Abstract
Type 2 diabetes mellitus (T2DM) is associated with the development and progression of cardiovascular disease (CVD). Statins have an established efficacy in the management of dyslipidemia primarily by decreasing the levels of low-density lipoprotein cholesterol and thus decreasing CVD risk. They also have a favorable safety profile. Despite the statin-mediated benefit of CVD risk reduction a residual CVD risk remains, especially in T2DM patients with high triglyceride (TG) and low high-density lipoprotein cholesterol (HDL-C) values. Fibrates decrease TG levels, increase HDL-C concentrations, and improve many other atherosclerosis-related variables. Fibrate/statin co-administration improves the overall lipoprotein profile in patients with mixed dyslipidemia and may reduce the residual CVD risk during statin therapy. However, limited data exists regarding the effects of statin/fibrate combination on CVD outcomes in patients with T2DM. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study the statin/fibrate combination did not significantly reduce the rate of CVD events compared with simvastatin/placebo in patients with T2DM. However, it did show a possible benefit in a pre-specified analysis in the subgroup of patients with high TG and low HDL-C levels. Furthermore, in the ACCORD study the simvastatin/fenofibrate combination significantly reduced the rate of progression of retinopathy compared with statin/placebo administration in patients with T2DM. The present review presents the available data regarding the effects of statin/fibrate combination in patients with T2DM and atherogenic mixed dyslipidemia.
Keywords: cardiovascular disease, type 2 diabetes, dyslipidemia, fibrate, HDL cholesterol, statin, triglycerides
Abbreviations: ACCORD - Action to Control Cardiovascular Risk in Diabetes; Apo - apolipoprotein; ASPEN - Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus; b.i.d - twice daily; CHD - coronary heart disease; CI - confidence interval; CVD - cardiovascular disease; DAIS - Diabetes Atherosclerosis Intervention Study; DIACOR - Diabetes and Combined Lipid Therapy Regimen study; GFR - glomerular filtration rate; FIELD - Fenofibrate Intervention and Event Lowering in Diabetes study; HbA1c - glycosolated hemoglobin; HDL-C - high-density lipoprotein cholesterol; HMG-CoA - 3-hydroxy-3-methylglutaryl coenzyme A; HOMA - homeostasis model assessment; HPS - Heart Protection Study; hsCRP - high-sensitivity C-reactive protein; IAUC - incremental area under the curve; LDL-C - low-density lipoprotein cholesterol; Lp(a) - lipoprotein (a); LpPLA2 - lipoprotein-associated phospholipase A2; NCEP ATP III - National Cholesterol Education Program Adult Treatment Panel; NS - not significant; OR - odds ratio; PCSK9 - proprotein convertase subtilisin kexin/type 9; PPAR - peroxisome proliferator activated receptor; PROVE-IT - Pravastatin or Atorvastatin Evaluation and Infection Therapy study; SENDCAP - Saint Mary's Ealing Northwick Park Diabetes Cardiovascular Disease Prevention; T2DM - type 2 diabetes mellitus; TG - triglyceride; t.i.d. - three times daily; TNF-α - tumor necrosis factor alpha; TNT - Treating to New Targets; VA-HIT - Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial; VLDL - very low-density lipoprotein
1. Introduction
The incidence of type 2 diabetes mellitus (T2DM), which is associated with high morbidity and mortality rates, is increasing dramatically [1]. Presently, over 10% of adults in the USA have T2DM, whereas predictions for the first third of the 21st century show an at least 2 times increase in the prevalence of T2DM in China, Middle East, Sub-Saharan Africa, India, rest of Asia, and Latin America. In economically advanced countries, the increase will be about 50% in 2030 [2].
Patients with carbohydrate metabolism disorders are at increased risk of developing cardiovascular disease (CVD) [3-8]. Current treatment guidelines focus on lowering low-density lipoprotein cholesterol (LDL-C) as the primary strategy for reducing CVD risk [9, 10]. Consequently, statins (i.e. 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors) constitute the cornerstone of dyslipidemia treatment [11]. However, patients (with or without T2DM) treated with hypolipidemic drugs usually have persistent lipid abnormalities [12-15]. It has also been shown that in large trials at least one in seven treated patients experienced CVD events over five years [16, 17]. These observations indicate that patients with T2DM have a residual CVD risk, which is now considered a target of hypolipidemic therapy. Therefore, combination drug treatment in T2DM patients is a useful approach [18, 19].
The residual CVD risk is usually attributed to the presence of mixed dyslipidemia with elevated triglyceride (TG) concentration and low levels of high-density lipoprotein cholesterol (HDL-C) [20-23]. It should also be mentioned that HDL has discrete subfractions, and its protein and lipid components can be modified by oxidative processes in T2DM, leading to impaired anti-atherogenic properties [24-28]. These particles with lower antioxidant and anti-inflammatory activity are considered "dysfunctional" [24, 29, 30].
Additional factors that contribute to the increased CVD risk in patients with mixed dyslipidemia and T2DM are, among other atherosclerosis-related variables, the preponderance of the atherogenic small dense LDL particles, the increased concentration of apolipoprotein (apo) C-III and C-II, and the increased levels of inflammation-related markers such as high-sensitivity C-reactive protein (hsCRP) and lipoprotein-associated phospholipase A2 (LpPLA2) [31-46]. Another consideration is that many patients have a secondary cause of hypertriglyceridemia and/or low HDL-C levels such as excessive alcohol intake, fatty diet, hypothyroidism, nephrotic syndrome, and chronic renal failure, or they receive drugs that alter lipid variables such as thiazide diuretics, beta-blockers, high-dose corticosteroids, isotretinoin, tamoxifen, high-dose estrogens, antipsychotics, anti-tumor necrosis factor agents, and antiretroviral drugs [47-70]. Various lifestyle, dietary, and pharmaceutical interventions have been proposed for the improvement of these atherosclerosis-related variables and consequently the reduction of residual CVD risk [71-100].
Fibrates constitute a class of drugs that seem promising for the reduction of residual CVD risk as they specifically decrease TG levels and elevate HDL-C concentrations [101]. The combination of statins and fibrates is an attractive option for the comprehensive management of high CVD risk patients with T2DM as it affects different aspects of lipoprotein metabolism. The effects of statin and fibrate co-administration in T2DM patients will be discussed in the following sections.
2. Methods
A PubMed/Scopus search was performed up to March 2013 using combinations of "diabetes" with the following keywords: cardiovascular risk, cholesterol, triglycerides, HDL-C, statin, simvastatin, atorvastatin, rosuvastatin, fluvastatin, lovastatin, pravastatin, pitavastatin, fibrate, fenofibrate, fenofibric acid, bezafibrate, gemfibrozil, lipid-lowering medications, and adverse effects. Randomized controlled trials, original papers, review articles, and case reports were included in the present review. References of these articles were scrutinized for relevant articles.
3. Effects of statins in patients with T2DM
Statins are the cornerstone of lipid lowering therapy, with many large-scale, randomized, placebo-controlled trials demonstrating powerful efficacy in preventing CVD outcomes [16, 102-113]. Their effect on CVD risk reduction is primarily attributable to their hypolipidemic properties but statins additionally exert pleiotropic effects [114-118].
The Heart Protection Study (HPS) included 5,963 adults (age 40-80 years) with T2DM and 14,573 with occlusive arterial disease (without T2DM), who were randomized to receive simvastatin 40 mg/day or placebo [119]. Pre-specified analyses included the rate of first major coronary events (non-fatal myocardial infarction or coronary heart disease (CHD) death) and of first major vascular events (major CHD event, stroke or revascularization). Simvastatin treatment resulted in an average reduction of LDL-C by 39 mg/dl (1.0 mmol/l) compared with placebo during the 5-year treatment period. Significant reductions (approximately 25%) in the rate of major CHD events, strokes and revascularizations were observed in all patients. In patients with T2DM a significant reduction of 22% (95% confidence interval (CI) 13-30, p < 0.0001) in major vascular events was observed. Additionally, a significant reduction of 33% (95% CI 17-46, p = 0.0003) was observed among the 2,912 T2DM participants without occlusive arterial disease. Notably, the reduction of major vascular events was also seen (-27%, 95% CI 13-40, p = 0.0007) in the 2,426 patients with T2DM and pre-treatment LDL-C levels <116 mg/dl (3.0 mmol/l) [119].
In the Collaborative Atorvastatin Diabetes Study (CARDS) 2,838 patients (age 40-75 years) with T2DM without high levels of LDL-C (<160 mg/dl (<4.14 mmol/l)) were randomized for a median of 3.9 years to atorvastatin 10 mg/day (n = 1,428) or placebo (n = 1,410) [120]. A significant reduction of 37% (95% CI 17-52, p = 0.001) in major CVD events was observed. When different types of events were analyzed separately, a significant reduction in the rate of acute CHD events (hazard ratio 0.64, 95% CI 0.45-0.91, p < 0.05), coronary revascularizations (hazard ratio 0.69, 95% CI 0.41-1.16, p = NS), and stroke (hazard ratio 0.52, 95% CI 0.31-0.89, p < 0.05) was shown. Notably, atorvastatin treatment resulted in a trend of significant reduction in death rates (-27%, p = 0.059) [120].
However, another study with atorvastatin in T2DM patients did not result in significant reductions of CVD events. The Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus (ASPEN) assessed the 4-year administration of atorvastatin 10 mg/day versus placebo on CVD prevention in 2,410 subjects with T2DM and LDL-C levels below contemporary guideline targets (i.e. LDL-C ≤ 140 mg/dl (3.6 mmol/l) in subjects with documented myocardial infarction or an interventional procedure >3 months before screening, or LDL-C ≤ 160 mg/dl (4.1 mmol/l) if not) [121]. The administration of atorvastatin led to a greater reduction of LDL-C (-29% versus placebo, p < 0.0001), total cholesterol and TG concentration, as well as a greater increase in HDL-C, compared with the placebo group (all p < 0.01). However, the composite primary end point rates were 13.7% and 15.0% for atorvastatin and placebo, respectively (hazard ratio 0.90, 95% CI 0.73-1.12, p = 0.34). Furthermore, no significant difference was found in the subset of 1,905 subjects without prior myocardial infarction or interventional procedure (10.4% with atorvastatin and 10.8% with placebo, hazard ratio 0.97, 95% CI 0.74-1.28, p = NS), or in the 505 subjects with prior myocardial infarction or interventional procedure (26.2% with atorvastatin and 30.8% with placebo; hazard ratio 0.82, 95% CI: 0.59-1.15, p = NS). The authors of the study commented that these results could be attributed to the types of subjects recruited, the nature of the primary endpoint, and the requirement for protocol changes because of newer treatment guidelines [121].
A prospective meta-analysis that included 18,686 patients (1,466 with type 1 and 17,220 with type 2 diabetes) from 14 randomized trials concluded that statin therapy should be considered for all individuals with T2DM who are at sufficiently high risk of vascular events[17]. Indeed, statin treatment safely reduced the 5-year incidence of major CHD events, coronary revascularization and stroke [17].
A meta-analysis of randomized controlled trials evaluated the effects of hypolipidemic therapy among patients with T2DM and non-diabetic subjects [122]. The data suggested that lipid-lowering drug treatment was similarly effective in patients with T2DM and in non-diabetic individuals. The risk reduction of major CHD events in primary prevention was 21% (95% CI 11-30, p < 0.0001) in patients with T2DM and 23% (95% CI 12-33, p = 0.0003) in non-diabetic patients, whereas in secondary prevention it was 21% (95% CI 10-31, p = 0.0005) and 23% (96% CI 19-26, p < = 0.0001), respectively. Furthermore, after adjustment for baseline characteristics, patients with T2DM experienced a greater benefit from lipid-lowering therapy compared with non-diabetic subjects in both primary and secondary prevention [122].
Generally, statins are safe drugs [123-127]. Of note, statin therapy has been associated with alterations of glucose homeostasis and insulin resistance [128]. A meta-analysis that included 13 statin trials with 91,140 participants showed that statin therapy is associated with an increased risk of 9% for incident T2DM [129]. However, this risk is substantially low compared with the reduction of CHD events observed with statin treatment.
4. Effects of fibrates in patients with T2DM
Fibrates belong to a class of drugs that activate peroxisome proliferator activated receptor α (PPAR-α) [101, 130]. Bezafibrate, gemfibrozil, and the newer agents, fenofibrate and fenofibric acid, are members of this family. This class of drugs is able to decrease the concentration of plasma TG by 30-50%, to increase levels of HDL-C by 2-20%, and to exert a number of pleiotropic effects [92, 101, 130-135]. As a result, these drugs may have a role in individuals at high risk of CVD events and combined dyslipidemia [136, 137]. In the Veterans Affairs High-Density Lipoprotein Cholesterol Intervention trial (VA-HIT), gemfibrozil significantly reduced the risk of major CVD events (CHD, death, stroke, or myocardial infarction) by 32% (p = 0.004) in T2DM patients with established CHD and low levels of HDL-C [138]. In the Saint Mary’s Ealing Northwick Park Diabetes Cardiovascular Disease Prevention (SENDCAP) study, bezafibrate significantly reduced the combined incidence of ischemic change on the resting electrocardiography and of documented myocardial infarction in 164 patients with T2DM without previous CHD (p = 0.01) [139].
Fenofibrate, is now the most commonly prescribed fibrate worldwide and is indicated for the treatment of combined dyslipidemia, remnant hyperlipidemia and hypertriglyceridemia [140]. Fenofibrate leads to a reduction of TG concentration by 30-50% and an increase of HDL-C by 2-20% [101, 130, 131, 141-143]. Furthermore, fenofibrate, similar to gemfibrozil, exerts a variable effect on LDL-C concentration that is a small decrease in patients with hypercholesterolemia or a slight increase in individuals with mixed dyslipidemia [130, 144-147]. It should be mentioned that the Friedewald equation becomes progressively unreliable when TG concentration exceeds 400 mg/dl (4.5 mmol/l). Hence, at higher TG levels, the Friedewald formula should not be used and other methods of LDL-C determination should be employed [148, 149]. The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial, the largest study that examined the benefits of fenofibrate in 9,795 patients with T2DM, provided mixed results. Fenofibrate (200 mg/day for 5 years) did not significantly reduce the risk of the primary trial outcome of major CHD events (-11%, p = NS), but it reduced the risk of total CVD events (-11%, p = 0.035) compared with placebo, mainly by the prevention of non-fatal myocardial infarctions (-24%, p = 0.01) and coronary revascularizations (-21%, p = 0.003) [142]. It should be mentioned that in the FIELD study significantly more patients in the placebo group (17%) compared with the fenofibrate group (8%, p < 0.0001) also received other lipid treatments, predominantly statins. As a result, the possible beneficial CVD effects of fenofibrate may have been weakened from the statin treatment in the placebo group [142]. Moreover, in the Diabetes Atherosclerosis Intervention Study (DAIS), a double-blind placebo-controlled trial that evaluated the angiographic progression of CHD in patients with T2DM (n = 418), fenofibrate treatment was associated with decreased angiographic progression of CHD [150].
