Abstract
OBJECTIVES: There is extensive but controversial evidence on the diverse effects of statins on the level of high-density lipoprotein cholesterol (HDL-C). Some of these effects may limit the benefits of statins in terms of cardiovascular risk reduction. To identify the conditions for beneficial effects, this study investigated the response to atorvastatin and simvastatin treatment in type 2 diabetic patients with elevated low-density lipoprotein cholesterol (LDL-C). METHODS: 2,872 subjects with type 2 diabetes from a disease management program were investigated. Patients with LDL-C ≥130 mg/dl or total cholesterol ≥200 mg/dl were put onto statin therapy by the National Health Insurance system in Taiwan. RESULTS: 1,080 patients who completed 1 year of statin treatment were analyzed. There were significant reductions in LDL-C in both the atorvastatin (37.1%) and simvastatin (34.3%) group after one year of treatment compared with baseline levels. Unexpectedly, the majority of diabetic patients who received atorvastatin or simvastatin did not show an increase in HDL-C levels. 59.8% of patients had a significant HDL-C reduction (ΔHDL-C ≤ -3%) after atorvastatin treatment. Multivariate logistic regression analysis showed that the following patients were at higher risk of HDL-C reduction after 12 months: (i) patients in whom statin therapy was initiated aged <65 years and who had a BMI ≥24 kg/m2, (ii) male patients with a baseline HDL-C >40 mg/dl, and (iii) female patients with a baseline HDL-C >50 mg/dl. However, diabetic patients with severe atherogenic dyslipidemia (LDL-C ≥130, TG ≥204, and HDL-C ≤34 mg/dl) obtained more benefits in terms of HDL-C change after statin therapy. CONCLUSIONS: Diabetic patients, except those with severe atherogenic dyslipidemia, are prone to a decrease in serum HDL-C level after statin treatment, particularly after atorvastatin treatment.
Keywords: high-density lipoprotein cholesterol, atorvastatin, simvastatin, lipid, type 2 diabetes
Abbreviations: AIM-HIGH - Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes trial; ANOVA - analysis of variance; ASCOT-LLA - Anglo-Scandinavian Cardiac Outcomes Trial - Lipid Lowering Arm; ASPEN - Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus; CARDS - Collaborative Atorvastatin Diabetes Study; CVD - cardiovascular disease; HbA1c - glycosylated hemoglobin; HDL-C - high-density lipoprotein cholesterol; HMG-CoA - 3-hydroxy-3-methylglutaryl-coenzyme A; HPS - Heart Protection Study; LDL-C - low-density lipoprotein cholesterol; MDRD - modification of diet in renal disease; SPSS - statistical package of social sciences; TC - total cholsterol; TG - triglycerides; TNT - Treating to New Targets study; UKPDS - UK Prospective Diabetes Study
1. Introduction
Patients with diabetes mellitus are at increased risk of cardiovascular diseases (CVD) [1]. In this regard, increased low-density lipoprotein cholesterol (LDL-C) is assumed to be associated with the pathogenesis of atherosclerosis [2]. Since the discovery of statins, which are potent LDL-C-lowering drugs, several large clinical trials have shown that they successfully lower the risk of CVD in diabetic patients [3-7]. Based on the well-documented clinical efficacy and safety profile of statins, the American Diabetes Association recommended achievement of the LDL-C target as first priority, and suggested that statins are the optimal therapeutic agents for the reduction of CVD risk [8]. Unfortunately, despite the fact that statins have been widely prescribed for diabetic patients with satisfactory LDL-C reduction, the risk of CVD remains substantial [9]. This may partly result from the persistently unsatisfactory serum concentrations of high-density lipoprotein cholesterol (HDL-C), triglycerides, and non-HDL cholesterol after statin treatment [10, 11].
The impact of low HDL-C or elevated non-HDL concentrations on CVD should not be overlooked. In the Framingham study, Kannel et al. reported that a low HDL-C level is the strongest factor linked to CVD [12]. In the UKPDS study, HDL-C was also identified as an important independent factor in predicting major CVD events in diabetic patients [13, 14]. Moreover, Jafri et al. showed that the association between low levels of HDL-C and increased CVD risk is unchanged, even under statin treatment [15]. These studies indicate the importance of serum HDL-C in CVD risk reduction.
Beyond their ability to lower LDL-C, statins have long been recognized as increasing serum HDL-C level by approximately 4-10% [16]. However, there may be discrepancies in diabetic patients. In the HPS study, diabetic patients treated with simvastatin showed only an approximately 1% increase in serum HDL-C level compared with placebo after 5 years of follow up [4]. In the ASCOT-LLA study, HDL-C showed an insignificant reduction (0.8%) in diabetic patients after 3.3 years of atorvastatin treatment [6]. In the CARDS study in particular, serum HDL-C was observed to be decreased by 9.4% after 3.9 years of atorvastatin treatment [5]. These milestone trials did not show the consistent result that long-term statin treatment results in an increase of serum HDL-C in diabetic patients.
Besides the inconsistent HDL-C response in diabetic patients, it should be noted that patients of different ethnicities may have dissimilar statin responses. In a double-blind randomized control trial, Goldberg et al. reported that black/Hispanic patients had less favorable lipid changes after atorvastatin or simvastatin/ezetimibe management than other races/ethnicities [17]. Furthermore, several articles have indicated that several gene polymorphisms could result in differences in lipid changes between statins [18-20]. On the basis of the above, our study aimed to evaluate the change in HDL-C level after 1 year of atorvastatin and simvastatin treatment in Chinese patients with type 2 diabetes mellitus.
