin excretion rate in Taiwanese type 2 diabetic patients. Kidney Int. 2005;67(4):1547–1553. doi: 10.1111/j.1523-1755.2005.00235.x. [DOI] [PubMed] [Google Scholar]
- 71.Pan J, Gao F, Bao Y, Zhang L, Tu Y, Jia W. Non-high-density lipoprotein cholesterol is associated more closely with albuminuria in Chinese type 2 diabetic patients with normal renal function, compared with traditional lipid parameters. J Clin Lipidol. 2012;6(4):382–387. doi: 10.1016/j.jacl.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 72.Tseng CH. Differential dyslipidemia associated with albuminuria in type 2 diabetic patients in Taiwan. Clin Biochem. 2009;42(10-11):1019–1024. doi: 10.1016/j.clinbiochem.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 73.Bose S, Bomback AS, Mehta NN, Chen SC, Li S, Whaley-Connell A, Benjamin J, McCullough PA. Dysglycemia but not lipids is associated with abnormal urinary albumin excretion in diabetic kidney disease: a report from the Kidney Early Evaluation Program (KEEP) BMC Nephrol. 2012;13:104. doi: 10.1186/1471-2369-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cases A, Coll E. Dyslipidemia and the progression of renal disease in chronic renal failure patients. Kidney Int Suppl. 2005;99:S87–S93. doi: 10.1111/j.1523-1755.2005.09916.x. [DOI] [PubMed] [Google Scholar]
- 75.Wei P, Grimm PR, Settles DC, Balwanz CR, Padanilam BJ, Sansom SC. Simvastatin reverses podocyte injury but not mesangial expansion in early stage type 2 diabetes mellitus. Ren Fail. 2009;31(6):503–513. doi: 10.1080/08860220902963848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu B, Shen H, Zhou J, Lin F, Hu Y. Effects of simvastatin on oxidative stress in streptozotocin-induced diabetic rats: a role for glomeruli protection. Nephron Exp Nephrol. 2005;101(1):e1–e8. doi: 10.1159/000085712. [DOI] [PubMed] [Google Scholar]
- 77.Lu L, Peng WH, Wang W, Wang LJ, Chen QJ, Shen WF. Effects of atorvastatin on progression of diabetic nephropathy and local RAGE and soluble RAGE expressions in rats. J Zhejiang Univ Sci B. 2011;12(8):652–659. doi: 10.1631/jzus.B1101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59(1):260–269. doi: 10.1046/j.1523-1755.2001.00487.x. [DOI] [PubMed] [Google Scholar]
- 79.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9343):2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 80.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Charlton-Menys V, DeMicco DA, Fuller JH. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS) Am J Kidney Dis. 2009;54(5):810–819. doi: 10.1053/j.ajkd.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 81.Luk AO, Yang X, Ma RC, Ng VW, Yu LW, Lau WW, Ozaki R, Chow FC, Kong AP, Tong PC. et al. Association of statin use and development of renal dysfunction in type 2 diabetes - the Hong Kong Diabetes Registry. Diabetes Res Clin Pract. 2010;88(3):227–233. doi: 10.1016/j.diabres.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 82.Athyros VG, Papageorgiou AA, Elisaf M, Mikhailidis DP. Statins and renal function in patients with diabetes mellitus. Curr Med Res Opin. 2003;19(7):615–617. doi: 10.1185/030079903125002315. [DOI] [PubMed] [Google Scholar]
- 83.Ansquer JC, Foucher C, Rattier S, Taskinen MR, Steiner G. Fenofibrate reduces progression to microalbuminuria over 3 years in a placebo-controlled study in type 2 diabetes: results from the Diabetes Atherosclerosis Intervention Study (DAIS) Am J Kidney Dis. 2005;45(3):485–493. doi: 10.1053/j.ajkd.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 84.KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am J Kidney Dis. 2012;60(5):850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 85.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 86.Fellstrom BC, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360(14):1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 87.Sharp Collaborative Group. Study of Heart and Renal Protection (SHARP): randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am Heart J. 2010;160(5):785–794. doi: 10.1016/j.ahj.