Fenofibrate is a pro-drug, which is converted in the liver to the active drug and then released into the plasma to activate PPAR-α in tissues [151, 152]. The newer fibrate formulation, fenofibric acid is not a pro-drug and does not undergo first-pass hepatic metabolism [153].
5. Effects of statin and fibrate combination on metabolic parameters in patients with mixed dyslipidemia and/or T2DM
In several clinical studies (Tables 1 and 2) the combination of statin/fibrate therapy resulted in pronounced decreases in LDL-C, TG and non-HDL-C, as well as increases in HDL-C, compared with either monotherapy [101, 154-156]. For example, a pooled subgroup analysis of three randomized, controlled, double-blind, 12-week trials that included 586 patients with mixed dyslipidemia and T2DM showed that the fenofibric acid/statin combination treatment was associated with a significantly greater improvement of lipid profile compared with either monotherapy [157]. Indeed, it was shown that the combination of fenofibric acid with low- or moderate-dose statin, led to a 5-fold higher percentage of patients achieving the combined optimal levels of LDL-C, non-HDL-C, apoB, TG and HDL-C [157].
Table 1. Selected studies of the combined treatment of statins with fenofibrate in T2DM patients.
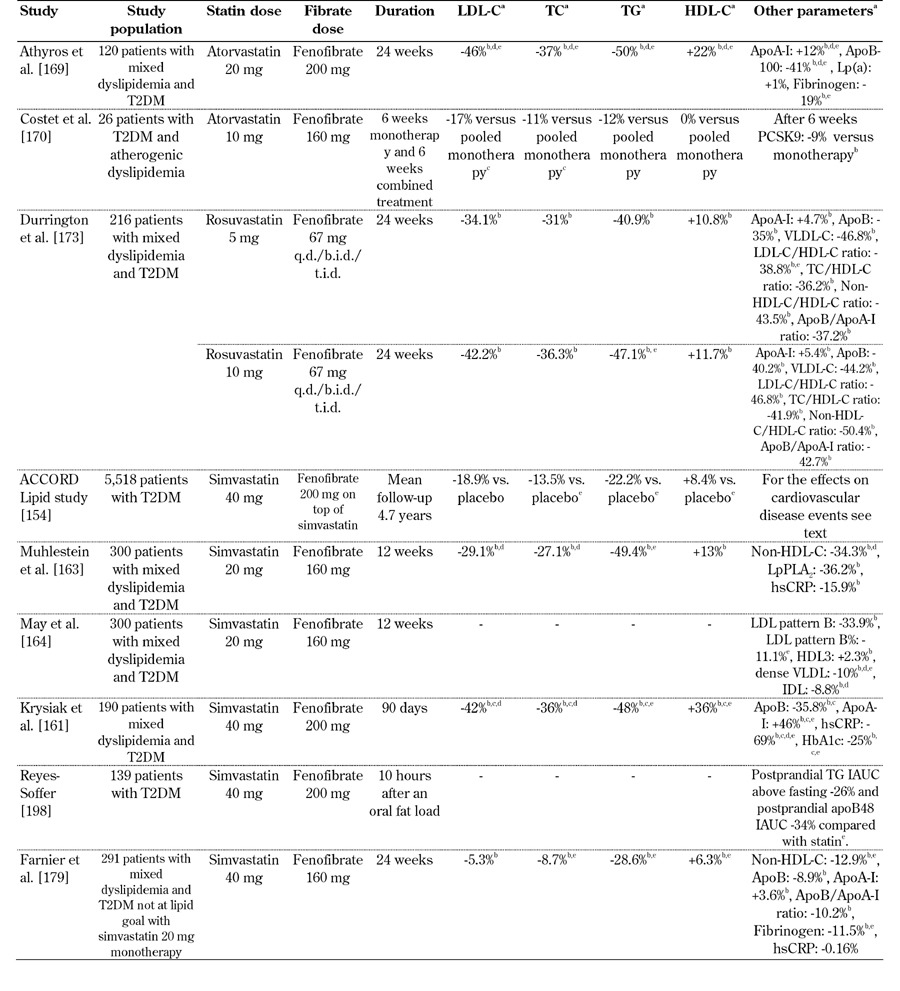
Legend: a Values express the increase or reduction versus baseline in the combination treatment, except otherwise mentioned. b p < 0.05 versus baseline. c p < 0.05 versus placebo. d p < 0.05 versus fibrate monotherapy. e p < 0.05 versus statin monotherapy. Abbreviations: T2DM - type 2 diabetes mellitus; LDL-C - low-density lipoprotein cholesterol; TC - total cholesterol; TG - triglycerides; HDL-C - high-density lipoprotein cholesterol; apo - apolipoprotein; Lp(a) - lipoprotein (a); PCSK9 - proprotein convertase subtilisin/kexin type 9; VLDL - very low-density lipoprotein; VLDL-C - VLDL cholesterol; hsCRP - high-sensitivity C-reactive protein; LpPLA2 - lipoprotein-associated phospholipase A2; IDL - intermediate-density lipoprotein; HbA1c - glycosylated hemoglobin; IAUC - incremental area under the curve; q.d./b.i.d./t.i.d - once/twice/three times daily.
Table 2. Selected studies of the combined treatment of statins with fenofibric acid or bezafibrate in T2DM patients.
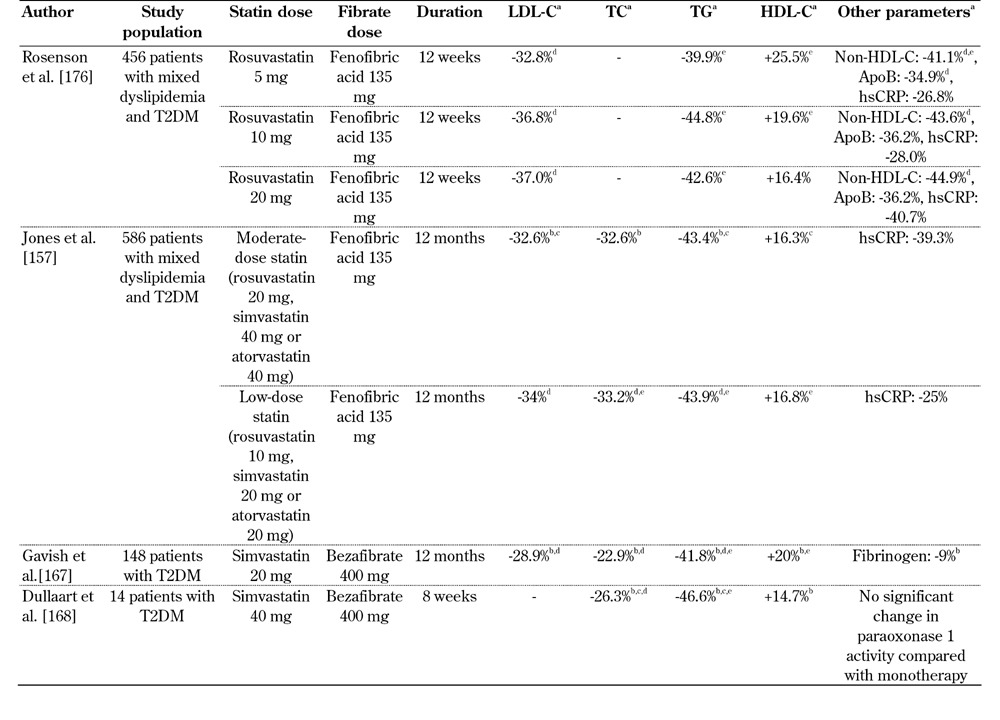
Legend: a Values express the increase or reduction versus baseline in the combination treatment, except otherwise mentioned. b p < 0.05 versus baseline. c p < 0.05 versus placebo. d p < 0.05 versus fibrate monotherapy. e p < 0.05 versus statin monotherapy. Abbreviations: T2DM - type 2 diabetes mellitus; LDL-C - low-density lipoprotein cholesterol; TC - total cholesterol; TG - triglycerides; HDL-C - high-density lipoprotein cholesterol; apo - apolipoprotein; hsCRP - high-sensitivity C-reactive protein.
5.1 Simvastatin plus fibrate combination treatment
A study assessed the efficacy of fenofibrate administered for 12 months in patients with mixed hyperlipidemia (n = 45, age 58.9 ± 11.3 years) [158]. Patients were included if they had plasma TG levels >300 mg/dl and LDL-C >160 mg/dl after at least 6 months on simvastatin treatment and received simvastatin 10 mg/day plus fenofibrate 200 mg/day (n = 5), simvastatin 20 mg/day plus fenofibrate 200 mg/day (n = 26), simvastatin 20 mg/day plus fenofibrate 300 mg/day (n = 11) and simvastatin 30 mg/day plus fenofibrate 200 mg/day (n = 3). The combination treatment resulted in reductions in total cholesterol (-18% with the lower doses (p < 0.05) to -39% with the higher doses (p < 0.05)), LDL-C (-21% (p = NS) to -39% (p < 0.05)), and TG levels (-35% (p < 0.05) to -56% (p < 0.01)), as well as an increase in HDL-C (+8% (p < 0.05) to +30% (p = NS)), respectively [158].
A multicenter, randomized, double-blind, active-controlled, 18-week study (Simvastatin plus Fenofibrate for Combined Hyperlipidaemia (SAFARI)) [159] included 411 patients with combined hyperlipidemia (age 21 - 68 years), of whom 71% had the metabolic syndrome as defined by The National Cholesterol Education Program Adult Treatment Panel III criteria [10]. After 12 weeks a significantly greater improvement in total cholesterol, TG, non-HDL-C, LDL-C, HDL-C, very low-density lipoprotein (VLDL) cholesterol (VLDL-C), VLDL-TG, apoA-I, and apoB levels was observed with combination therapy compared with simvastatin monotherapy (all p < 0.01) [159].
The efficacy of fenofibrate 145 mg, simvastatin 40 mg or their combination was compared in 241 patients with T2DM and combined dyslipidemia [160]. After 12 months of treatment, fenofibrate plus simvastatin had a more favorable effect in decreasing total cholesterol, LDL-C, TG and apoB levels and in increasing HDL-C and apoA-I concentration compared with monotherapy. Moreover, hsCRP was decreased more with combination therapy than with simvastatin [160].
In a double-blind study, 196 patients with recently diagnosed and previously untreated T2DM and mixed dyslipidemia received metformin, and were further randomized to simvastatin 40 mg/day, fenofibrate 200 mg/day, their combination, or placebo [161]. After 90 days of treatment, simvastatin monotherapy and the combined therapy group resulted in greater changes in total cholesterol, LDL-C, and apoB levels compared with fenofibrate monotherapy. In addition, combination treatment improved TG, apoA-I, glucose, homeostasis model assessment (HOMA) index, and glycosylated hemoglobin (HbA1c) more than simvastatin monotherapy (all p < 0.05). The combination treatment also resulted in a more pronounced effect on plasma hsCRP and lymphocyte release of interleukin-2, interferon-gamma, and tumor necrosis factor alpha (TNF-a) compared with simvastatin or fenofibrate monotherapy [161].
Another randomized clinical study examined the effectiveness of fenofibrate plus simvastatin combination given for 6 months on an alternate-day regimen (simvastatin 10 mg every other day and fenofibrate 250 mg on the days that simvastatin was not taken) in patients (n = 69, age 56 ± 7 years) with mixed hyperlipidemia [162]. Patients in the alternate-day regimen and in the every-day combination treatment experienced similar decreases in total cholesterol (-31% versus 31%), TG (-55% versus -54%), LDL-C (-34% versus -36%), and apoB (-20% versus -18%) plasma levels, as well as similar increase in HDL-C and apoA-I levels (p = NS for all comparisons). Of note, at the end of the follow-up period total cholesterol, TG, HDL-C and LDL-C levels were within normal limits in almost all patients in both groups [162].
The DIACOR study (Diabetes and Combined Lipid Therapy Regimen) examined the effects on inflammatory biomarkers of simvastatin 20 mg, fenofibrate 160 mg and their combination in 300 patients with mixed dyslipidemia and T2DM [163]. After 12 weeks of treatment, significant median changes were observed in hsCRP levels and LpPLA2 activity compared with baseline levels (-14.6%, p = 0.004 and -16.8%, p < 0.0001, respectively), but these changes did not differ significantly with monotherapy. When only patients with high baseline levels of hsCRP (>2.0 mg/l) were examined, a greater reduction was shown in all treatment groups compared with baseline levels (fenofibrate: -18.9%, p = 0.002, simvastatin: -24.8%, p < 0.0001, combination: -27.3%, p = 0.002). Likewise, further reductions were observed in patients with increased baseline LpPLA2 levels (fenofibrate: -41.3%, simvastatin: -47.5%, combination: -46.8%, p < 0.0001 for all versus baseline) [163]. In addition, the combination of fenofibrate with simvastatin significantly improved the lipid profile when compared with fenofibrate monotherapy and led to greater reductions in TG levels when compared with simvastatin alone [163].
In an extension of the DIACOR study, lipoprotein subparticle profiles were evaluated [164]. After 12 weeks, the combination therapy and fenofibrate monotherapy led to significant reductions in the LDL pattern B (-11.1% and -13.7%, respectively, p < 0.0001 for both) and to significant increases in the percentage of buoyant LDL-C constituting total LDL-C [164]. Thus, fenofibrate and combination therapy favored the shift from LDL pattern B to the more buoyant, less atherogenic LDL. When the same parameters were examined in patients with TG levels >170 mg/dl at baseline, it was observed that they tended to have greater reductions in LDL pattern B than those with TG levels <170 mg/dl [164].
A multicenter, double-blind, placebo-controlled trial included patients (n = 611, age 18-79 years) with mixed hyperlipidemia (including patients with T2DM with LDL-C of 100 to 180 mg/dl) who were randomized for 12 weeks in a 3:3:3:1 ratio to ezetimibe/simvastatin 10/20 mg plus fenofibrate 160 mg, ezetimibe/simvastatin 10/20 mg, fenofibrate 160 mg or placebo [165]. The triple combination significantly decreased TG, non-HDL-C and apoB levels (-50.0%, -50.5%, and -44.7%, respectively) compared with all other treatments. The triple combination significantly reduced LDL-C levels (-45.8%) compared with the other treatments except the ezetimibe/simvastatin administration (-47.1%), whereas it significantly increased HDL-C (+18.7%) concentration compared with the other treatments except the fenofibrate administration (+18.2%) [165].