2. Methods
2.1 Subjects
From October 2006 to May 2012, a total of 2872 type 2 diabetic patients participated in a comprehensive diabetic care program in a specialized diabetic outpatient clinic [21, 22]. At enrollment in the program, the patients' HbA1c, total cholesterol, LDL-C, HDL-C, triglycerides, and serum creatinine levels were assessed, and their urine was examined for albuminuria. At the annual evaluation, these measurements were re-examined. Hypertension was diagnosed if one of the following criteria were present:
- Repeatedly measured systolic blood pressure >140 mmHg
- Diastolic pressure >90 mmHg at a clinic visit
- If the patient had already been prescribed anti-hypertensive medication at the time of enrollment
Among these patients, statins were prescribed to patients with LDL-C ≥130 mg/dl or total cholesterol ≥200 mg/dl, which is recommended by the National Health Insurance system in Taiwan. Patients with TG ≥500 mg/dl who were prescribed fenofibrate were excluded. Patients who had been prescribed 10 mg atorvastatin and 20 mg simvastatin for at least 12 months were included in the present study. Blood samples were collected after at least 8 hrs of overnight fasting. HbA1c was measured by ion-exchange high-performance liquid chromatography (VARIANT II Turbo, Bio-Rad, Hercules, CA, US). Urinary albumin concentrations were measured by immunoturbidmetry (Beckman Instruments, Galway, Ireland) [24]. The estimated glomerular filtration rate (eGFR) was calculated by the traditional 4-variable MDRD equation. A biochemical automatic analyzer (Beckman-Coulter, Fullerton, CA, US) was used to analyze blood samples. Serum levels of total cholesterol, LDL-C, and triglycerides were measured by a standard enzymatic method. Serum HDL-C was measured by direct enzymatic methods using commercial kits (Cholestest N-HDL, Sekisui Medical, Tokyo, Japan) with less than 3% intra-assay and inter-assay coefficients of variance [22-24]. Our laboratory analyses were performed under internal and external quality control at the laboratory of the College of American Pathologists. The study was approved by the Institutional Review Board of Tri-Service General Hospital, Taipei, Taiwan.
2.2 Statistical analysis
Continuous variables were presented as the mean ± S.D. Student's t-tests were performed for group comparisons. As the distribution of serum triglyceride levels was skewed, logarithmically-transformed values were used for statistical analysis. Trend analyses were performed by ANOVA with linear contrast between the groups. Chi-square tests were conducted to compare categorical variables. Univariate logistic regression analyses were used to identify significant baseline determinates of the reduction in serum HDL-C (i.e., ΔHDL-C ≤-3%). Multivariate forward stepwise logistic regression analysis was applied to identify independent variables predicting a significant reduction in serum HDL-C. SPSS version 14.0 (SPSS, Chicago, IL) was used for statistical calculations. A p-value <0.05 was considered to be significant.
3. Results
Out of 2,872 diabetic patients, a total of 1,080 patients (atorvastatin/simvastatin: 754/326) with elevated total cholesterol or LDL-C who completed 1 year of statin treatment were analyzed. Table 1 shows the baseline clinical characteristics of the study population. There were no significant differences in the physical and biological measurements between the atorvastatin and simvastatin groups before statin treatment. After 1 year of statin treatment, there was a significant decrease in HbA1c, total cholesterol (TC), LDL-C, and the ratio of TC to HDL-C (TC/HDL-C) in both the atorvastatin and simvastatin group (Table 2). There was a significant decrease in TG, HDL-C, and the ratio of TG to HDL-C (TG/HDL-C) in the atorvastatin group, while the changes in these metabolic factors were not statistically significant in the simvastatin group. With the exception of TC/HDL, the atorvastatin group showed a greater decrease in all lipid parameters, including HDL-C, compared with the percentage changes in lipid profile in the simvastatin group (data not shown).
Table 1. Baseline characteristics of the 1080 type 2 diabetic patients in the atorvastatin and simvastatin treatment groups.