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 88.Holdaas H, Holme I, Schmieder RE, Jardine AG, Zannad F, Norby GE, Fellstrom BC. Rosuvastatin in diabetic hemodialysis patients. J Am Soc Nephrol. 2011;22(7):1335–1341. doi: 10.1681/ASN.2010090987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J. et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Graham I, Atar D, Borch-Johnsen K, Boysen G, Burell G, Cifkova R, Dallongeville J, De Backer G, Ebrahim S, Gjelsvik B. et al. European guidelines on cardiovascular disease prevention in clinical practice: full text. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) Eur J Cardiovasc Prev Rehabil. 2007;14(Suppl 2):S1–S113. doi: 10.1097/01.hjr.0000277983.23934.c9. [DOI] [PubMed] [Google Scholar]
- 91.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16(2):489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 93.Yokoyama H, Aoki T, Imahori M, Kuramitsu M. Subclinical atherosclerosis is increased in type 2 diabetic patients with microalbuminuria evaluated by intima-media thickness and pulse wave velocity. Kidney Int. 2004;66(1):448–454. doi: 10.1111/j.1523-1755.2004.00752.x. [DOI] [PubMed] [Google Scholar]
- 94.Yu Y, Suo L, Yu H, Wang C, Tang H. Insulin resistance and endothelial dysfunction in type 2 diabetes patients with or without microalbuminuria. Diabetes Res Clin Pract. 2004;65(2):95–104. doi: 10.1016/j.diabres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 95.Wu T, McGrath KC, Death AK. Cardiovascular disease in diabetic nephropathy patients: cell adhesion molecules as potential markers? Vasc Health Risk Manag. 2005;1(4):309–316. doi: 10.2147/vhrm.2005.1.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stehouwer CD, Gall MA, Twisk JW, Knudsen E, Emeis JJ, Parving HH. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. 2002;51(4):1157–1165. doi: 10.2337/diabetes.51.4.1157. [DOI] [PubMed] [Google Scholar]
- 97.Jensen T, Bjerre-Knudsen J, Feldt-Rasmussen B, Deckert T. Features of endothelial dysfunction in early diabetic nephropathy. Lancet. 1989;1(8636):461–463. doi: 10.1016/s0140-6736(89)91365-2. [DOI] [PubMed] [Google Scholar]
- 98.Bruno CM, Meli S, Marcinno M, Ierna D, Sciacca C, Neri S. Plasma endothelin-1 levels and albumin excretion rate in normotensive, microalbuminuric type 2 diabetic patients. J Biol Regul Homeost Agents. 2002;16(2):114–117. [PubMed] [Google Scholar]
- 99.Hermans MM, Henry R, Dekker JM, Kooman JP, Kostense PJ, Nijpels G, Heine RJ, Stehouwer CD. Estimated glomerular filtration rate and urinary albumin excretion are independently associated with greater arterial stiffness: the Hoorn Study. J Am Soc Nephrol. 2007;18(6):1942–1952. doi: 10.1681/ASN.2006111217. [DOI] [PubMed] [Google Scholar]
- 100.Stehouwer CD, Henry RM, Dekker JM, Nijpels G, Heine RJ, Bouter LM. Microalbuminuria is associated with impaired brachial artery, flow-mediated vasodilation in elderly individuals without and with diabetes: further evidence for a link between microalbuminuria and endothelial dysfunction - the Hoorn Study. Kidney Int Suppl. 2004;(92):S42–S44. doi: 10.1111/j.1523-1755.2004.09211.x. [DOI] [PubMed] [Google Scholar]
- 101.Hermans MM, Henry RM, Dekker JM, Nijpels G, Heine RJ, Stehouwer CD. Albuminuria, but not estimated glomerular filtration rate, is associated with maladaptive arterial remodeling: the Hoorn Study. J Hypertens. 2008;26(4):791–797. doi: 10.1097/HJH.0b013e3282f50066. [DOI] [PubMed] [Google Scholar]
- 102.Ko GT, So WY, Chan NN, Chan WB, Tong PC, Li J, Yeung V, Chow CC, Ozaki R, Ma RC. et al. Prediction of cardiovascular and total mortality in Chinese type 2 diabetic patients by the WHO definition for the metabolic syndrome. Diabetes Obes Metab. 2006;8(1):94–104. doi: 10.1111/j.1463-1326.2005.00475.x. [DOI] [PubMed] [Google Scholar]
- 103.Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. A systematic overview of the literature. Arch Intern Med. 1997;157(13):1413–1418. [PubMed] [Google Scholar]