A study randomized 621 patients with mixed dyslipidemia to fenofibric acid, simvastatin or their combination [166]. The combination of fenofibric acid 135 mg/day with simvastatin 40 mg/day resulted in a greater increase in HDL-C (+18.9% versus +8.5%, p < 0.001) and a greater decrease in TG levels (-42.7% versus -22.4%, p < 0.001) compared with simvastatin 40 mg/day, as well as a greater reduction in LDL-C levels compared with fenofibric acid monotherapy (-25.3% versus -4.0%, p < 0.001). The combination of fenofibric acid with simvastatin 20 mg/day produced similar results. Furthermore, the combination of fenofibric acid with simvastatin 40 mg/day produced a greater reduction in VLDL-C (p = 0.005) and similar reductions in non-HDL-C, apoB, total cholesterol, and hsCRP compared with simvastatin 40 mg/day [166].
The combination of simvastatin with bezafibrate was assessed in one study, in which 48 patients receiving bezafibrate slow release (400 mg/day) and 100 patients receiving simvastatin 20 mg/day were given for 1 year the combination of both drugs [167]. After 1 year, a significant reduction in the concentration of total cholesterol (-23%), TG (-23%), LDL-C (-29%), and fibrinogen (-10%, all p < 0.05) compared with bezafibrate monotherapy and a significant increase in HDL-C levels (+25%, p < 0.05) compared with simvastatin monotherapy was observed. Of note, it was shown that during the initial 6 months of monotherapy the CVD event rate was 9.5% but it was reduced to 2% during the combination treatment [167].
A small study in 14 patients with T2DM showed that administration of simvastatin 40 mg/day combined with bezafibrate 400 mg/day over 8 weeks increased HDL-C and apoA-I levels (p < 0.05), but did not induce any significant increase in arylesterase (p = 0.24) or paraoxonase activity (p = 0.37) [168].
5.2 Atorvastatin plus fibrate combination treatment
In a study by Athyros et al., 120 patients with T2DM and mixed hyperlipidemia, who were free of CHD, were randomized to atorvastatin (20 mg/day, n = 40), micronized fenofibrate (200 mg/day, n = 40), or atorvastatin 20 mg/day plus fenofibrate 200 mg/day (n = 40) [169]. The combination treatment led to greater reductions compared with atorvastatin or fenofibrate monotherapy (all p < 0.05) in the plasma concentration of LDL-C (-46% versus -40% and -15%, respectively), total cholesterol (-37% versus -31% and -16%, respectively), TGs (-50% versus -30% and -41%, respectively), and apoB (-33.3% versus -24.5% and -18.7%, respectively). The atorvastatin/fenofibrate combination resulted in a significant increase in HDL-C concentration (+22%; p < 0.0001 versus baseline; p < 0.05 versus atorvastatin), whereas the atorvastatin administration led to minor effects (+9%; p < 0.0001 versus baseline) and fenofibrate monotherapy resulted in a non-significant increase (+16%; p = NS). The atorvastatin/fenofibrate combination reduced the 10-year probability of myocardial infarction from 21.6% to 4.2% (p < 0.005 versus both monotherapies) [169].
In a randomized, open-label, cross-over study in patients with T2DM and atherogenic dyslipidemia, after treatment with either atorvastatin 10 mg or fenofibrate 160 mg for 6 weeks, patients received the combination of them for another 6 weeks. Combination therapy after 6 weeks of treatment led to a further decrease in LDL-C by 17% (p = 0.05) but did not show further benefit in total cholesterol, TG, HDL-C and proprotein convertase subtilisin kexin/type 9 (PCSK9) levels [170].
In a study that assessed the combination of fenofibric acid with atorvastatin in patients with mixed hyperlipidemia (n = 577), the fenofibric acid/atorvastatin combination led to significantly greater reduction in TG (-42.1% versus -23.2%, p < 0.001) and a significantly greater increase in HDL-C levels (+12.6% versus +5.3%, p = 0.01) compared with atorvastatin monotherapy, as well as a greater reduction in LDL-C concentration compared with fenofibric acid monotherapy (-35.4% versus -3.4%, p < 0.001) [171].
In another 12-week double-blind study, a total of 543 patients with mixed hyperlipidemia received atorvastatin 40 mg/day plus ezetimibe 10 mg/day and were randomized to fenofibric acid 135 mg/day or placebo [172]. The triple combination (fenofibric acid plus atorvastatin and ezetimibe) produced a significantly greater improvement in HDL-C (+13.0% versus +4.2%, p < 0.001) and TG levels (-57.3% versus -39.7%, p < 0.001), as well as a significantly greater effect on non-HDL-C, apoB, apoA-I, apoC-III, VLDL-C, and hsCRP, than atorvastatin/ezetimibe combination alone [172].
5.3 Rosuvastatin plus fibrate combination treatment
A randomized, parallel-group, multicenter trial, evaluated the effects of rosuvastatin plus fenofibrate in patients with T2DM and combined hyperlipidemia [173]. The study population (n = 216) was randomized to placebo/rosuvastatin, placebo/fenofibrate, rosuvastatin 5 mg/fenofibrate and rosuvastatin 10 mg/fenofibrate. At 24 weeks, combination therapy of rosuvastatin 10 mg with fenofibrate significantly reduced TG levels compared with the placebo/rosuvastatin group (-47.1% versus -30.3%, p = 0.001). Regarding LDL-C concentration, the greater reductions were observed in placebo/rosuvastatin (-46.7%) compared with rosuvastatin 10 mg/fenofibrate (-42.2%, p = NS), placebo/fenofibrate (+0.7%, p < 0.001) and rosuvastatin 5 mg/fenofibrate (-34.1%, p < 0.001) [173].
In a study that randomized 1,377 patients with mixed hyperlipidemia for 12 weeks to either fenofibric acid 135 mg, rosuvastatin 10, 20, or 40 mg, or fenofibric acid plus rosuvastatin 10 or 20 mg, fenofibric acid plus rosuvastatin 20 mg produced greater improvements in TG (-42.9% versus -25.6%, p < 0.001) and HDL-C (+19.0% versus +10.3%, p < 0.001) levels compared with rosuvastatin 20 mg monotherapy [174]. Furthermore, a significantly greater LDL-C reduction (-38.8% versus -6.5%, p < 0.001) compared with fenofibric acid monotherapy was seen. The comparison of fenofibric acid plus rosuvastatin 10 mg with rosuvastatin 10 mg or with fenofibric acid monotherapy produced similar results. Fenofibric acid plus rosuvastatin 10 or 20 mg resulted in significantly greater reduction of hsCRP compared with the corresponding dose of rosuvastatin monotherapy (p <0.05) [174].
In another randomized, double-blind study, the combination of rosuvastatin 5 mg/day with fenofibric acid 135 mg/day for 12 weeks in patients with mixed dyslipidemia (n = 758) produced a significantly greater increase in HDL-C plasma concentration (+23.0% versus +12.4%, p < 0.001) and a significantly greater reduction in TG levels (-40.3% versus -17.5%, p < 0.001) compared with rosuvastatin monotherapy. Also, this combination caused a greater reduction in LDL-C levels (-28.7% versus -4.1%, p < 0.001) compared with fenofibric acid monotherapy [175].
In a post hoc analysis of the two aforementioned trials [174, 175], 456 patients with T2DM and mixed dyslipidemia were treated with rosuvastatin (5, 10, or 20 mg), fenofibric acid 135 mg, or the combination of them for 12 weeks [176]. The realization of LDL-C <100 mg/dl, HDL-C >40/50 mg/dl in men and women, respectively, TG <150 mg/dl, non-HDL-C <130 mg/dl, and apoB <90 mg/dl and the combined targets of the above parameters were evaluated. Combination therapy with rosuvastatin plus fenofibric acid resulted in a significantly greater proportion of T2DM patients achieving the above individual and combined lipid targets than the corresponding dose rosuvastatin monotherapies [176].
A recent trial randomized patients to receive combination of fenofibric acid 135 mg/day with rosuvastatin 5, 10, or 20 mg/day or to monotherapy with simvastatin 40 mg/day [177]. The combination treatments produced significantly greater improvements in LDL-C, HDL-C, non-HDL-C, apoB, TG, hsCRP, total cholesterol and apoC-III compared with simvastatin 40 mg/day. Of note, optimal levels for LDL-C (<100 mg/dl, p < 0.001), non-HDL-C (<130 mg/dl, p < 0.001), apoB (<90 mg/dl, p ≤ 0.02), and TG (<150 mg/dl, p < 0.001) were achieved in a significantly higher proportion of patients in each fenofibric acid/rosuvastatin group compared with monotherapy with simvastatin 40 mg/day [177].
In a post-hoc analysis of two 12-week controlled studies and a 52-week extension study, patients were treated with fenofibric acid 135 mg, rosuvastatin 5, 10, 20, or 40 mg or rosuvastatin 5, 10, or 20 mg plus fenofibric acid 135 mg in the controlled studies, and with rosuvastatin 20 mg plus fenofibric acid 135 mg in the extension study [178]. When patients who had high hsCRP (≥2 mg/l) levels after 12 weeks of rosuvastatin 10, 20, or 40 mg monotherapy were examined, it was shown that hsCRP was reduced by approximately 36% after switching to rosuvastatin 20 mg plus fenofibric acid 135 mg for up to 52 weeks and approximately 36% of patients shifted from hsCRP levels ≥2 mg/l to a concentration <2 mg/l [178].
5.4 Pravastatin plus fibrate combination treatment
In a multicenter randomized, double-blind, parallel-arm study, a fixed-dose of pravastatin 40 mg/fenofibrate 160 mg or simvastatin 20 mg monotherapy was administered over 12 weeks in 291 patients with T2DM and mixed hyperlipidemia who were receiving simvastatin 20 mg and were not achieving lipid goals [179]. After 12 weeks of treatment, fixed-dose combination led to significantly greater improvements in non-HDL-C (-12.9%), TG (-28.6%), total cholesterol (-8.7%) and HDL-C concentration (+6.3%) compared with simvastatin (-6.8%, +5%, -5.2%, +1.8% respectively, all p< 0.05). In this study, the proportion of patients who achieved the non-HDL-C goal <130 mg/dl at week 12 was significantly greater in the fixed-dose group (42.4%) compared with simvastatin monotherapy (24.1%, p = 0.001). Similarly, the fenofibrate/pravastatin combination led to a significantly greater proportion of patients achieving the combined end-point of non-HDL-C <130 mg/dl and LDL-C <100 mg/dl compared with simvastatin monotherapy (41% versus 26%, p < 0.05) [179].
Another study of the same group compared a triple therapy of pravastatin 40 mg/fenofibrate 160 mg plus ezetimibe 10 mg with dual therapy of simvastatin 20 mg plus ezetimibe 10 mg over 12 weeks in 273 patients with T2DM, mixed hyperlipidemia, and CVD [180]. After the initial 12-week treatment, all patients received the triple therapy for another 12-week period. After 12 weeks, the triple therapy was better in terms of reducing TG levels (-14.6% compared with dual therapy, p = 0.007), but dual therapy was better at terms of LDL-C decrease (-5.3% compared with triple therapy, p = 0.05). At week 24 the fenofibrate/pravastatin plus ezetimibe triple therapy resulted in significant reductions in the levels of non-HDL-C, TG, LDL-C, and apoB and significant increases in HDL-C and apoA-I levels compared with the baseline (on simvastatin 20) lipid levels [180].
5.5 Fluvastatin plus fibrate combination treatment
A 12-month randomized double-blind trial assessed the combination of fluvastatin 80 mg with fenofibrate 200 mg in 48 patients with combined hyperlipidemia, T2DM and CVD [181]. After 12 months, a greater effect of the combination therapy on the levels of LDL-C, total cholesterol, and HDL-C was observed (-35%, -32%, +34%, respectively) compared with fluvastatin monotherapy (-25%, -17%, +14%, respectively, all p < 0.05 between groups) [181].
Another study compared the effect on LDL subfractions of fluvastatin/fenofibrate combination (80/200 mg) or simvastatin 20 mg/ezetimibe combination (20/10 mg) in 56 patients with metabolic syndrome and/or T2DM [182]. The simvastatin/ezetimibe combination produced greater reduction in total cholesterol and LDL-C levels in the whole population compared with fluvastatin/fenofibrate. No effect of therapy on LDL subfraction was shown in patients with no predominance of small dense LDL subfractions, but when patients with increased small dense LDL subfractions were assessed a greater improvement of TG levels and LDL radius of fluvastatin/fenofibrate compared with simvastatin/ezetimibe treatment was observed [182].
5.6 Pitavastatin plus fibrate combination treatment
Pitavastatin has not been studied in combination with fibrates in patients with T2DM.
6. Effects of statin-fibrate combination on microvascular complications in patients with T2DM
The effect of fenofibrate on the incidence of laser treatment for diabetic retinopathy was examined as an endpoint in the main analysis of FIELD [142]. Fenofibrate led to lower numbers of patients receiving laser treatment for retinopathy compared with placebo (178 versus 253, difference between groups 1.6%, p = 0.0003). A significant benefit of fenofibrate administration was observed in the rate of first or any laser treatment for maculopathy or for proliferative retinopathy without macular involvement. A FIELD sub-study showed that the progression of diabetic retinopathy was reduced significantly in patients with retinopathy at baseline [183]. A beneficial effect of fenofibrate treatment on non-traumatic amputations was also shown in another FIELD sub-study [184].
The effects of an intensive glycemia and lipid treatment and of a standard therapy on the progression of diabetic retinopathy were assessed in a subgroup of 2,856 participants of the ACCORD trial [185]. After 4 years, the rate of progression of diabetic retinopathy was 6.5% with the simvastatin/fenofibrate intensive dyslipidemia therapy, and 10.2% with simvastatin/placebo (adjusted odds ratio (OR) 0.60, 95% CI 0.42-0.87, p = 0.006), whereas it was 7.3% with intensive glycemia treatment and 10.4% with standard therapy (adjusted OR 0.67, 95% CI 0.51-0.87, p = 0.003) [185].
Fenofibrate administration is associated with an increase in creatinine serum levels [186, 187]. Possible explanations for this increase include a decrease in creatinine clearance, an impairment of vasodilatory prostaglandin generation or an increase of the metabolic production of creatinine [186-189]. On the other hand, the increase of serum creatinine with fenofibrate is transient and reversible even without treatment discontinuation [190]. Furthermore, evidence exists that fenofibrate exerts a protective action against pathological changes in diabetic nephropathy and hypertensive glomerulosclerosis [190].