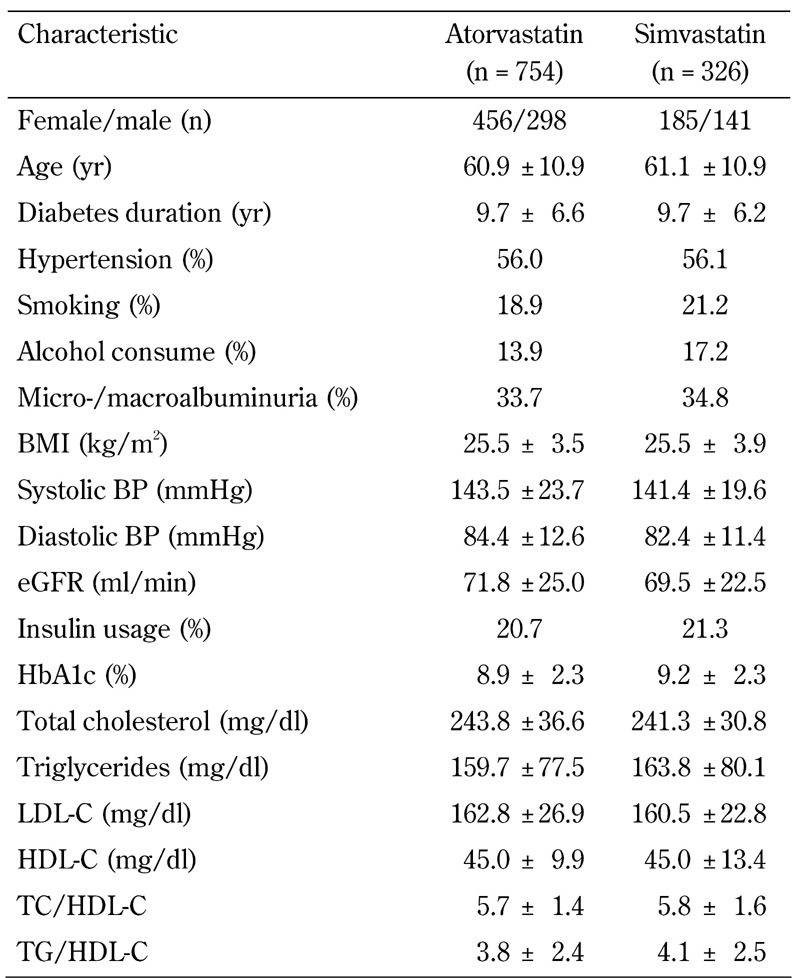
Legend: Data are mean ± SD, or percentages. Abbreviations: BMI – body mass index; BP – blood pressure; eGFR – estimated glomerular filtration rate; HbA1c – glycosylated hemoglobin; HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; TC – total cholesterol; TG – triglycerides.
Table 2. Lipid parameters of the 1080 type 2 diabetic patients at baseline and after 12 months of atorvastatin or simvastatin treatment.
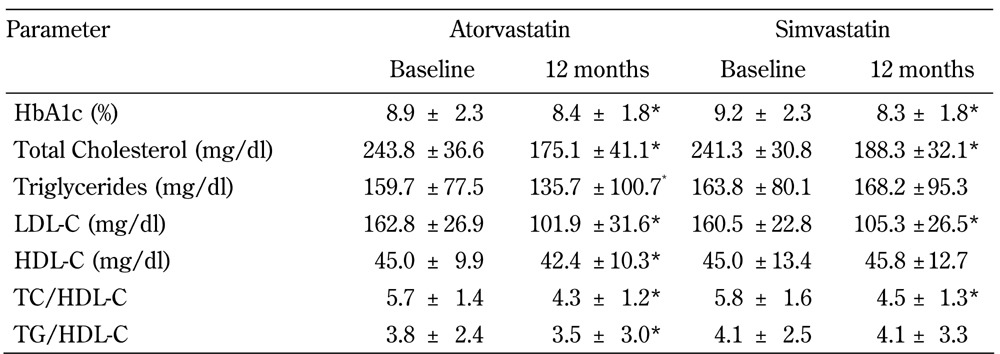
Legend: Data are means ± SD. * Significant different compared with baseline in the atorvastatin or simvastatin group (p < 0.05). Abbreviations: HbA1c – glycosylated hemoglobin; HDL-C – high-density lipoprotein cholesterol; LDL-C – low-density lipoprotein cholesterol; TC – total cholesterol; TG – triglycerides.
To identify the clinical differences in HDL-C response after 1 year of atorvastatin or simvastatin treatment, we stratified the patients into 3 groups (see Tables 3 and 4, and Figure 1):
Table 3. Characteristics of the type 2 diabetic subjects stratified according to the percentage change in the 12-month HDL-cholesterol level after atorvastatin treatment (n = 754).
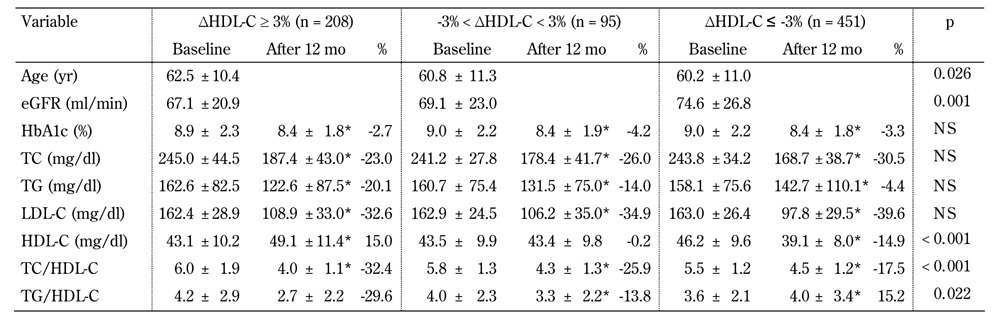
Legend: Data are mean ± SD, or percentage. Percent change calculated as (data measured at 12 months - data measured at baseline)/data measured at baseline. * significant difference compared with baseline (p < 0.05). P-values for baseline comparisons. Abbreviations: eGFR - estimated glomerular filtration rate; HbA1c - glycosylated hemoglobin; HDL-C - high-density lipoprotein cholesterol; LDL-C - low-density lipoprotein cholesterol; NS - not significant; TC - total cholesterol; TG - triglycerides.
Table 4. Characteristics of the type 2 diabetic subjects stratified according to the percentage change in the 12-month HDL-cholesterol level after simvastatin treatment (n = 326).