- 104.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Halle JP, Young J, Rashkow A, Joyce C. et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286(4):421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 105.Rein P, Vonbank A, Saely CH, Beer S, Jankovic V, Boehnel C, Breuss J, Risch L, Fraunberger P, Drexel H. Relation of albuminuria to angiographically determined coronary arterial narrowing in patients with and without type 2 diabetes mellitus and stable or suspected coronary artery disease. Am J Cardiol. 2011;107(8):1144–1148. doi: 10.1016/j.amjcard.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 106.So WY, Kong AP, Ma RC, Ozaki R, Szeto CC, Chan NN, Ng V, Ho CS, Lam CW, Chow CC. et al. Glomerular filtration rate, cardiorenal end points, and all-cause mortality in type 2 diabetic patients. Diabetes care. 2006;29(9):2046–2052. doi: 10.2337/dc06-0248. [DOI] [PubMed] [Google Scholar]
- 107.Ninomiya T, Perkovic V, de Galan BE, Zoungas S, Pillai A, Jardine M, Patel A, Cass A, Neal B, Poulter N. et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J Am Soc Nephrol. 2009;20(8):1813–1821. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tseng CH, Chong CK, Tseng CP, Tai TY. The association between urinary albumin excretion and ankle-brachial index in elderly Taiwanese patients with type 2 diabetes mellitus. Age Ageing. 2008;37(1):77–82. doi: 10.1093/ageing/afm148. [DOI] [PubMed] [Google Scholar]
- 109.Tseng CH, Tseng CP, Tai TY, Chong CK. Effect of angiotensin blockade on the association between albuminuria and peripheral arterial disease in elderly Taiwanese patients with type 2 diabetes mellitus. Circ J. 2005;69(8):965–970. doi: 10.1253/circj.69.965. [DOI] [PubMed] [Google Scholar]
- 110.Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet. 2000;355(9200):253–259. [PubMed] [Google Scholar]
- 111.Gerstein HC. Reduction of cardiovascular events and microvascular complications in diabetes with ACE inhibitor treatment: HOPE and MICRO-HOPE. Diabetes Metab Res Rev. 2002;18(Suppl 3):S82–S85. doi: 10.1002/dmrr.285. [DOI] [PubMed] [Google Scholar]
- 112.Shahinfar S, Dickson TZ, Ahmed T, Zhang Z, Ramjit D, Smith RD, Brenner BM. Losartan in patients with type 2 diabetes and proteinuria: observations from the RENAAL Study. Kidney Int Suppl. 2002;82:S64–S67. doi: 10.1046/j.1523-1755.62.s82.13.x. [DOI] [PubMed] [Google Scholar]
- 113.de Zeeuw D, Remuzzi G, Parving HH, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation. 2004;110(8):921–927. doi: 10.1161/01.CIR.0000139860.33974.28. [DOI] [PubMed] [Google Scholar]
- 114.Ibsen H, Lindholm LH, Pedersen OL, Dahlof B, Kjeldsen S. The effect of losartan versus atenolol on cardiovascular morbidity and mortality in patients with diabetes mellitus in the LIFE-study. Ugeskr Laeger. 2003;165(5):459–462. [PubMed] [Google Scholar]
- 115.Lindholm LH, Ibsen H, Dahlof B, Devereux RB, Beevers G, de Faire U, Fyhrquist F, Julius S, Kjeldsen SE, Kristiansson K. et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):1004–1010. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- 116.Holtkamp FA, de Zeeuw D, de Graeff PA, Laverman GD, Berl T, Remuzzi G, Packham D, Lewis JB, Parving HH, Lambers Heerspink HJ. Albuminuria and blood pressure, independent targets for cardioprotective therapy in patients with diabetes and nephropathy: a post hoc analysis of the combined RENAAL and IDNT trials. Eur Heart J. 2011;32(12):1493–1499. doi: 10.1093/eurheartj/ehr017. [DOI] [PubMed] [Google Scholar]
- 117.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 118.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 119.de Boer IH, Rue TC, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Sun W, Zinman B, Brunzell JD, White NH. et al. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications cohort. Arch Intern Med. 2011;171(5):412–420. doi: 10.1001/archinternmed.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D. et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358(24):2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 121.Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr. et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]