In the FIELD study, fenofibrate reduced the rate of progression to albuminuria [142]. In the ACCORD Lipid study, serum creatinine levels were increased from 0.93 to 1.10 mg/dl in the fenofibrate group, whereas in the placebo group, they were increased from 0.93 to 1.04 mg/dl (p < 0.05) [154]. Furthermore, significantly more patients in the fenofibrate group experienced at least one increased creatinine measurement (>1.3 mg/dl if women, >1.5 mg/dl if men), whereas discontinuation because of a decrease in the estimated glomerular filtration rate (GFR) was observed in 2.4% of patients in the fenofibrate group and 1.1% in the placebo group (p < 0.05). However, these differences did not result in a significant difference in the incidence of end-stage renal disease and the need for dialysis between treatment groups (75 patients in the fenofibrate group versus 77 in the placebo group). Notably, a lower incidence of microalbuminuria (38.2% versus 41.6%, p = 0.01) and macroalbuminuria (10.5% versus 12.3%, p = 0.04) was observed in the fenofibrate group compared with the placebo group [154].
7. Effects of statin-fibrate combination therapy on CVD risk in patients with T2DM
There is limited clinical evidence examining the beneficial effects of statin plus fibrate combination on CVD events. In the ACCORD trial [154], patients with T2DM (n = 5518) who were already treated with open-label simvastatin were randomly allocated to receive fenofibrate or placebo. The primary outcome of the study was the first occurrence of a major CVD event such as nonfatal myocardial infarction, nonfatal stroke, or death from CVD causes. The mean duration of follow-up was 4.7 years for the primary outcome. By the end of the study, the fenofibrate/simvastatin combination resulted in greater improvements of total cholesterol, TG and HDL-C (p = 0.02, p < 0.001, p = 0.01, respectively) when compared with the placebo/simvastatin group. On the other hand, there was no significant difference in the reduction of LDL-C levels between treatment groups (p = 0.16). The annual rate of the primary outcome was 2.2% in the fenofibrate group and 2.4% in the placebo group (p = 0.32). In addition, there was no significant difference in the rates of secondary outcomes between groups (primary outcome plus revascularization or hospitalization for congestive heart failure, p = 0.30; major CHD event, p = 0.26; non-fatal myocardial infarction, p = 0.039; any stroke, p = 0.80; non-fatal stroke, p = 0.48; death from any cause, p = 0.33; death from CVD cause, p = 0.26; fatal or nonfatal congestive heart failure, p = 0.1). As a consequence, the combination of fenofibrate and simvastatin did not significantly reduce the rate of major CVD events compared with simvastatin alone. Similar results were seen comparing patients <65 years of age (n = 3,660) versus those ≥65 years (n = 1,858) [154].
These observations suggest that the addition of fenofibrate to simvastatin did not produce a significant decrease in the rate of CVD events. However, the ACCORD Lipid study had some limitations; the enrollment of patients did not achieve the predetermined power and simvastatin was given in open-label fashion.
It should also be mentioned that in a pre-specified analysis in the subgroup of ACCORD patients (regardless of age) with high baseline TG (≥204 mg/dl) and low baseline HDL-C (≤34 mg/d) values, there was a 28% (p < 0.05) reduction in the relative risk of CVD events in the fenofibrate plus simvastatin group. It should be mentioned that the effects of the addition of fenofibrate in the subgroup of patients with high TG and low HDL-C values are similar to previous subgroup analyses in other fibrate trials [136, 191-196]. For example, a significant reduction of CVD risk (-27% relative risk reduction, 95% CI 9-42, p = 0.005) was seen with fenofibrate in patients with marked dyslipidemia in a sub-analysis of the FIELD trial [197].
A recent study examined the effects of simvastatin plus fenofibrate or placebo in postprandial concentrations of plasma TG, apoB48, and apoC-III, over 10 hours, after an oral fat load in 139 subjects (mean age 61 years) of the ACCORD Lipid trial [198]. The fenofibrate plus simvastatin administration resulted in a significant reduction in TG incremental area under the curve (IAUC) compared with the placebo plus simvastatin group (572 (352-907) versus 770 (429-1,420) mg/dl/h (adjusted p = 0.008)), as well as in a significant reduction in plasma apoB48 IAUC (23.2 ± 16.3 versus 35.2 ± 28.6 μg/ml/h (adjusted p = 0.008)). However, plasma apoC-III IAUC was not different between fenofibrate and placebo groups, despite the fact that plasma apoC-III concentrations were reduced by 10-20% with fenofibrate compared with placebo during the 10-hour study. Notably, although the fenofibrate-induced reduction of postprandial TG IAUC was constant across the entire range of fasting TG levels, the postprandial apoB48 IAUC was only reduced in subjects with increased fasting TG levels [198]. The fact that the levels of atherogenic apoB48 were reduced only in individuals with increased fasting TG levels may provide an explanation for the results of the overall ACCORD Lipid trial, which suggested a benefit from fenofibrate plus simvastatin treatment only in individuals with mixed dyslipidemia.
Overall, based on current evidence, it seems that the administration of fibrates in patients with atherogenic dyslipidemia, either as monotherapy or combined with statins, is associated with a reduction of CVD risk. Nonetheless, this effect was not significant in patients without mixed dyslipidemia [199].
8. Safety
The combination of statin with fibrates is generally believed to increase the risk of myopathy and rhabdomyolysis [127, 200-202]. Renal and/or hepatic insufficiency, increased age, and administration of several medications are suggested risk factors for the development of these adverse events [203-205].
A meta-analysis that estimated the safety of statin with fenofibrate combination included 1,628 subjects who participated in a total of 6 studies [206]. The results showed that the rates of serious adverse events (2.0% versus 1.5%, p = 0.71) and adverse events related to study drug (10.9% versus 11.0%, p = 0.95), as well as the rate of discontinuation due to any adverse events (4.5% versus 3.1%, p = 0.20) or any adverse events (42% versus 41%, p = 0.82) were not significantly different between statin and statin/fenofibrate combination. Myopathy or rhabdomyolysis did not appear in any of the 1,628 subjects included in this meta-analysis. However, a significantly greater incidence of alanine aminotransferase and/or aspartate aminotransferase ≥3 times the upper limit of normal was observed in the combination treatment compared with statin monotherapy (3.1% versus 0.2%, p = 0.0009) [206]. It should be mentioned that the fenofibrate or fenofibric acid plus statin combination is safer compared with gemfibrozil plus statin combination [207, 208]. Hence, gemfibrozil should not be combined with a statin and preferential treatment with other fibrates should be given.
9. Conclusions
Statin therapy has been shown to be both effective and safe in the treatment of hyperlipidemia. However, in high CVD risk patients such as T2DM patients there is a considerable residual CVD risk despite statin therapy. Indeed, the National Cholesterol Education Program Adult Treatment Panel (NCEP ATP III) guidelines establish goals for both LDL-C and non-HDL-C in high-risk patients [209]. As a result, it is of great importance to identify these patients and take the necessary steps to minimize as much as possible this residual CVD risk. To achieve this, a higher titration of statin dosage could be given in order to achieve the desired goals. Indeed, a high-dose statin therapy has been associated with improved outcomes [210]. However, this increase of statin dosage is not always desirable or even safe. Indeed, statin side effects such as myopathy are dose related [211]. Furthermore, although they are very efficacious in terms of CVD event reduction, higher statin dosages are associated with a greater incidence of T2DM development [212, 213].
Beyond LDL-C levels, HDL-C and TG have an important role in CVD. Indeed, among subjects with LDL-C <70 mg/dl in the Treating to New Targets (TNT) study, those with low HDL-C levels had a greater incidence of CVD events compared with subjects with high HDL-C levels [214]. Similarly, among subjects with LDL-C <70 mg/dl in the Pravastatin or Atorvastatin Evaluation and Infection Therapy (PROVE-IT) study a concentration of TG >150 mg/dl was associated with a 27% higher CVD risk compared with TG levels <150 mg/dl [215]. In addition, the combination of elevated TG and decreased HDL-C levels was associated with increased CVD risk independently of LDL-C levels. Therefore, the combination of a statin with a fibrate may have an important role to play in the minimization of residual CVD risk during statin treatment.
Based on the current evidence, statin/fibrate combination therapy favorably alters the lipid profile of T2DM patients characterized by high TG and low HDL-C profile, which is associated with increased CVD risk. The micro- and macrovascular benefits associated with the statin/fibrate administration makes this combination an attractive treatment choice for the T2DM patient. However, to date, limited data exists on the effect of statin/fibrate combinations on “hard” clinical outcomes in T2DM patients with high TG and low HDL-C values. Based on current evidence, statins continue to be the cornerstone of dyslipidemia management. A fibrate could be added in patients with mixed dyslipidemia that do not reach the desired non-HDL-C or TG and/or HDL-C concentrations. Certainly, larger prospective studies are needed, specifically those designed to assess the effect of statin/fibrate combination on CVD morbidity and mortality of T2DM subjects with mixed dyslipidemia.
Disclosures: There were no declared conflicts of interest. This manuscript has been prepared independently. Some of the authors have attended conferences sponsored by various pharmaceutical companies.
References
- 1.American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31:596–615. doi: 10.2337/dc08-9017. [DOI] [PubMed] [Google Scholar]
- 2.Ginter E, Simko V. Type 2 diabetes mellitus, pandemic in 21st century. Adv Exp Med Biol. 2012;771:42–50. doi: 10.1007/978-1-4614-5441-0_6. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Coresh J, Golden SH, Brancati FL, Folsom AR, Steffes MW. Glycemic control and coronary heart disease risk in persons with and without diabetes: the atherosclerosis risk in communities study. Arch Intern Med. 2005;165:1910–1916. doi: 10.1001/archinte.165.16.1910. [DOI] [PubMed] [Google Scholar]
- 4.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362:800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerstein HC, Swedberg K, Carlsson J, McMurray JJ, Michelson EL, Olofsson B, Pfeffer MA, Yusuf S. The hemoglobin A1c level as a progressive risk factor for cardiovascular death, hospitalization for heart failure, or death in patients with chronic heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Arch Intern Med. 2008;168:1699–1704. doi: 10.1001/archinte.168.15.1699. [DOI] [PubMed] [Google Scholar]
- 6.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332:73–78. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filippatos TD, Rizos EC, Gazi IF, Lagos K, Agouridis A, Mikhailidis DP, Elisaf MS. Differences in metabolic parameters and cardiovascular risk between American Diabetes Association and World Health Organization definition of impaired fasting glucose in European Caucasian subjects: a cross-sectional study. Arc Med Sci. 2013 doi: 10.5114/aoms.2013.38671. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Filippatos TD, Rizos EC, Tsimihodimos V, Gazi IF, Tselepis AD, Elisaf MS. Small high-density lipoprotein (HDL) subclasses are increased with decreased activity of HDL-associated phospholipase A2 in subjects with prediabetes. Lipids. 2013;48(6):547–555. doi: 10.1007/s11745-013-3787-1. [DOI] [PubMed] [Google Scholar]
- 9.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 10.Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Jones PH. Statins as the cornerstone of drug therapy for dyslipidemia: monotherapy and combination therapy options. Am Heart J. 2004;148(Suppl 1):S9–S13. doi: 10.1016/j.ahj.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 12.Hermans MP, Castro Cabezas M, Strandberg T, Ferrieres J, Feely J, Elisaf M, Michel G, Sansoy V. Centralized Pan-European survey on the under-treatment of hypercholesterolaemia (CEPHEUS): overall findings from eight countries. Curr Med Res Opin. 2010;26:445–454. doi: 10.1185/03007990903500565. [DOI] [PubMed] [Google Scholar]
- 13.Elisaf MS, Nikas N. Centralized Pan-European survey on the undertreatment of hypercholesterolemia in patients using lipid lowering drugs - the CEPHEUS-Greece survey. Angiology. 2010;61:465–474. doi: 10.1177/0003319710366432. [DOI] [PubMed] [Google Scholar]
- 14.da Silva PM, Cardoso SM. Persistent lipid abnormalities in patients treated with statins: Portuguese results of the Dyslipidemia International Study (DYSIS) Rev Port Cardiol. 2011;30:47–63. [PubMed] [Google Scholar]