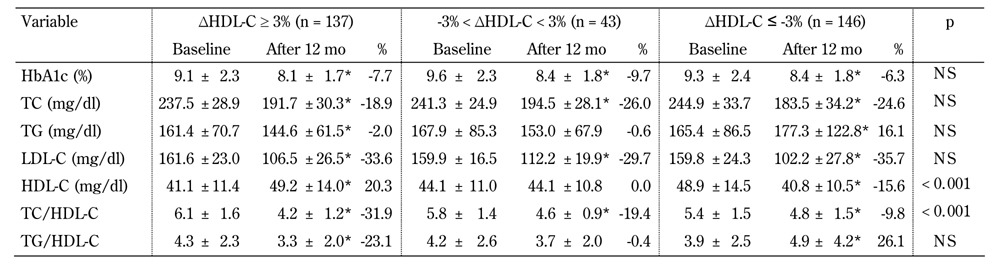
Legend: Data are mean ± SD, or percentage. Percent change calculated as (data measured at 12 months - data measured at baseline)/data measured at baseline. * significant difference compared with baseline (p < 0.05). P-values for baseline comparisons. Abbreviations: eGFR - estimated glomerular filtration rate; HbA1c - glycosylated hemoglobin; HDL-C - high-density lipoprotein cholesterol; LDL-C - low-density lipoprotein cholesterol; NS - not significant; TC - total cholesterol; TG - triglycerides.
Figure 1.
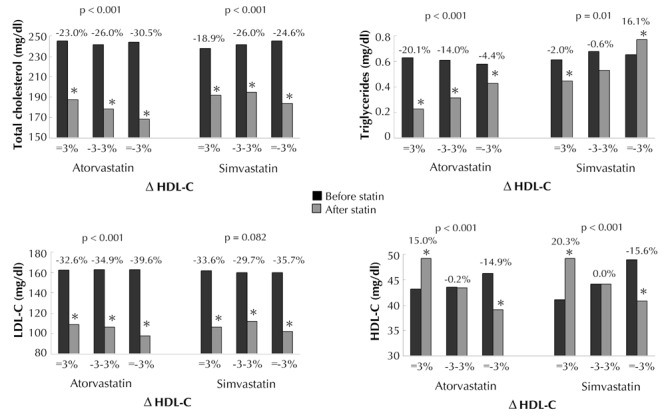
Basal total cholesterol, triglycerides, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and changes in these parameters in the diabetic patients after 1 year of atorvastatin or simvastatin treatment, stratified by the percentile changes in HDL-C (i.e. ΔHDL-C ≥ 3%, -3% < ΔHDL-C < -3%, and ΔHDL-C ≤-3%). P-value: trend analysis for percentage change. * p < 0.05 compared with baseline.
1. ΔHDL-C ≤ -3%
2. -3% < ΔHDL-C < 3%
3. ΔHDL-C ≥3%
The prevalence of ΔHDL-C ≤ -3%, -3% < ΔHDL-C < 3%, and ΔHDL-C ≥3% in the atorvastatin group was 59.8%, 12.6%, and 27.6%, respectively. The prevalence of ΔHDL-C ≤ -3%, -3% < ΔHDL-C < 3%, and ΔHDL-C ≥3% in the simvastatin group was 44.8%, 13.2%, and 42.0%, respectively. Patients who took atorvastatin had a higher prevalence of ΔHDL-C ≤ -3% than those who took simvastatin (p < 0.001). The atorvastatin users who tended to have a better HDL-C response were older (p = 0.026) and had a lower eGFR (p = 0.001), while there were no differences in the basic physical characteristics among the different HDL-C responders in the simvastatin group (data not shown).
With regards to baseline metabolic factors, regardless of atorvastatin or simvastatin treatment, patients with a better HDL-C response had a lower serum HDL-C, but higher TC/HDL-C initially. We also found that patients with a better HDL-C response had a lower decrement in TC, but higher decrements in serum TG, TC/HDL-C, and TG/ HDL-C in both the atorvastatin and simvastatin treatment groups. Regarding the percentage change in LDL-C, a significant trend associated with HDL-C response was found in the atorvastatin group only, not in the simvastatin group.
In the univariate logistic regression analyses (Table 5, model 1), patients aged <65 years, with a BMI ≥24 kg/m2, baseline serum HDL-C >50 mg/dl in females or serum HDL-C >40 mg/dl in males and who took atorvastatin treatment showed a positive association with the percentage reduction in HDL-C (ΔHDL-C ≤ -3%). In contrast, TC/HDL-C >5 and increased TG associated with low HDL-C (i.e., TG >150 mg/dl associated with HDL-C <50 mg/dl in females or HDL-C <40 mg/dl in males; TG ≥204 mg/dl and HDL-C ≤34 mg/dl) showed a negative association. In multivariate forward stepwise logistic regression analysis (Table 5, model 2), we investigated the independent factors associated with the change in HDL-C after statin treatment. Among patients aged <65 years and with a BMI ≥24 kg/m2, baseline serum HDL-C >50 mg/dl in females or serum HDL-C >40 mg/dl in males were significantly associated with HDL-C changes. Patients who took atorvastatin rather than simvastatin also had a higher risk of ΔHDL-C ≤ -3%. Interestingly, we found that patients with TG ≥204 mg/dl and HDL-C ≤34 mg/dl, but not females with TG >150 mg/dl and HDL-C ≤50 mg/dl or males with serum HDL-C ≤40 mg/dl, were protected against ΔHDL-C ≤ -3%.
Table 5. Logistic regression analysis of baseline variables regarding the reduction of HDL-C (i.e. ΔHDL-C ≤-3%) after 12 months of statin treatment.