- 15.Leiter LA, Lundman P, da Silva PM, Drexel H, Junger C, Gitt AK. Persistent lipid abnormalities in statin-treated patients with diabetes mellitus in Europe and Canada: results of the Dyslipidaemia International Study. Diabet Med. 2011;28:1343–1351. doi: 10.1111/j.1464-5491.2011.03360.x. [DOI] [PubMed] [Google Scholar]
- 16.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R. et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 17.Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 18.Ling H, Burns TL, Hilleman DE. Novel strategies for managing dyslipidemia: treatment beyond statins. Postgrad Med. 2012;124:43–54. doi: 10.3810/pgm.2012.11.2612. [DOI] [PubMed] [Google Scholar]
- 19.Filippatos TD, Elisaf MS. Combination drug treatment in obese diabetic patients. World J Diabetes. 2010;1:8–11. doi: 10.4239/wjd.v1.i1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alsheikh-Ali AA, Lin JL, Abourjaily P, Ahearn D, Kuvin JT, Karas RH. Prevalence of low high-density lipoprotein cholesterol in patients with documented coronary heart disease or risk equivalent and controlled low-density lipoprotein cholesterol. Am J Cardiol. 2007;100:1499–1501. doi: 10.1016/j.amjcard.2007.06.058. [DOI] [PubMed] [Google Scholar]
- 21.Foger B. Lipid lowering therapy in type 2 diabetes. Wien Med Wochenschr. 2011;161:289–296. doi: 10.1007/s10354-011-0908-4. [DOI] [PubMed] [Google Scholar]
- 22.Sarwar N, Danesh J, Eiriksdottir G, Sigurdsson G, Wareham N, Bingham S, Boekholdt SM, Khaw KT, Gudnason V. Triglycerides and the risk of coronary heart disease: 10,158 incident cases among 262,525 participants in 29 Western prospective studies. Circulation. 2007;115:450–458. doi: 10.1161/CIRCULATIONAHA.106.637793. [DOI] [PubMed] [Google Scholar]
- 23.Gazi IF, Milionis HJ, Filippatos TD, Tsimihodimos V, Kostapanos MS, Doumas M, Tselepis AD, Elisaf M. Hypertriglyceridaemic waist phenotype criteria and prevalent metabolic triad in women. Diabetes Metab Res Rev. 2008;24:223–230. doi: 10.1002/dmrr.784. [DOI] [PubMed] [Google Scholar]
- 24.Francis GA. The complexity of HDL. Biochim Biophys Acta. 2010;1801:1286–1293. doi: 10.1016/j.bbalip.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 25.Nobecourt E, Jacqueminet S, Hansel B, Chantepie S, Grimaldi A, Chapman MJ, Kontush A. Defective antioxidative activity of small dense HDL3 particles in type 2 diabetes: relationship to elevated oxidative stress and hyperglycaemia. Diabetologia. 2005;48:529–538. doi: 10.1007/s00125-004-1655-5. [DOI] [PubMed] [Google Scholar]
- 26.Morgantini C, Natali A, Boldrini B, Imaizumi S, Navab M, Fogelman AM, Ferrannini E, Reddy ST. Anti-inflammatory and antioxidant properties of HDLs are impaired in type 2 diabetes. Diabetes. 2011;60:2617–2623. doi: 10.2337/db11-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lagos KG, Filippatos TD, Tsimihodimos V, Gazi IF, Rizos C, Tselepis AD, Mikhailidis DP, Elisaf MS. Alterations in the high density lipoprotein phenotype and HDL-associated enzymes in subjects with metabolic syndrome. Lipids. 2009;44:9–16. doi: 10.1007/s11745-008-3251-9. [DOI] [PubMed] [Google Scholar]
- 28.Tsimihodimos V, Gazi I, Filippatos T, Kostapanos M, Lagos K, Kostara C, Tellis CC, Elisaf M, Tselepis AD. Plasma triglyceride levels and body mass index values are the most important determinants of prebeta-1 HDL concentrations in patients with various types of primary dyslipidemia. Atherosclerosis. 2010;208:506–511. doi: 10.1016/j.atherosclerosis.2009.07.038. [DOI] [PubMed] [Google Scholar]
- 29.Ragbir S, Farmer JA. Dysfunctional high-density lipoprotein and atherosclerosis. Curr Atheroscler Rep. 2010;12:343–348. doi: 10.1007/s11883-010-0091-x. [DOI] [PubMed] [Google Scholar]
- 30.Otocka-Kmiecik A, Mikhailidis DP, Nicholls SJ, Davidson M, Rysz J, Banach M. Dysfunctional HDL: A novel important diagnostic and therapeutic target in cardiovascular disease? Prog Lipid Res. 2012;51:314–324. doi: 10.1016/j.plipres.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Packard C, Caslake M, Shepherd J. The role of small, dense low density lipoprotein (LDL): a new look. Int J Cardiol. 2000;74(Suppl 1):S17–S22. doi: 10.1016/s0167-5273(99)00107-2. [DOI] [PubMed] [Google Scholar]
- 32.Gazi IF, Tsimihodimos V, Tselepis AD, Elisaf M, Mikhailidis DP. Clinical importance and therapeutic modulation of small dense low-density lipoprotein particles. Expert Opin Biol Ther. 2007;7:53–72. doi: 10.1517/14712598.7.1.53. [DOI] [PubMed] [Google Scholar]
- 33.Gazi IF, Filippatos TD, Tsimihodimos V, Saougos VG, Liberopoulos EN, Mikhailidis DP, Tselepis AD, Elisaf M. The hypertriglyceridemic waist phenotype is a predictor of elevated levels of small, dense LDL cholesterol. Lipids. 2006;41:647–654. doi: 10.1007/s11745-006-5015-8. [DOI] [PubMed] [Google Scholar]
- 34.Gazi I, Tsimihodimos V, Filippatos T, Bairaktari E, Tselepis AD, Elisaf M. Concentration and relative distribution of low-density lipoprotein subfractions in patients with metabolic syndrome defined according to the National Cholesterol Education Program criteria. Metabolism. 2006;55:885–891. doi: 10.1016/j.metabol.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 35.Tellis CC, Tselepis AD. The role of lipoprotein-associated phospholipase A2 in atherosclerosis may depend on its lipoprotein carrier in plasma. Biochim Biophys Acta. 2009;1791:327–338. doi: 10.1016/j.bbalip.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Gazi I, Lourida ES, Filippatos T, Tsimihodimos V, Elisaf M, Tselepis AD. Lipoprotein-associated phospholipase A2 activity is a marker of small, dense LDL particles in human plasma. Clin Chem. 2005;51:2264–2273. doi: 10.1373/clinchem.2005.058404. [DOI] [PubMed] [Google Scholar]
- 37.Hingorani AD, Shah T, Casas JP, Humphries SE, Talmud PJ. C-reactive protein and coronary heart disease: predictive test or therapeutic target? Clin Chem. 2009;55:239–255. doi: 10.1373/clinchem.2008.115923. [DOI] [PubMed] [Google Scholar]
- 38.Kei AA, Filippatos TD, Tsimihodimos V, Elisaf MS. A review of the role of apolipoprotein C-II in lipoprotein metabolism and cardiovascular disease. Metabolism. 2012;61:906–921. doi: 10.1016/j.metabol.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Filippatos TD, Tsimihodimos V, Kostapanos M, Kostara C, Bairaktari E, Kiortsis DN, Tselepis AD, Elisaf M. Small dense LDL cholesterol and apolipoproteins C-II and C-III in non-diabetic obese subjects with metabolic syndrome. Arch Med Sci. 2008;4:263–269. [Google Scholar]
- 40.Seifalian AM, Filippatos TD, Joshi J, Mikhailidis DP. Obesity and arterial compliance alterations. Curr Vasc Pharmacol. 2010;8:155–168. doi: 10.2174/157016110790886956. [DOI] [PubMed] [Google Scholar]
- 41.Milionis HJ, Filippatos TD, Loukas T, Bairaktari ET, Tselepis AD, Elisaf MS. Serum lipoprotein(a) levels and apolipoprotein(a) isoform size and risk for first-ever acute ischaemic nonembolic stroke in elderly individuals. Atherosclerosis. 2006;187:170–176. doi: 10.1016/j.atherosclerosis.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 42.Milionis HJ, Filippatos TD, Derdemezis CS, Kalantzi KJ, Goudevenos J, Seferiadis K, Mikhailidis DP, Elisaf MS. Excess body weight and risk of first-ever acute ischaemic non-embolic stroke in elderly subjects. Eur J Neurol. 2007;14:762–769. doi: 10.1111/j.1468-1331.2007.01849.x. [DOI] [PubMed] [Google Scholar]
- 43.Filippatos TD, Randeva HS, Derdemezis CS, Elisaf MS, Mikhailidis DP. Visfatin/PBEF and atherosclerosis-related diseases. Curr Vasc Pharmacol. 2010;8:12–28. doi: 10.2174/157016110790226679. [DOI] [PubMed] [Google Scholar]
- 44.Filippatos TD, Tsimihodimos V, Derdemezis CS, Gazi IF, Saougos V, Mikhailidis DP, Tselepis AD, Elisaf MS. Increased plasma visfatin concentration is a marker of an atherogenic metabolic profile. Nutr Metab Cardiovasc Dis. 2013;23:330–336. doi: 10.1016/j.numecd.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Filippatos TD, Derdemezis CS, Gazi IF, Lagos K, Kiortsis DN, Tselepis AD, Elisaf MS. Increased plasma visfatin levels in subjects with the metabolic syndrome. Eur J Clin Invest. 2008;38:71–72. doi: 10.1111/j.1365-2362.2007.01904.x. [DOI] [PubMed] [Google Scholar]
- 46.Filippatos TD, Derdemezis CS, Kiortsis DN, Tselepis AD, Elisaf MS. Increased plasma levels of visfatin/pre-B cell colony-enhancing factor in obese and overweight patients with metabolic syndrome. J Endocrinol Invest. 2007;30:323–326. doi: 10.1007/BF03346300. [DOI] [PubMed] [Google Scholar]
- 47.Spence JD, Huff M, Barnett PA. Effects of indapamide versus hydrochlorothiazide on plasma lipids and lipoproteins in hypertensive patients: a direct comparison. Can J Clin Pharmacol. 2000;7:32–37. [PubMed] [Google Scholar]
- 48.Reneland R, Alvarez E, Andersson PE, Haenni A, Byberg L, Lithell H. Induction of insulin resistance by beta-blockade but not ACE-inhibition: long-term treatment with atenolol or trandolapril. J Hum Hypertens. 2000;14:175–180. doi: 10.1038/sj.jhh.1000964. [DOI] [PubMed] [Google Scholar]
- 49.Lindholm LH, Persson M, Alaupovic P, Carlberg B, Svensson A, Samuelsson O. Metabolic outcome during 1 year in newly detected hypertensives: results of the Antihypertensive Treatment and Lipid Profile in a North of Sweden Efficacy Evaluation (ALPINE study) J Hypertens. 2003;21:1563–1574. doi: 10.1097/01.hjh.0000084723.53355.76. [DOI] [PubMed] [Google Scholar]
- 50.Boquist S, Ruotolo G, Hellenius ML, Danell-Toverud K, Karpe F, Hamsten A. Effects of a cardioselective beta-blocker on postprandial triglyceride-rich lipoproteins, low density lipoprotein particle size and glucose-insulin homeostasis in middle-aged men with modestly increased cardiovascular risk. Atherosclerosis. 1998;137:391–400. doi: 10.1016/s0021-9150(97)00278-5. [DOI] [PubMed] [Google Scholar]
- 51.Zane LT, Leyden WA, Marqueling AL, Manos MM. A population-based analysis of laboratory abnormalities during isotretinoin therapy for acne vulgaris. Arch Dermatol. 2006;142:1016–1022. doi: 10.1001/archderm.142.8.1016. [DOI] [PubMed] [Google Scholar]
- 52.Flynn WJ, Freeman PG, Wickboldt LG. Pancreatitis associated with isotretinoin-induced hypertriglyceridemia. Ann Intern Med. 1987;107:63. doi: 10.7326/0003-4819-107-1-63. [DOI] [PubMed] [Google Scholar]
- 53.Gupta S, Tandon VR, Kapoor B, Gupta A, Gupta GD, Khajuria V. Effects of tamoxifen therapy on plasma lipid profile in patients of breast cancer. J Assoc Physicians India. 2006;54:183–186. [PubMed] [Google Scholar]
- 54.Meyer JM, Davis VG, Goff DC, McEvoy JP, Nasrallah HA, Davis SM, Rosenheck RA, Daumit GL, Hsiao J, Swartz MS. et al. Change in metabolic syndrome parameters with antipsychotic treatment in the CATIE Schizophrenia Trial: prospective data from phase 1. Schizophr Res. 2008;101:273–286. doi: 10.1016/j.schres.2007.12.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kohen I, Manu P. Rapidly worsening hypertriglyceridemia during treatment with risperidone. Am J Ther. 2010;17:216–218. doi: 10.1097/MJT.0b013e318197eadf. [DOI] [PubMed] [Google Scholar]
- 56.Nagamine T. Olanzapine-induced elevation of serum triglyceride levels in a normal weight patient with schizophrenia. Intern Med. 2008;47:181–182. doi: 10.2169/internalmedicine.47.0557. [DOI] [PubMed] [Google Scholar]
- 57.Pollono EN, Lopez-Olivo MA, Lopez JA, Suarez-Almazor ME. A systematic review of the effect of TNF-alpha antagonists on lipid profiles in patients with rheumatoid arthritis. Clin Rheumatol. 2010;29:947–955. doi: 10.1007/s10067-010-1405-7. [DOI] [PubMed] [Google Scholar]
- 58.Sandhu S, Al-Sarraf A, Taraboanta C, Frohlich J, Francis GA. Incidence of pancreatitis, secondary causes, and treatment of patients referred to a specialty lipid clinic with severe hypertriglyceridemia: a retrospective cohort study. Lipids Health Dis. 2011;10:157. doi: 10.1186/1476-511X-10-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kiortsis DN, Mavridis AK, Filippatos TD, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on lipoprotein profile in patients with rheumatoid arthritis and ankylosing spondylitis. J Rheumatol. 2006;33:921–923. [PubMed] [Google Scholar]
- 60.Oh RC, Lanier JB. Management of hypertriglyceridemia. Am Fam Physician. 2007;75:1365–1371. [PubMed] [Google Scholar]
- 61.Pejic RN, Lee DT. Hypertriglyceridemia. J Am Board Fam Med. 2006;19:310–316. doi: 10.3122/jabfm.19.3.310. [DOI] [PubMed] [Google Scholar]
- 62.Kolovou GD, Anagnostopoulou KK, Kostakou PM, Bilianou H, Mikhailidis DP. Primary and secondary hypertriglyceridaemia. Curr Drug Targets. 2009;10:336–343. doi: 10.2174/138945009787846452. [DOI] [PubMed] [Google Scholar]
- 63.Seneff S, Wainwright G, Mascitelli L. Is the metabolic syndrome caused by a high fructose, and relatively low fat, low cholesterol diet? Arch Med Sci. 2011;7:8–20. doi: 10.5114/aoms.2011.20598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pavlidis AN, Kolovou GD, Anagnostopoulou KK, Petrou PC, Cokkinos DV. Postprandial metabolic heterogeneity in men with primary dyslipidaemia. Arch Med Sci. 2010;6:879–886. doi: 10.5114/aoms.2010.19295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gazi IF, Apostolou FA, Liberopoulos EN, Filippatos TD, Tellis CC, Elisaf MS, Tselepis AD. Leptospirosis is associated with markedly increased triglycerides and small dense low-density lipoprotein and decreased high-density lipoprotein. Lipids. 2011;46:953–960. doi: 10.1007/s11745-011-3580-y. [DOI] [PubMed] [Google Scholar]