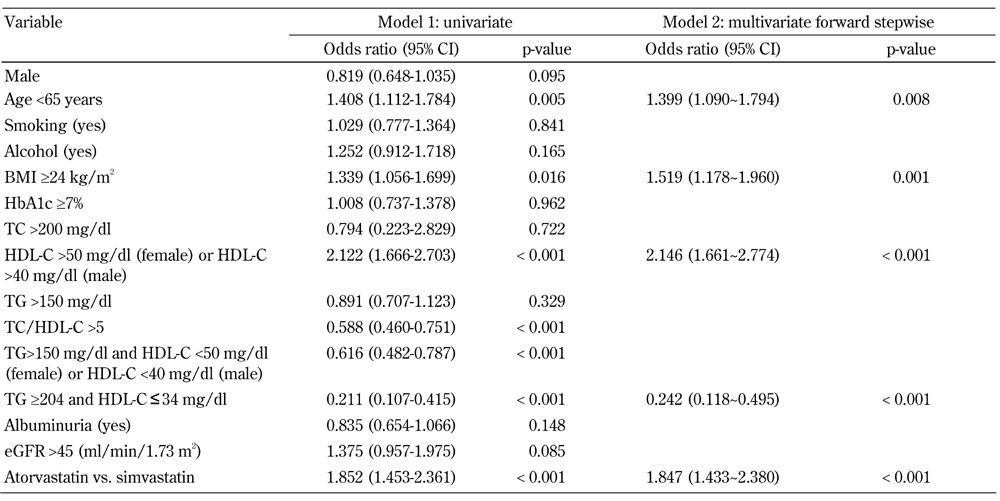
Legend: R2 = 0.160 for atorvastatin vs. simvastatin, model 2. Abbreviations: eGFR - estimated glomerular filtration rate; HbA1c - glycosylated hemoglobin; HDL-C - high-density lipoprotein cholesterol; LDL-C - low-density lipoprotein cholesterol; NS - not significant; TC - total cholesterol; TG - triglycerides.
4. Discussion
A recent meta-analysis, which includes several short-term studies (4-12 weeks), indicate that diabetic patients benefit by increasing serum HDL-C after statin treatment [25]. In contrast, our results do not support a positive HDL-C change after 1 year of atorvastatin and simvastatin treatment in Chinese diabetic patients.
In the HPS study, patients treated with simvastatin showed an approximately 1% increase in serum HDL-C level [4]. In the CARDS study, patients treated with atorvastatin showed a 9% decrease in serum HDL-C level [5]. In the ASCOT-LLA and the TNT study, serum HDL-C barely changed [6, 7]. Although the ASPEN study demonstrated a significant improvement in serum HDL-C, this amounted to an approximately 2% elevation only [26]. All of these large long-term statin trials show an underperformance in causing HDL-C changes in diabetic patients. Our results are in accordance with these large studies, and suggest that the improvements seen in serum HDL-C in diabetic patients under “long-term” atorvastatin and simvastatin treatment should be reassessed.
The following results may be a source of some indirect support for our results. In a pan-European survey of diabetic patients, 89% of whom were on statin treatment, Bruckert et al. reported that 45% of patients had a low HDL-C [11]. In a high-risk CVD population, in which 65% of patients were managed by statin treatment, Alsheikh-Ali et al. reported that over 60% of patients had a low HDL-C across all levels of LDL-C [10]. Surprisingly, the prevalence of low HDL-C was nearly 80% in those patients with LDL-C ≤70mg/dl [10]. Moreover, low serum HDL-C was equally prevalent in statin users vs. non-statin users (67% vs. 64%; p = NS) [10]. These epidemiological studies may shed a different light on the improvement of low HDL-C by statins in the real world.
There are several explanations for the diminished HDL-C elevation found after statin treatment in diabetic patients (Figure 2). Firstly, several enzymes, including lipoprotein lipase, hepatic lipase, and phospholipid transfer protein, involved in HDL-C metabolism and remodeling are reported to be impaired in an insulin-resistant milieu [27]. These enzymatic abnormalities may attenuate the positive effect of statins on HDL-C in diabetic patients. Secondly, it is known that the liver could be the major source of the cholesterol that circulates in HDL-C [28], and prolonging the inhibition of HMG-CoA reductase by statins may result in the depletion of hepatic cholesterol, leading to decreased production of HDL-C [16]. Our results regarding HDL-C response after statin treatment may also be partly affected by ethnicity differences.
Figure 2. Proposed mechanisms of HDL lowering in type 2 diabetic patients.
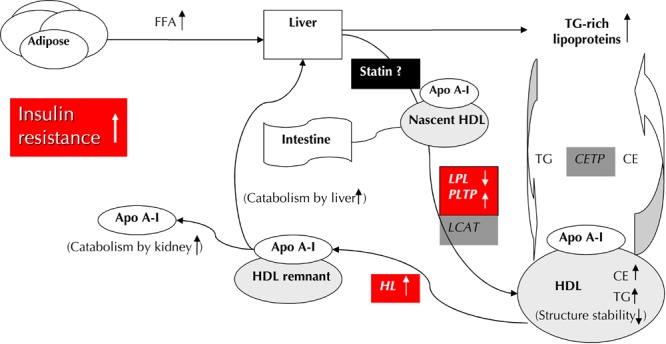
Increased insulin resistance at the adipocyte causes increased release of free fatty acid (FFA) flux into the liver, and stimulates the assembly and secretion of triglyceride (TG)-rich lipoprotein. Cholesterol ester transfer protein (CETP) mediates the transfer of TG from TG-rich lipoproteins to HDL in exchange for cholesterol ester (CE). The TG-rich HDL particles, characterized by structural instability and loosely bound Apo-AI, are voluntarily hydrolyzed by hepatic lipase (HL), which results in more rapid clearance of free Apo-AI and HDL remnants. In association with impaired lipoprotein lipase (LPL) and phospholipid transfer protein (PLTP) activity in the remodeling of HDL, the constellation of the above factors may lead to decreased serum HDL concentrations in type 2 diabetic patients. There are two possible explanations for the postulated mechanism of our HDL-C response after 1 year of statin therapy: 1. The impaired enzymatic activity involving HDL metabolism may attenuate the positive effect of statin in changing the HDL concentration. 2. The source of cholesterol in HDL may be decreased by the prolonged inhibition of hepatic cholesterol synthesis with statin therapy.
It should be noted that our understanding of the response to statins has been predominantly derived from Caucasian populations, However, in Chinese diabetic patients, Chu et al. showed that serum HDL-C decreased insignificantly by approximately 1.4-5.2% after 10-40 mg of atorvastatin treatment [29]. In Japanese diabetic patients, Shimabukuru et al. demonstrated a significant 4.2% reduction in serum HDL-C after a 6-month atorvastatin treatment [30]. In Turkish diabetic patients, Akalin et al. reported a non-significant change in serum HDL-C after statin treatment [31]. Associated with our results, the effectiveness of statins in altering serum HDL-C level in diabetic patients with diverse ethnicities may need further clarification.
Our results show that simvastatin changed HDL-C levels in diabetic patients more effectively than atorvastatin. In a meta-analysis, atorvastatin was not shown to be superior, and even showed some inferiority, in terms of increasing HDL-C across all dosage comparisons as compared with simvastatin [32]. In a recently published study, simvastatin showed a higher increase in the percentage change in HDL-C than atorvastatin across all levels of identical dosage in diabetic patients [25]. Also, in contrast to the consistency of effect shown by simvastatin in altering serum HDL-C, the study demonstrated that a smaller increase in serum HDL-C was obtained with higher atorvastatin doses [25]. Although, the differences in structure between simvastatin and atorvastatin may contribute to the differences in changes in serum HDL-C [33], our results may also be partly due to the differences in the pharmacogenetic responses to simvastatin and atorvastatin in the Chinese population [19, 20, 34]. On the basis of the above, simvastatin may be more effective in changing serum HDL-C level than atorvastatin in Chinese diabetic patients.
Our results may suggest that severe atherogenic dyslipidemia (TG ≥204 mg/dl and HDL-C ≤34 mg/dl) could be determinant for the change in serum HDL-C after statin treatment. Increased triglycerides and low HDL-C levels characterizing diabetic patients result from a disequilibrium of lipid metabolism in an insulin-resistant milieu (Figure 2). Thus, diabetic patients with severe atherogenic dyslipidemia manifest this serious metabolic dysfunction, and these patients may experience more benefit from medical correction. Recently, Shimabukuro et al. found that the ability of pitavastatin to increase serum HDL-C could not be replicated in a small diabetic group with a mean HDL-C >50mg/dl and TG <150mg/dl [30]. Together with the results of Ginsberg et al., who reported that only diabetic patients with TG ≥204 mg/dl associated with HDL-C ≤34 mg/dl had a reduced risk of CVD under intensive lipid-lowering therapy [35], these findings support our results that diabetic patients with severe atherogenic dyslipidemia may have a better clinical response under dyslipidemia therapy.
The significance of the clinical implications of statins in changing serum HDL-C may be questioned, but it should be noted that serum HDL-C may be an independent predictor of CVD risk even under statin treatment [15]. Furthermore, Brown et al. clearly indicated that the simple addition of the percentage reduction in LDL-C and the percentage increase in HDL-C is far more effective than either lipoprotein component alone [36]. The solid clinical benefits of statins and the disappointing results obtained by adding niacin to statin treatment, as demonstrated in the AIM-HIGH study [37], emphasize the potential importance of differences between statins related to the changes in serum HDL-C level they induce.
Although our report is the first to show the differences in long-term HDL-C response between atorvastatin and simvastatin in Chinese diabetic patients, the study has several limitations. Firstly, the effects of physical activity, alcohol intake, and diet compliance on HDL-C could not be accurately reported. Secondly, our study participants were recruited from a single clinical unit, which may limit extrapolation of our results to the general Chinese diabetic population. Thirdly, our study may be biased by the coefficients of variation associated with measurements of serum HDL-C concentration. Fourthly, we did not clarify the pharmacogenetic effects seen in our study. Despite these limitations, our results were based on a large diabetic cohort, and we believe that our study may remind clinicians to pay attention to HDL-C response after long-term statin treatment.
Our results make two major contributions to the current literature. Firstly, our study indicated that most of the Chinese diabetic patients did not benefit in terms of serum HDL-C after 1 year of atorvastatin or simvastatin treatment. Secondly, the majority of the Chinese diabetic patients with an increased LDL-C level, except for those with severe hypertriglyceridemia and low serum HDL-C, may experience a decrease in serum HDL-C after statin treatment, particularly after atorvastatin. In conclusion, diabetic patients, apart from those with profound atherogenic dyslipidemia, may experience a decrease in serum HDL-C level after statin treatment, particularly after atorvastatin treatment.