- 66.Derdemezis CS, Filippatos TD, Voulgari PV, Tselepis AD, Drosos AA, Kiortsis DN. Effects of a 6-month infliximab treatment on plasma levels of leptin and adiponectin in patients with rheumatoid arthritis. Fundam Clin Pharmacol. 2009;23:595–600. doi: 10.1111/j.1472-8206.2009.00717.x. [DOI] [PubMed] [Google Scholar]
- 67.Derdemezis CS, Filippatos TD, Voulgari PV, Tselepis AD, Drosos AA, Kiortsis DN. Leptin and adiponectin levels in patients with ankylosing spondylitis. The effect of infliximab treatment. Clin Exp Rheumatol. 2010;28:880–883. [PubMed] [Google Scholar]
- 68.Filippatos TD, Liberopoulos EN, Pavlidis N, Elisaf MS, Mikhailidis DP. Effects of hormonal treatment on lipids in patients with cancer. Cancer Treat Rev. 2009;35:175–184. doi: 10.1016/j.ctrv.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 69.Filippatos TD, Derdemezis CS, Gazi IF, Nakou ES, Mikhailidis DP, Elisaf MS. Orlistat-associated adverse effects and drug interactions: a critical review. Drug Saf. 2008;31:53–65. doi: 10.2165/00002018-200831010-00005. [DOI] [PubMed] [Google Scholar]
- 70.Filippatos TD, Derdemezis CS, Voulgari PV, Tsimihodimos V, Elisaf MS, Tselepis AD, Drosos AA. Effects of 12 months of treatment with disease-modifying anti-rheumatic drugs on low and high density lipoprotein subclass distribution in patients with early rheumatoid arthritis: a pilot study. Scand J Rheumatol. 2013;42(3):169–175. doi: 10.3109/03009742.2012.745013. [DOI] [PubMed] [Google Scholar]
- 71.Nakou ES, Filippatos TD, Agouridis AP, Kostara C, Bairaktari ET, Elisaf MS. The effects of ezetimibe and/or orlistat on triglyceride-rich lipoprotein metabolism in obese hypercholesterolemic patients. Lipids. 2010;45:445–450. doi: 10.1007/s11745-010-3409-0. [DOI] [PubMed] [Google Scholar]
- 72.Agouridis AP, Filippatos TD, Tsimihodimos V, Elisaf MS. Combinations of ezetimibe with nonstatin drug regimens affecting lipid metabolism. Expert Rev Cardiovasc Ther. 2011;9:355–366. doi: 10.1586/erc.11.4. [DOI] [PubMed] [Google Scholar]
- 73.Rizzo M, Rini GB, Berneis K. Effects of statins, fibrates, rosuvastatin, and ezetimibe beyond cholesterol: the modulation of LDL size and subclasses in high-risk patients. Adv Ther. 2007;24:575–582. doi: 10.1007/BF02848780. [DOI] [PubMed] [Google Scholar]
- 74.Ai M, Otokozawa S, Asztalos BF, Nakajima K, Stein E, Jones PH, Schaefer EJ. Effects of maximal doses of atorvastatin versus rosuvastatin on small dense low-density lipoprotein cholesterol levels. Am J Cardiol. 2008;101:315–318. doi: 10.1016/j.amjcard.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 75.Kalogirou M, Tsimihodimos V, Gazi I, Filippatos T, Saougos V, Tselepis AD, Mikhailidis DP, Elisaf M. Effect of ezetimibe monotherapy on the concentration of lipoprotein subfractions in patients with primary dyslipidaemia. Curr Med Res Opin. 2007;23:1169–1176. doi: 10.1185/030079907x188062. [DOI] [PubMed] [Google Scholar]
- 76.Kostapanos MS, Milionis HJ, Filippatos TD, Nakou ES, Bairaktari ET, Tselepis AD, Elisaf MS. A 12-week, prospective, open-label analysis of the effect of rosuvastatin on triglyceride-rich lipoprotein metabolism in patients with primary dyslipidemia. Clin Ther. 2007;29:1403–1414. doi: 10.1016/j.clinthera.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 77.Florentin M, Tselepis AD, Elisaf MS, Rizos CV, Mikhailidis DP, Liberopoulos EN. Effect of non-statin lipid lowering and anti-obesity drugs on LDL subfractions in patients with mixed dyslipidaemia. Curr Vasc Pharmacol. 2010;8:820–830. doi: 10.2174/157016110793563825. [DOI] [PubMed] [Google Scholar]
- 78.Filippatos TD, Mikhailidis DP. Lipid-lowering drugs acting at the level of the gastrointestinal tract. Curr Pharm Des. 2009;15:490–516. doi: 10.2174/138161209787315738. [DOI] [PubMed] [Google Scholar]
- 79.Filippatos T, Derdemezis C, Elisaf M. Effects of orlistat, alone or combined with hypolipidemic drugs, on cardiovascular risk factors. Clinical Lipidology. 2009;4:331–341. [Google Scholar]
- 80.Tzotzas T, Filippatos TD, Triantos A, Bruckert E, Tselepis AD, Kiortsis DN. Effects of a low-calorie diet associated with weight loss on lipoprotein-associated phospholipase A2 (Lp-PLA2) activity in healthy obese women. Nutr Metab Cardiovasc Dis. 2008;18:477–482. doi: 10.1016/j.numecd.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 81.Nakou E, Filippatos TD, Liberopoulos EN, Tselepis AD, Kiortsis DN, Mikhailidis DP, Elisaf MS. Effects of sibutramine plus verapamil sustained release/trandolapril combination on blood pressure and metabolic variables in obese hypertensive patients. Expert Opin Pharmacother. 2008;9:1629–1639. doi: 10.1517/14656566.9.10.1629. [DOI] [PubMed] [Google Scholar]
- 82.Nakou ES, Filippatos TD, Georgoula M, Kiortsis DN, Tselepis AD, Mikhailidis DP, Elisaf MS. The effect of orlistat and ezetimibe, alone or in combination, on serum LDL and small dense LDL cholesterol levels in overweight and obese patients with hypercholesterolaemia. Curr Med Res Opin. 2008;24:1919–1929. doi: 10.1185/03007990802177150. [DOI] [PubMed] [Google Scholar]
- 83.Nakou ES, Filippatos TD, Kiortsis DN, Derdemezis CS, Tselepis AD, Mikhailidis DP, Elisaf MS. The effects of ezetimibe and orlistat, alone or in combination, on high-density lipoprotein (HDL) subclasses and HDL-associated enzyme activities in overweight and obese patients with hyperlipidaemia. Expert Opin Pharmacother. 2008;9:3151–3158. doi: 10.1517/14656560802548430. [DOI] [PubMed] [Google Scholar]
- 84.Agouridis AP, Tsimihodimos V, Filippatos TD, Tselepis AD, Elisaf MS. High doses of rosuvastatin are superior to low doses of rosuvastatin plus fenofibrate or n-3 fatty acids in mixed dyslipidemia. Lipids. 2011;46:521–528. doi: 10.1007/s11745-011-3538-0. [DOI] [PubMed] [Google Scholar]
- 85.Agouridis AP, Tsimihodimos V, Filippatos TD, Dimitriou AA, Tellis CC, Elisaf MS, Mikhailidis DP, Tselepis AD. The effects of rosuvastatin alone or in combination with fenofibrate or omega 3 fatty acids on inflammation and oxidative stress in patients with mixed dyslipidemia. Expert Opin Pharmacother. 2011;12:2605–2611. doi: 10.1517/14656566.2011.591383. [DOI] [PubMed] [Google Scholar]
- 86.Filippatos TD, Elisaf MS. Combination drug treatment in patients with non-alcoholic fatty liver disease. World J Hepatol. 2010;2:139–142. doi: 10.4254/wjh.v2.i4.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Filippatos TD, Elisaf MS. Role of ezetimibe in non-alcoholic fatty liver disease. World J Hepatol. 2011;3:265–267. doi: 10.4254/wjh.v3.i10.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Derdemezis C, Filippatos T, Tselepis A, Mikhailidis D, Elisaf M. Effects of ezetimibe, either alone or in combination with atorvastatin, on serum visfatin levels: a pilot study. Expert Opin Pharmacother. 2008;9:1829–1837. doi: 10.1517/14656566.9.11.1829. [DOI] [PubMed] [Google Scholar]
- 89.Derdemezis CS, Filippatos TD, Mikhailidis DP, Elisaf MS. Review article: effects of plant sterols and stanols beyond low-density lipoprotein cholesterol lowering. J Cardiovasc Pharmacol Ther. 2010;15:120–134. doi: 10.1177/1074248409357921. [DOI] [PubMed] [Google Scholar]
- 90.Kiortsis DN, Filippatos TD, Elisaf MS. The effects of orlistat on metabolic parameters and other cardiovascular risk factors. Diabetes Metab. 2005;31:15–22. doi: 10.1016/s1262-3636(07)70161-1. [DOI] [PubMed] [Google Scholar]
- 91.Milionis HJ, Rizos E, Kostapanos M, Filippatos TD, Gazi IF, Ganotakis ES, Goudevenos J, Mikhailidis DP, Elisaf MS. Treating to target patients with primary hyperlipidaemia: comparison of the effects of ATOrvastatin and ROSuvastatin (the ATOROS study) Curr Med Res Opin. 2006;22:1123–1131. doi: 10.1185/030079906X112462. [DOI] [PubMed] [Google Scholar]
- 92.Filippatos TD, Gazi IF, Liberopoulos EN, Athyros VG, Elisaf MS, Tselepis AD, Kiortsis DN. The effect of orlistat and fenofibrate, alone or in combination, on small dense LDL and lipoprotein-associated phospholipase A2 in obese patients with metabolic syndrome. Atherosclerosis. 2007;193:428–437. doi: 10.1016/j.atherosclerosis.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 93.Kostapanos MS, Derdemezis CS, Filippatos TD, Milionis HJ, Kiortsis DN, Tselepis AD, Elisaf MS. Effect of rosuvastatin treatment on plasma visfatin levels in patients with primary hyperlipidemia. Eur J Pharmacol. 2008;578:249–252. doi: 10.1016/j.ejphar.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 94.Kostapanos MS, Milionis HJ, Filippatos TD, Christogiannis LG, Bairaktari ET, Tselepis AD, Elisaf MS. Dose-dependent effect of rosuvastatin treatment on HDL-subfraction phenotype in patients with primary hyperlipidemia. J Cardiovasc Pharmacol Ther. 2009;14:5–13. doi: 10.1177/1074248408331031. [DOI] [PubMed] [Google Scholar]
- 95.Florentin M, Liberopoulos EN, Filippatos TD, Kostara C, Tselepis A, Mikhailidis DP, Elisaf M. Effect of rimonabant, micronised fenofibrate and their combination on cardiometabolic risk factors in overweight/obese patients: a pilot study. Expert Opin Pharmacother. 2008;9:2741–2750. doi: 10.1517/14656566.9.16.2741. [DOI] [PubMed] [Google Scholar]
- 96.Milionis HJ, Gazi IF, Filippatos TD, Tzovaras V, Chasiotis G, Goudevenos J, Seferiadis K, Elisaf MS. Starting with rosuvastatin in primary hyperlipidemia - is there more than lipid lowering? Angiology. 2005;56:585–592. doi: 10.1177/000331970505600510. [DOI] [PubMed] [Google Scholar]
- 97.Filippatos TD, Kiortsis DN, Liberopoulos EN, Mikhailidis DP, Elisaf MS. A review of the metabolic effects of sibutramine. Curr Med Res Opin. 2005;21:457–468. doi: 10.1185/030079905X38132. [DOI] [PubMed] [Google Scholar]
- 98.Kiortsis DN, Tsouli S, Filippatos TD, Konitsiotis S, Elisaf MS. Effects of sibutramine and orlistat on mood in obese and overweight subjects: a randomised study. Nutr Metab Cardiovasc Dis. 2008;18:207–210. doi: 10.1016/j.numecd.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 99.Jialal I, Amess W, Kaur M. Management of hypertriglyceridemia in the diabetic patient. Curr Diab Rep. 2010;10:316–320. doi: 10.1007/s11892-010-0124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Filippatos T, Elisaf M. Recommendations for severe hypertriglyceridemia treatment, are there new strategies? Curr Vasc Pharmacol. 2013 doi: 10.2174/15701611113119990133. In press. [DOI] [PubMed] [Google Scholar]
- 101.Filippatos T, Milionis HJ. Treatment of hyperlipidaemia with fenofibrate and related fibrates. Expert Opin Investig Drugs. 2008;17:1599–1614. doi: 10.1517/13543784.17.10.1599. [DOI] [PubMed] [Google Scholar]
- 102.Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565–571. doi: 10.1016/S0140-6736(12)61190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC. et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 104.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 105.Athyros VG, Papageorgiou AA, Mercouris BR, Athyrou VV, Symeonidis AN, Basayannis EO, Demitriadis DS, Kontopoulos AG. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus 'usual' care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Curr Med Res Opin. 2002;18:220–228. doi: 10.1185/030079902125000787. [DOI] [PubMed] [Google Scholar]
- 106.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711–1718. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 107.Donner-Banzhoff N, Sonnichsen A. Statins and primary prevention of cardiovascular events. BMJ. 2008;337:a2576. doi: 10.1136/bmj.a2576. [DOI] [PubMed] [Google Scholar]
- 108.Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, Perri D. Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol. 2008;52:1769–1781. doi: 10.1016/j.jacc.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 109.Amarenco P, Labreuche J. Lipid management in the prevention of stroke: review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. 2009;8:453–463. doi: 10.1016/S1474-4422(09)70058-4. [DOI] [PubMed] [Google Scholar]
- 110.Delahoy PJ, Magliano DJ, Webb K, Grobler M, Liew D. The relationship between reduction in low-density lipoprotein cholesterol by statins and reduction in risk of cardiovascular outcomes: an updated meta-analysis. Clin Ther. 2009;31:236–244. doi: 10.1016/j.clinthera.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 111.Mills EJ, Wu P, Chong G, Ghement I, Singh S, Akl EA, Eyawo O, Guyatt G, Berwanger O, Briel M. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170,255 patients from 76 randomized trials. QJM. 2011;104:109–124. doi: 10.1093/qjmed/hcq165. [DOI] [PubMed] [Google Scholar]
- 112.Ross SD, Allen IE, Connelly JE, Korenblat BM, Smith ME, Bishop D, Luo D. Clinical outcomes in statin treatment trials: a meta-analysis. Arch Intern Med. 1999;159:1793–1802. doi: 10.1001/archinte.159.15.1793. [DOI] [PubMed] [Google Scholar]
- 113.Zhou Z, Rahme E, Pilote L. Are statins created equal? Evidence from randomized trials of pravastatin, simvastatin, and atorvastatin for cardiovascular disease prevention. Am Heart J. 2006;151:273–281. doi: 10.1016/j.ahj.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 114.Athyros VG, Kakafika AI, Tziomalos K, Karagiannis A, Mikhailidis DP. Pleiotropic effects of statins - clinical evidence. Curr Pharm Des. 2009;15:479–489. doi: 10.2174/138161209787315729. [DOI] [PubMed] [Google Scholar]