Disclosures: The authors report no conflict of interests.
References
- 1.Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164:1422–1426. doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 3.Pyorala K, Pedersen TR, Kjekshus J, Faergeman O, Olsson AG, Thorgeirsson G. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease. A subgroup analysis of the Scandinavian Simvastatin Survival Study (4S) Diabetes Care. 1997;20:614–620. doi: 10.2337/diacare.20.4.614. [DOI] [PubMed] [Google Scholar]
- 4.Collins R, Armitage J, Parish S, Sleigh P, Peto R Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361:2005–2016. doi: 10.1016/s0140-6736(03)13636-7. [DOI] [PubMed] [Google Scholar]
- 5.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 6.Sever PS, Poulter NR, Dahlof B, Wedel H, Collins R, Beevers G, Caulfield M, Kjeldsen SE, Kristinsson A, McInnes GT. et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial - lipid-lowering arm (ASCOT-LLA) Diabetes Care. 2005;28:1151–1157. doi: 10.2337/diacare.28.5.1151. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd J, Barter P, Carmena R, Deedwania P, Fruchart JC, Haffner S, Hsia J, Breazna A, LaRosa J, Grundy S. et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the Treating to New Targets (TNT) study. Diabetes Care. 2006;29:1220–1226. doi: 10.2337/dc05-2465. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Standards of medical care in diabetes 2012. Diabetes Care. 2012;35(Suppl 1):S11–S63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman J. Beyound LDL-cholesterol reduction: the way ahead in managing dyslipidaemia. Eur Heart J Suppl. 2005;7:F56–F62. [Google Scholar]
- 10.Alsheikh-Ali AA, Lin JL, Abourjaily P, Ahearn D, Kuvin JT, Karas RH. Prevalence of low high-density lipoprotein cholesterol in patients with documented coronary heart disease or risk equivalent and controlled low-density lipoprotein cholesterol. Am J Cardiol. 2007;100:1499–1501. doi: 10.1016/j.amjcard.2007.06.058. [DOI] [PubMed] [Google Scholar]
- 11.Bruckert E, Baccara-Dinet M, Eschwege E. Low HDL-cholesterol is common in European Type 2 diabetic patients receiving treatment for dyslipidaemia: data from a pan-European survey. Diabet Med. 2007;24:388–391. doi: 10.1111/j.1464-5491.2007.02111.x. [DOI] [PubMed] [Google Scholar]
- 12.Kannel WB, Castelli WP, Gordon T. Cholesterol in the prediction of atherosclerotic disease. New perspectives based on the Framingham study. Ann Intern Med. 1979;90:85–91. doi: 10.7326/0003-4819-90-1-85. [DOI] [PubMed] [Google Scholar]
- 13.Stevens RJ, Kothari V, Adler AI, Stratton IM. The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56) Clin Sci (Lond) 2001;101:671–679. [PubMed] [Google Scholar]
- 14.Kothari V, Stevens RJ, Adler AI, Stratton IM, Manley SE, Neil HA, Holman RR. UKPDS 60: risk of stroke in type 2 diabetes estimated by the UK Prospective Diabetes Study risk engine. Stroke. 2002;33:1776–1781. doi: 10.1161/01.str.0000020091.07144.c7. [DOI] [PubMed] [Google Scholar]
- 15.Jafri H, Alsheikh-Ali AA, Karas RH. Meta-analysis: statin therapy does not alter the association between low levels of high-density lipoprotein cholesterol and increased cardiovascular risk. Ann Intern Med. 2010;153:800–808. doi: 10.7326/0003-4819-153-12-201012210-00006. [DOI] [PubMed] [Google Scholar]
- 16.McTaggart F, Jones P. Effects of statins on high-density lipoproteins: a potential contribution to cardiovascular benefit. Cardiovasc Drugs Ther. 2008;22:321–338. doi: 10.1007/s10557-008-6113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg RB, Guyton JR, Mazzone T, Weinstock RS, Polis AB, Tipping D, Tomassini JE, Tershakovec AM. Relationships between metabolic syndrome and other baseline factors and the efficacy of ezetimibe/simvastatin and atorvastatin in patients with type 2 diabetes and hypercholesterolemia. Diabetes Care. 2010;33:1021–1024. doi: 10.2337/dc09-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Y, Zhang LR, Fu Q. CYP3A4*1G polymorphism is associated with lipid-lowering efficacy of atorvastatin but not of simvastatin. Eur J Clin Pharmacol. 2008;64:877–882. doi: 10.1007/s00228-008-0502-x. [DOI] [PubMed] [Google Scholar]
- 19.Li YP, Zhang LR, Jia M, Hu XJ. CYP3AP1*3 allele is associated with lipid-lowering efficacy of simvastatin and atorvastatin in Chinese women. J Clin Pharmacol. 2011;51:181–188. doi: 10.1177/0091270010370589. [DOI] [PubMed] [Google Scholar]
- 20.Kolovou G, Kolovou V, Mihas C, Giannakopoulou V, Vasiliadis I, Boussoula E, Kollia A, Boutsikou M, Katsiki N, Mavrogeni S. Cholesteryl Ester Transfer Protein and ATP-Binding Cassette Transporter A1 Genotype Alter the Atorvastatin and Simvastatin Efficacy: Time for Genotype-Guided Therapy? Angiology. 2012 doi: 10.1177/0003319712444594. In press. [DOI] [PubMed] [Google Scholar]
- 21.Chang YH, Chang DM, Lin KC, Shin SJ, Lee YJ. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a meta-analysis and systemic review. Diabetes Metab Res Rev. 2011;27:515–527. doi: 10.1002/dmrr.1201. [DOI] [PubMed] [Google Scholar]
- 22.Chang YH, Chang DM, Lin KC, Hsieh CH, Lee YJ. High-density lipoprotein cholesterol and the risk of nephropathy in type 2 diabetic patients. Nutr Metab Cardiovasc Dis. 2013;23:751–757. doi: 10.1016/j.numecd.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, Shin SJ, Lee YJ. ENPP1 K121Q polymorphism is not related to type 2 diabetes mellitus, features of metabolic syndrome, and diabetic cardiovascular complications in a Chinese population. Rev Diabet Stud. 2006;3:21–30. doi: 10.1900/RDS.2006.3.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller WG, Myers GL, Sakurabayashi I, Bachmann LM, Caudill SP, Dziekonski A, Edwards S, Kimberly MM, Korzun WJ, Leary ET. et al. Seven direct methods for measuring HDL and LDL cholesterol compared with ultracentrifugation reference measurement procedures. Clin Chem. 2010;56:977–986. doi: 10.1373/clinchem.2009.142810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karlson BW, Barter PJ, Palmer MK, Lundman P, Nicholls SJ. Comparison of the effects of different statins and doses on lipid levels in patients with diabetes: results from VOYAGER. Nutr Metab Cardiovasc Dis. 2012;22:697–703. doi: 10.1016/j.numecd.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Knopp RH, d'Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN) Diabetes Care. 2006;29:1478–1485. doi: 10.2337/dc05-2415. [DOI] [PubMed] [Google Scholar]
- 27.Borggreve SE, De Vries R, Dullaart RP. Alterations in high-density lipoprotein metabolism and reverse cholesterol transport in insulin resistance and type 2 diabetes mellitus: role of lipolytic enzymes, lecithin:cholesterol acyltransferase and lipid transfer proteins. Eur J Clin Invest. 2003;33:1051–1069. doi: 10.1111/j.1365-2362.2003.01263.x. [DOI] [PubMed] [Google Scholar]
- 28.Brewer HB Jr, Remaley AT, Neufeld EB, Basso F, Joyce C. Regulation of plasma high-density lipoprotein levels by the ABCA1 transporter and the emerging role of high-density lipoprotein in the treatment of cardiovascular disease. Arterioscler Thromb Vasc Biol. 2004;24:1755–1760. doi: 10.1161/01.ATV.0000142804.27420.5b. [DOI] [PubMed] [Google Scholar]
- 29.Chu CH, Lee JK, Lam HC, Lu CC, Sun CC, Wang MC, Chuang MJ, Wei MC. Atorvastatin does not affect insulin sensitivity and the adiponectin or leptin levels in hyperlipidemic type 2 diabetes. J Endocrinol Invest. 2008;31:42–47. doi: 10.1007/BF03345565. [DOI] [PubMed] [Google Scholar]
- 30.Shimabukuro M, Higa M, Tanaka H, Shimabukuro T, Yamakawa K, Masuzaki H. Distinct effects of pitavastatin and atorvastatin on lipoprotein subclasses in patients with type 2 diabetes mellitus. Diabet Med. 2011;28:856–864. doi: 10.1111/j.1464-5491.2011.03240.x. [DOI] [PubMed] [Google Scholar]
- 31.Akalin A, Temiz G, Akcar N, Sensoy B. Short term effects of atorvastatin on endothelial functions and oxidized LDL levels in patients with type 2 diabetes. Endocr J. 2008;55:861–866. doi: 10.1507/endocrj.k07e-121. [DOI] [PubMed] [Google Scholar]
- 32.Rogers SL, Magliano DJ, Levison DB, Webb K, Clarke PJ, Grobler MP, Liew D. A dose-specific meta-analysis of lipid changes in randomized controlled trials of atorvastatin and simvastatin. Clin Ther. 2007;29:242–252. doi: 10.1016/j.clinthera.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Arnaboldi L, Corsini A. Do structural differences in statins correlate with clinical efficacy? Curr Opin Lipidol. 2010;21:298–304. doi: 10.1097/MOL.0b013e32833b776c. [DOI] [PubMed] [Google Scholar]
- 34.Huang Y, Wu Y, Liu R, Fan P, Zhang J, Wang F, Luo X, Liu Y, Liu B, Bai H. Differential effect of ATP binding cassette transporter A1 R219K and cholesteryl ester transfer protein TaqIB genotypes on HDL-C levels in overweight/obese and non-obese Chinese subjects. Acta cardiologica. 2011;66:231–237. doi: 10.1080/ac.66.2.2071256. [DOI] [PubMed] [Google Scholar]
- 35.Ginsberg HN, Elam MB, Lovato LC, Crouse JR 3rd, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J. et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown BG, Stukovsky KH, Zhao XQ. Simultaneous low-density lipoprotein-C lowering and high-density lipoprotein-C elevation for optimum cardiovascular disease prevention with various drug classes, and their combinations: a meta-analysis of 23 randomized lipid trials. Curr Opin Lipidol. 2006;17:631–636. doi: 10.1097/MOL.0b013e32800ff750. [DOI] [PubMed] [Google Scholar]
- 37.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]