- 115.Filippatos TD, Mikhailidis DP. Statins and heart failure. Angiology. 2008;59:58S–61S. doi: 10.1177/0003319708319643. [DOI] [PubMed] [Google Scholar]
- 116.Wang ZH, Liu XL, Zhong M, Zhang LP, Shang YY, Hu XY, Li L, Zhang Y, Deng JT, Zhang W. Pleiotropic effects of atorvastatin on monocytes in atherosclerotic patients. J Clin Pharmacol. 2010;50:311–319. doi: 10.1177/0091270009340889. [DOI] [PubMed] [Google Scholar]
- 117.Murrow JR, Sher S, Ali S, Uphoff I, Patel R, Porkert M, Le NA, Jones D, Quyyumi AA. The differential effect of statins on oxidative stress and endothelial function: atorvastatin versus pravastatin. J Clin Lipidol. 2012;6:42–49. doi: 10.1016/j.jacl.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 118.Krysiak R, Gdula-Dymek A, Bachowski R, Okopien B. Pleiotropic effects of atorvastatin and fenofibrate in metabolic syndrome and different types of pre-diabetes. Diabetes Care. 2010;33:2266–2270. doi: 10.2337/dc10-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 120.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 121.Knopp RH, d'Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN) Diabetes Care. 2006;29:1478–1485. doi: 10.2337/dc05-2415. [DOI] [PubMed] [Google Scholar]
- 122.Costa J, Borges M, David C, Vaz Carneiro A. Efficacy of lipid lowering drug treatment for diabetic and non-diabetic patients: meta-analysis of randomised controlled trials. BMJ. 2006;332:1115–1124. doi: 10.1136/bmj.38793.468449.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Leung A, Schaefer EW, Tempelhof MW, Stone NJ. Emphasizing statin safety in the hospitalized patient: a review. Am J Med. 2012;125:845–853. doi: 10.1016/j.amjmed.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 124.Mills EJ, Wu P, Chong G, Ghement I, Singh S, Akl EA, Eyawo O, Guyatt G, Berwanger O, Briel M. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170,255 patients from 76 randomized trials. QJM. 2011;104:109–124. doi: 10.1093/qjmed/hcq165. [DOI] [PubMed] [Google Scholar]
- 125.Stein EA, Vidt DG, Shepherd J, Cain VA, Anzalone D, Cressman MD. Renal safety of intensive cholesterol-lowering treatment with rosuvastatin: a retrospective analysis of renal adverse events among 40,600 participants in the rosuvastatin clinical development program. Atherosclerosis. 2012;221:471–477. doi: 10.1016/j.atherosclerosis.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 126.Josan K, Majumdar SR, McAlister FA. The efficacy and safety of intensive statin therapy: a meta-analysis of randomized trials. CMAJ. 2008;178:576–584. doi: 10.1503/cmaj.070675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kiortsis DN, Filippatos TD, Mikhailidis DP, Elisaf MS, Liberopoulos EN. Statin-associated adverse effects beyond muscle and liver toxicity. Atherosclerosis. 2007;195:7–16. doi: 10.1016/j.atherosclerosis.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 128.Kostapanos MS, Liamis GL, Milionis HJ, Elisaf MS. Do statins beneficially or adversely affect glucose homeostasis? Curr Vasc Pharmacol. 2010;8:612–631. doi: 10.2174/157016110792006879. [DOI] [PubMed] [Google Scholar]
- 129.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW. et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 130.Elisaf M. Effects of fibrates on serum metabolic parameters. Curr Med Res Opin. 2002;18:269–276. doi: 10.1185/030079902125000516. [DOI] [PubMed] [Google Scholar]
- 131.Tsimihodimos V, Miltiadous G, Daskalopoulou SS, Mikhailidis DP, Elisaf MS. Fenofibrate: metabolic and pleiotropic effects. Curr Vasc Pharmacol. 2005;3:87–98. doi: 10.2174/1570161052773942. [DOI] [PubMed] [Google Scholar]
- 132.Filippatos TD, Kiortsis DN, Liberopoulos EN, Georgoula M, Mikhailidis DP, Elisaf MS. Effect of orlistat, micronised fenofibrate and their combination on metabolic parameters in overweight and obese patients with the metabolic syndrome: the FenOrli study. Curr Med Res Opin. 2005;21:1997–2006. doi: 10.1185/030079905x75078. [DOI] [PubMed] [Google Scholar]
- 133.Filippatos TD, Derdemezis CS, Georgoula M, Kiortsis DN, Tselepis AD, Elisaf MS. Fenofibrate and orlistat, alone or in combination, do not alter plasma visfatin levels in subjects with metabolic syndrome. J Med Sci Res. 2007;2:9–10. [Google Scholar]
- 134.Filippatos TD, Tsimihodimos V, Kostapanos M, Kostara C, Bairaktari ET, Kiortsis DN, Elisaf MS. Analysis of 6-month effect of orlistat administration, alone or in combination with fenofibrate, on triglyceride-rich lipoprotein metabolism in overweight and obese patients with metabolic syndrome. J Clin Lipidol. 2008;2:279–284. doi: 10.1016/j.jacl.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 135.Filippatos TD, Liberopoulos EN, Kostapanos M, Gazi IF, Papavasiliou EC, Kiortsis DN, Tselepis AD, Elisaf MS. The effects of orlistat and fenofibrate, alone or in combination, on high-density lipoprotein subfractions and pre-beta1-HDL levels in obese patients with metabolic syndrome. Diabetes Obes Metab. 2008;10:476–483. doi: 10.1111/j.1463-1326.2007.00733.x. [DOI] [PubMed] [Google Scholar]
- 136.Jun M, Foote C, Lv J, Neal B, Patel A, Nicholls SJ, Grobbee DE, Cass A, Chalmers J, Perkovic V. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet. 2010;375:1875–1884. doi: 10.1016/S0140-6736(10)60656-3. [DOI] [PubMed] [Google Scholar]
- 137.Agouridis AP, Filippatos TD, Derdemezis CS, Mikhailidis DP, Elisaf MS. Combination of fenofibrate with non-statin drug regimens. Curr Pharm Des. 2010;16:3401–3416. doi: 10.2174/138161210793563464. [DOI] [PubMed] [Google Scholar]
- 138.Rubins HB, Robins SJ, Collins D, Nelson DB, Elam MB, Schaefer EJ, Faas FH, Anderson JW. Diabetes, plasma insulin, and cardiovascular disease: subgroup analysis from the Department of Veterans Affairs high-density lipoprotein intervention trial (VA-HIT) Arch Intern Med. 2002;162:2597–2604. doi: 10.1001/archinte.162.22.2597. [DOI] [PubMed] [Google Scholar]
- 139.Elkeles RS, Diamond JR, Poulter C, Dhanjil S, Nicolaides AN, Mahmood S, Richmond W, Mather H, Sharp P, Feher MD. Cardiovascular outcomes in type 2 diabetes. A double-blind placebo-controlled study of bezafibrate: the St. Mary's, Ealing, Northwick Park Diabetes Cardiovascular Disease Prevention (SENDCAP) Study. Diabetes Care. 1998;21:641–648. doi: 10.2337/diacare.21.4.641. [DOI] [PubMed] [Google Scholar]
- 140.Keating GM, Ormrod D. Micronised fenofibrate: an updated review of its clinical efficacy in the management of dyslipidaemia. Drugs. 2002;62:1909–1944. doi: 10.2165/00003495-200262130-00013. [DOI] [PubMed] [Google Scholar]
- 141.Milionis HJ, Elisaf MS, Mikhailidis DP. Treatment of dyslipidaemias in patients with established vascular disease: a revival of the fibrates. Curr Med Res Opin. 2000;16:21–32. doi: 10.1185/0300799009117004. [DOI] [PubMed] [Google Scholar]
- 142.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P. et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 143.Tziomalos K, Athyros VG. Fenofibrate: a novel formulation (Triglide) in the treatment of lipid disorders: a review. Int J Nanomedicine. 2006;1:129–147. doi: 10.2147/nano.2006.1.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sorisky A, Ooi TC, Simo IE, Meuffels M, Hindmarsh JT, Nair R. Change in composition of high density lipoprotein during gemfibrozil therapy. Atherosclerosis. 1987;67:181–189. doi: 10.1016/0021-9150(87)90278-4. [DOI] [PubMed] [Google Scholar]
- 145.Kahri J, Vuorinen-Markkola H, Tilly-Kiesi M, Lahdenpera S, Taskinen MR. Effect of gemfibrozil on high density lipoprotein subspecies in non-insulin dependent diabetes mellitus. Relations to lipolytic enzymes and to the cholesteryl ester transfer protein activity. Atherosclerosis. 1993;102:79–89. doi: 10.1016/0021-9150(93)90086-a. [DOI] [PubMed] [Google Scholar]
- 146.Gnasso A, Lehner B, Haberbosch W, Leiss O, von Bergmann K, Augustin J. Effect of gemfibrozil on lipids, apoproteins, and postheparin lipolytic activities in normolipidemic subjects. Metabolism. 1986;35:387–393. doi: 10.1016/0026-0495(86)90125-3. [DOI] [PubMed] [Google Scholar]
- 147.Avellone G, Di Garbo V, Panno AV, Cordova R, Lepore R, Strano A. Changes induced by gemfibrozil on lipidic, coagulative and fibrinolytic pattern in patients with type IV hyperlipoproteinemia. Int Angiol. 1988;7:270–277. [PubMed] [Google Scholar]
- 148.Ahsan SK. Dyslipidemia: clinical approaches, evaluation of methods and strategies for standardization. Indian J Med Sci. 1997;51:420–425. [PubMed] [Google Scholar]
- 149.Gazi I, Tsimihodimos V, Filippatos TD, Saougos VG, Bairaktari ET, Tselepis AD, Elisaf M. LDL cholesterol estimation in patients with the metabolic syndrome. Lipids Health Dis. 2006;5:8. doi: 10.1186/1476-511X-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomised study. Lancet. 2001;357:905–910. [PubMed] [Google Scholar]
- 151.Chapman MJ. Pharmacology of fenofibrate. Am J Med. 1987;83:21–25. doi: 10.1016/0002-9343(87)90867-9. [DOI] [PubMed] [Google Scholar]
- 152.Moutzouri E, Kei A, Elisaf MS, Milionis HJ. Management of dyslipidemias with fibrates, alone and in combination with statins: role of delayed-release fenofibric acid. Vasc Health Risk Manag. 2010;6:525–539. doi: 10.2147/vhrm.s5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Trilipix. Abbott Laboratories; North Chicago, IL: [Accessed on May 26, 2010]. http://www.trilipixpro.com. [Google Scholar]
- 154.Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J. et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Filippatos TD, Elisaf MS. Fenofibrate plus simvastatin (fixed-dose combination) for the treatment of dyslipidaemia. Expert Opin Pharmacother. 2011;12:1945–1958. doi: 10.1517/14656566.2011.593509. [DOI] [PubMed] [Google Scholar]
- 156.Filippatos TD. A review of time courses and predictors of lipid changes with fenofibric acid-statin combination. Cardiovasc Drugs Ther. 2012;26:245–255. doi: 10.1007/s10557-012-6394-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jones PH, Cusi K, Davidson MH, Kelly MT, Setze CM, Thakker K, Sleep DJ, Stolzenbach JC. Efficacy and safety of fenofibric acid co-administered with low- or moderate-dose statin in patients with mixed dyslipidemia and type 2 diabetes mellitus: results of a pooled subgroup analysis from three randomized, controlled, double-blind trials. Am J Cardiovasc Drugs. 2010;10:73–84. doi: 10.2165/10061630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 158.Stefanutti C, Bucci A, Di Giacomo S, Fraone N, Pace A, Mareri M, Musca A, Mammarella A. Efficacy, safety and tolerability of combined low-dose simvastatin-fenofibrate treatment in primary mixed hyperlipidaemia. Clin Drug Investig. 2004;24:465–477. doi: 10.2165/00044011-200424080-00005. [DOI] [PubMed] [Google Scholar]
- 159.Grundy SM, Vega GL, Yuan Z, Battisti WP, Brady WE, Palmisano J. Effectiveness and tolerability of simvastatin plus fenofibrate for combined hyperlipidemia (the SAFARI trial) Am J Cardiol. 2005;95:462–468. doi: 10.1016/j.amjcard.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 160.Derosa G, Maffioli P, Salvadeo SA, Ferrari I, Gravina A, Mereu R, Palumbo I, D'Angelo A, Cicero AF. Fenofibrate, simvastatin and their combination in the management of dyslipidaemia in type 2 diabetic patients. Curr Med Res Opin. 2009;25:1973–1983. doi: 10.1185/03007990903073159. [DOI] [PubMed] [Google Scholar]
- 161.Krysiak R, Gdula-Dymek A, Okopien B. Effect of simvastatin and fenofibrate on cytokine release and systemic inflammation in type 2 diabetes mellitus with mixed dyslipidemia. Am J Cardiol. 2011;107:1010–1018 e1011. doi: 10.1016/j.amjcard.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 162.Kayikcioglu M, Ozerkan F, Soydan I. Effectiveness and safety of alternate-day simvastatin and fenofibrate on mixed hyperlipidemia. Am J Cardiol. 1999;83:1135–1137. doi: 10.1016/s0002-9149(99)00030-2. [DOI] [PubMed] [Google Scholar]
- 163.Muhlestein JB, May HT, Jensen JR, Horne BD, Lanman RB, Lavasani F, Wolfert RL, Pearson RR, Yannicelli HD, Anderson JL. The reduction of inflammatory biomarkers by statin, fibrate, and combination therapy among diabetic patients with mixed dyslipidemia: the DIACOR (Diabetes and Combined Lipid Therapy Regimen) study. J Am Coll Cardiol. 2006;48:396–401. doi: 10.1016/j.jacc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 164.May HT, Anderson JL, Pearson RR, Jensen JR, Horne BD, Lavasani F, Yannicelli HD, Muhlestein JB. Comparison of effects of simvastatin alone versus fenofibrate alone versus simvastatin plus fenofibrate on lipoprotein subparticle profiles in diabetic patients with mixed dyslipidemia (from the Diabetes and Combined Lipid Therapy Regimen Study) Am J Cardiol. 2008;101:486–489. doi: 10.1016/j.amjcard.2007.09.095. [DOI] [PubMed] [Google Scholar]
- 165.Farnier M, Roth E, Gil-Extremera B, Mendez GF, Macdonell G, Hamlin C, Perevozskaya I, Davies MJ, Kush D, Mitchel YB. Efficacy and safety of the coadministration of ezetimibe/simvastatin with fenofibrate in patients with mixed hyperlipidemia. Am Heart J. 2007;153(2):335. doi: 10.1016/j.ahj.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 166.Mohiuddin SM, Pepine CJ, Kelly MT, Buttler SM, Setze CM, Sleep DJ, Stolzenbach JC. Efficacy and safety of ABT-335 (fenofibric acid) in combination with simvastatin in patients with mixed dyslipidemia: a phase 3, randomized, controlled study. Am Heart J. 2009;157:195–203. doi: 10.1016/j.ahj.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 167.Gavish D, Leibovitz E, Shapira I, Rubinstein A. Bezafibrate and simvastatin combination therapy for diabetic dyslipidaemia: efficacy and safety. J Intern Med. 2000;247:563–569. doi: 10.1046/j.1365-2796.2000.00646.x. [DOI] [PubMed] [Google Scholar]
- 168.Dullaart RP, de Vries R, Voorbij HA, Sluiter WJ, van Tol A. Serum paraoxonase-I activity is unaffected by short-term administration of simvastatin, bezafibrate, and their combination in type 2 diabetes mellitus. Eur J Clin Invest. 2009;39:200–203. doi: 10.1111/j.1365-2362.2009.02090.x. [DOI] [PubMed] [Google Scholar]
- 169.Athyros VG, Papageorgiou AA, Athyrou VV, Demitriadis DS, Kontopoulos AG. Atorvastatin and micronized fenofibrate alone and in combination in type 2 diabetes with combined hyperlipidemia. Diabetes Care. 2002;25:1198–1202. doi: 10.2337/diacare.25.7.1198. [DOI] [PubMed] [Google Scholar]
- 170.Costet P, Hoffmann MM, Cariou B, Guyomarc'h Delasalle B, Konrad T, Winkler K. Plasma PCSK9 is increased by fenofibrate and atorvastatin in a non-additive fashion in diabetic patients. Atherosclerosis. 2010;212:246–251. doi: 10.1016/j.atherosclerosis.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 171.Goldberg AC, Bays HE, Ballantyne CM, Kelly MT, Buttler SM, Setze CM, Sleep DJ, Stolzenbach JC. Efficacy and safety of ABT-335 (fenofibric acid) in combination with atorvastatin in patients with mixed dyslipidemia. Am J Cardiol. 2009;103:515–522. doi: 10.1016/j.amjcard.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 172.Jones PH, Goldberg AC, Knapp HR, Kelly MT, Setze CM, Stolzenbach JC, Sleep DJ. Efficacy and safety of fenofibric acid in combination with atorvastatin and ezetimibe in patients with mixed dyslipidemia. Am Heart J. 2010;160:759–766. doi: 10.1016/j.ahj.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 173.Durrington PN, Tuomilehto J, Hamann A, Kallend D, Smith K. Rosuvastatin and fenofibrate alone and in combination in type 2 diabetes patients with combined hyperlipidaemia. Diabetes Res Clin Pract. 2004;64:137–151. doi: 10.1016/j.diabres.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 174.Jones PH, Davidson MH, Kashyap ML, Kelly MT, Buttler SM, Setze CM, Sleep DJ, Stolzenbach JC. Efficacy and safety of ABT-335 (fenofibric acid) in combination with rosuvastatin in patients with mixed dyslipidemia: a phase 3 study. Atherosclerosis. 2009;204:208–215. doi: 10.1016/j.atherosclerosis.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 175.Roth EM, Rosenson RS, Carlson DM, Fukumoto SM, Setze CM, Blasetto JW, Khurmi NS, Stolzenbach JC, Williams LA. Efficacy and safety of rosuvastatin 5 mg in combination with fenofibric acid 135 mg in patients with mixed dyslipidemia - a phase 3 study. Cardiovasc Drugs Ther. 2010;24:421–428. doi: 10.1007/s10557-010-6266-4. [DOI] [PubMed] [Google Scholar]
- 176.Rosenson RS, Carlson DM, Kelly MT, Setze CM, Hirshberg B, Stolzenbach JC, Williams LA. Achievement of lipid targets with the combination of rosuvastatin and fenofibric acid in patients with type 2 diabetes mellitus. Cardiovasc Drugs Ther. 2011;25:47–57. doi: 10.1007/s10557-010-6273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Roth EM, McKenney JM, Kelly MT, Setze CM, Carlson DM, Gold A, Stolzenbach JC, Williams LA, Jones PH. Efficacy and safety of rosuvastatin and fenofibric acid combination therapy versus simvastatin monotherapy in patients with hypercholesterolemia and hypertriglyceridemia: a randomized, double-blind study. Am J Cardiovasc Drugs. 2010;10:175–186. doi: 10.2165/11533430-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 178.Ballantyne CM, Davidson MH, Setze CM, Kelly MT. Effects of combination therapy with rosuvastatin and fenofibric acid in patients with mixed dyslipidemia and high-sensitivity C-reactive protein (>/= 2 mg/l) J Clin Lipidol. 2011;5:401–407. doi: 10.1016/j.jacl.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 179.Farnier M, Steinmetz A, Retterstol K, Csaszar A. Fixed-dose combination fenofibrate/pravastatin 160/40 mg versus simvastatin 20 mg monotherapy in adults with type 2 diabetes and mixed hyperlipidemia uncontrolled with simvastatin 20 mg: a double-blind, randomized comparative study. Clin Ther. 2011;33:1–12. doi: 10.1016/j.clinthera.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 180.Farnier M, Retterstol K, Steinmetz A, Csaszar A. Comparative efficacy and safety of fenofibrate/pravastatin plus ezetimibe triple therapy and simvastatin/ezetimibe dual therapy in type 2 diabetic patients with mixed hyperlipidaemia and cardiovascular disease. Diab Vasc Dis Res. 2012;9:205–215. doi: 10.1177/1479164111430715. [DOI] [PubMed] [Google Scholar]
- 181.Derosa G, Cicero AE, Bertone G, Piccinni MN, Ciccarelli L, Roggeri DE. Comparison of fluvastatin + fenofibrate combination therapy and fluvastatin monotherapy in the treatment of combined hyperlipidemia, type 2 diabetes mellitus, and coronary heart disease: a 12-month, randomized, double-blind, controlled trial. Clin Ther. 2004;26:1599–1607. doi: 10.1016/j.clinthera.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 182.Winkler K, Schewe T, Putz G, Odunc N, Schafer G, Siegel E, Geisen U, Abletshauser C, Hoffmann MM. Fluvastatin/fenofibrate vs. simvastatin/ezetimibe in patients with metabolic syndrome: different effects on LDL-profiles. Eur J Clin Invest. 2009;39:463–470. doi: 10.1111/j.1365-2362.2009.02126.x. [DOI] [PubMed] [Google Scholar]
- 183.Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E. et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 184.Rajamani K, Colman PG, Li LP, Best JD, Voysey M, D'Emden MC, Laakso M, Baker JR, Keech AC. Effect of fenofibrate on amputation events in people with type 2 diabetes mellitus (FIELD study): a prespecified analysis of a randomised controlled trial. Lancet. 2009;373:1780–1788. doi: 10.1016/S0140-6736(09)60698-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, Hubbard L, Esser BA, Lovato JF, Perdue LH. et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tsimihodimos V, Bairaktari E, Elisaf M. Fibrate-induced increase in serum urea and creatinine levels. Nephrol Dial Transplant. 2002;17:682. doi: 10.1093/ndt/17.4.682. [DOI] [PubMed] [Google Scholar]
- 187.Tsimihodimos V, Miltiadous G, Bairaktari E, Elisaf M. Possible mechanisms of the fibrate-induced increase in serum creatinine. Clin Nephrol. 2002;57:407–408. [PubMed] [Google Scholar]
- 188.Hottelart C, El Esper N, Rose F, Achard JM, Fournier A. Fenofibrate increases creatininemia by increasing metabolic production of creatinine. Nephron. 2002;92:536–541. doi: 10.1159/000064083. [DOI] [PubMed] [Google Scholar]
- 189.Forsblom C, Hiukka A, Leinonen ES, Sundvall J, Groop PH, Taskinen MR. Effects of long-term fenofibrate treatment on markers of renal function in type 2 diabetes: the FIELD Helsinki substudy. Diabetes Care. 2010;33:215–220. doi: 10.2337/dc09-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kostapanos MS, Florentin M, Elisaf MS. Fenofibrate and the kidney: an overview. Eur J Clin Invest. 2013;43(5):522–531. doi: 10.1111/eci.12068. [DOI] [PubMed] [Google Scholar]
- 191.Manninen V, Tenkanen L, Koskinen P, Huttunen JK, Manttari M, Heinonen OP, Frick MH. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study. Implications for treatment. Circulation. 1992;85:37–45. doi: 10.1161/01.cir.85.1.37. [DOI] [PubMed] [Google Scholar]
- 192.Sacks FM, Carey VJ, Fruchart JC. Combination lipid therapy in type 2 diabetes. N Engl J Med. 2010;363:692–694. doi: 10.1056/NEJMc1006407. [DOI] [PubMed] [Google Scholar]
- 193.Tenenbaum A, Motro M, Fisman EZ, Tanne D, Boyko V, Behar S. Bezafibrate for the secondary prevention of myocardial infarction in patients with metabolic syndrome. Arch Intern Med. 2005;165:1154–1160. doi: 10.1001/archinte.165.10.1154. [DOI] [PubMed] [Google Scholar]
- 194.Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation. 2000;102:21–27. doi: 10.1161/01.cir.102.1.21. [DOI] [PubMed] [Google Scholar]
- 195.Rubins HB, Robins SJ, Collins D, Fye CL, Anderson JW, Elam MB, Faas FH, Linares E, Schaefer EJ, Schectman G. et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N Engl J Med. 1999;341:410–418. doi: 10.1056/NEJM199908053410604. [DOI] [PubMed] [Google Scholar]
- 196.Frick MH, Elo O, Haapa K, Heinonen OP, Heinsalmi P, Helo P, Huttunen JK, Kaitaniemi P, Koskinen P, Manninen V. et al. Helsinki Heart Study: primary-prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N Engl J Med. 1987;317:1237–1245. doi: 10.1056/NEJM198711123172001. [DOI] [PubMed] [Google Scholar]
- 197.Scott R, O'Brien R, Fulcher G, Pardy C, D'Emden M, Tse D, Taskinen MR, Ehnholm C, Keech A. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care. 2009;32:493–498. doi: 10.2337/dc08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Reyes-Soffer G, Ngai CI, Lovato L, Karmally W, Ramakrishnan R, Holleran S, Ginsberg HN. Effect of combination therapy with fenofibrate and simvastatin on postprandial lipemia in the ACCORD lipid trial. Diabetes Care. 2013;36:422–428. doi: 10.2337/dc11-2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Tenenbaum A, Fisman EZ. "If it ain't broke, don't fix it": a commentary on the positive-negative results of the ACCORD Lipid study. Cardiovasc Diabetol. 2010;9:24. doi: 10.1186/1475-2840-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Enger C, Gately R, Ming EE, Niemcryk SJ, Williams L, McAfee AT. Pharmacoepidemiology safety study of fibrate and statin concomitant therapy. Am J Cardiol. 2010;106:1594–1601. doi: 10.1016/j.amjcard.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 201.Franssen R, Vergeer M, Stroes ES, Kastelein JJ. Combination statin-fibrate therapy: safety aspects. Diabetes Obes Metab. 2009;11:89–94. doi: 10.1111/j.1463-1326.2008.00917.x. [DOI] [PubMed] [Google Scholar]
- 202.Amend KL, Landon J, Thyagarajan V, Niemcryk S, McAfee A. Incidence of hospitalized rhabdomyolysis with statin and fibrate use in an insured US population. Ann Pharmacother. 2011;45:1230–1239. doi: 10.1345/aph.1Q110. [DOI] [PubMed] [Google Scholar]
- 203.Schech S, Graham D, Staffa J, Andrade SE, La Grenade L, Burgess M, Blough D, Stergachis A, Chan KA, Platt R. et al. Risk factors for statin-associated rhabdomyolysis. Pharmacoepidemiol Drug Saf. 2007;16:352–358. doi: 10.1002/pds.1287. [DOI] [PubMed] [Google Scholar]
- 204.Taher TH, Dzavik V, Reteff EM, Pearson GJ, Woloschuk BL, Francis GA. Tolerability of statin-fibrate and statin-niacin combination therapy in dyslipidemic patients at high risk for cardiovascular events. Am J Cardiol. 2002;89:390–394. doi: 10.1016/s0002-9149(01)02258-5. [DOI] [PubMed] [Google Scholar]
- 205.Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 206.Guo J, Meng F, Ma N, Li C, Ding Z, Wang H, Hou R, Qin Y. Meta-analysis of safety of the coadministration of statin with fenofibrate in patients with combined hyperlipidemia. Am J Cardiol. 2012;110:1296–1301. doi: 10.1016/j.amjcard.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 207.Jones PH, Davidson MH. Reporting rate of rhabdomyolysis with fenofibrate plus statin versus gemfibrozil plus any statin. Am J Cardiol. 2005;95:120–122. doi: 10.1016/j.amjcard.2004.08.076. [DOI] [PubMed] [Google Scholar]
- 208.Bays HE, Jones PH, Mohiuddin SM, Kelly MT, Sun H, Setze CM, Buttler SM, Sleep DJ, Stolzenbach JC. Long-term safety and efficacy of fenofibric acid in combination with statin therapy for the treatment of patients with mixed dyslipidemia. J Clin Lipidol. 2008;2:426–435. doi: 10.1016/j.jacl.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 209.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 210.Cannon CP, Steinberg BA, Murphy SA, Mega JL, Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J Am Coll Cardiol. 2006;48:438–445. doi: 10.1016/j.jacc.2006.04.070. [DOI] [PubMed] [Google Scholar]
- 211.Rallidis LS, Fountoulaki K, Anastasiou-Nana M. Managing the underestimated risk of statin-associated myopathy. Int J Cardiol. 2012;159:169–176. doi: 10.1016/j.ijcard.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 212.Navarese EP, Buffon A, Andreotti F, Kozinski M, Welton N, Fabiszak T, Caputo S, Grzesk G, Kubica A, Swiatkiewicz I. et al. Meta-analysis of impact of different types and doses of statins on new-onset diabetes mellitus. Am J Cardiol. 2013;111(8):1123–1130. doi: 10.1016/j.amjcard.2012.12.037. [DOI] [PubMed] [Google Scholar]
- 213.Waters DD, Ho JE, Boekholdt SM, DeMicco DA, Kastelein JJ, Messig M, Breazna A, Pedersen TR. Cardiovascular event reduction versus new-onset diabetes during atorvastatin therapy: effect of baseline risk factors for diabetes. J Am Coll Cardiol. 2013;61:148–152. doi: 10.1016/j.jacc.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 214.Wenger NK, Lewis SJ, Herrington DM, Bittner V, Welty FK Treating to New Targets Study Steering Committee and Investigators. Outcomes of using high- or low-dose atorvastatin in patients 65 years of age or older with stable coronary heart disease. Ann Intern Med. 2007;147:1–9. doi: 10.7326/0003-4819-147-1-200707030-00002. [DOI] [PubMed] [Google Scholar]
- 215.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]


