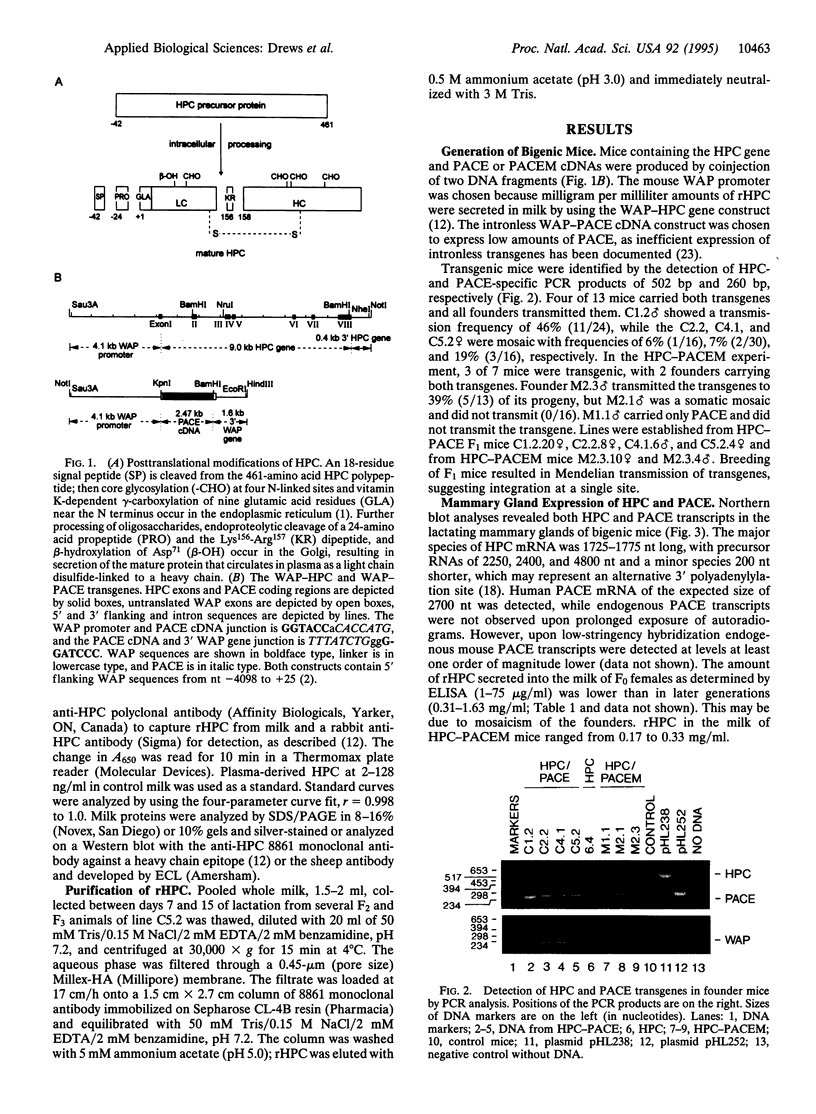
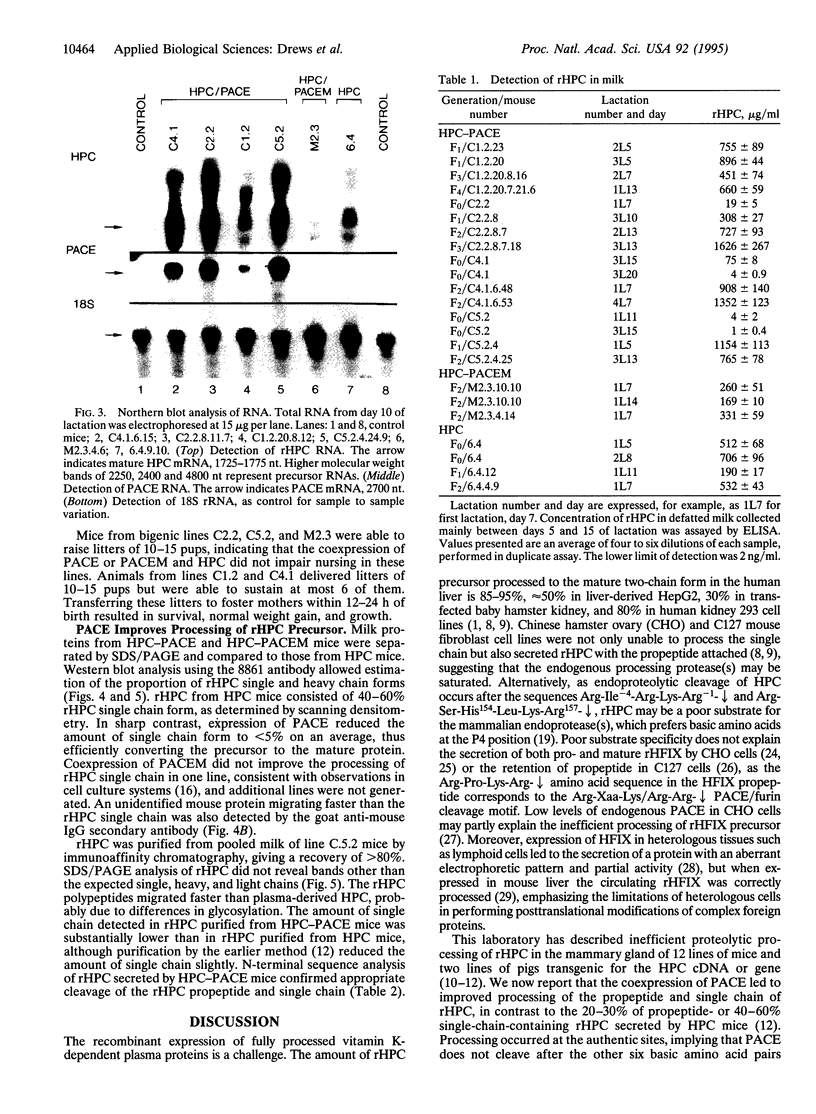
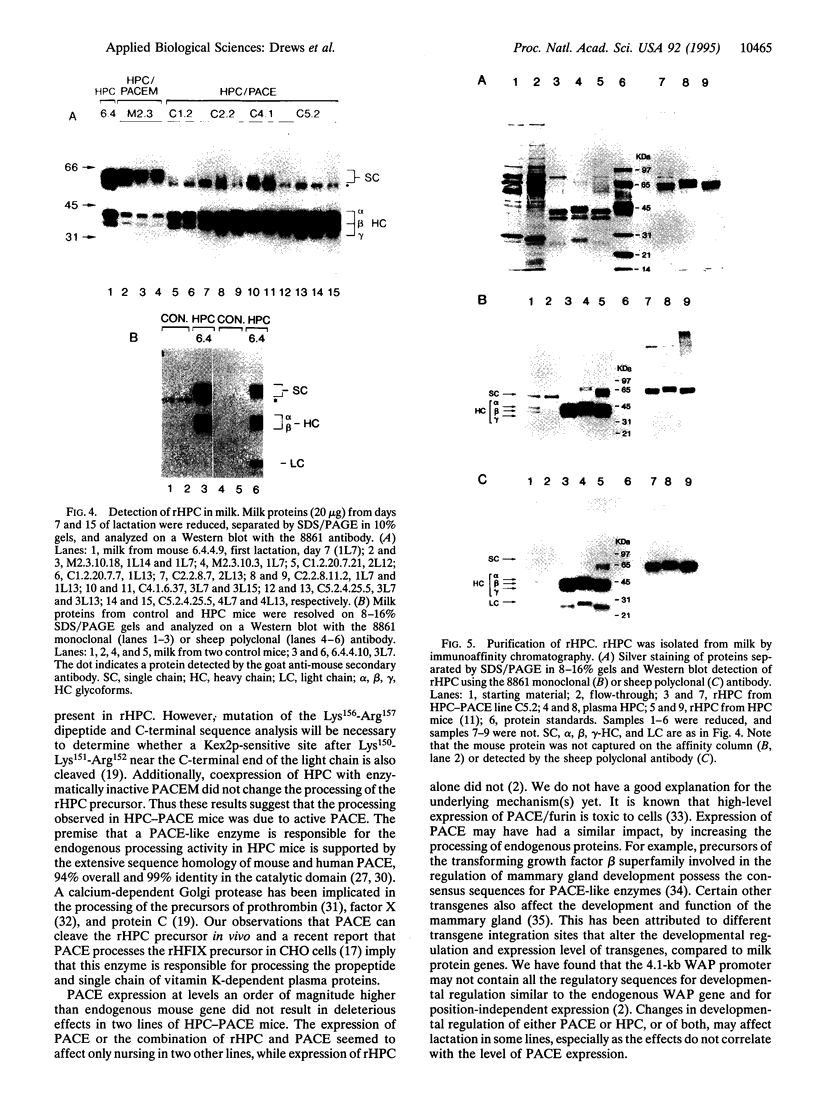
Abstract
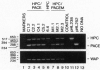
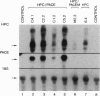
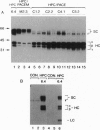
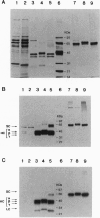
Endoproteolytic processing of the human protein C (HPC) precursor to its mature form involves cleavage of the propeptide after amino acids Lys-2-Arg-1 and removal of a Lys156-Arg157 dipeptide connecting the light and heavy chains. This processing was inefficient in the mammary gland of transgenic mice and pigs. We hypothesized that the protein processing capacity of specific animal organs may be improved by the coexpression of selected processing enzymes. We tested this by targeting expression of the human proprotein processing enzyme, named paired basic amino acid cleaving enzyme (PACE)/furin, or an enzymatically inactive mutant, PACEM, to the mouse mammary gland. In contrast to mice expressing HPC alone, or to HPC/PACEM bigenic mice, coexpression of PACE with HPC resulted in efficient conversion of the precursor to mature protein, with cleavage at the appropriate sites. These results suggest the involvement of PACE in the processing of HPC in vivo and represent an example of the engineering of animal organs into bioreactors with enhanced protein processing capacity.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayoubi T. A., Creemers J. W., Roebroek A. J., Van de Ven W. J. Expression of the dibasic proprotein processing enzyme furin is directed by multiple promoters. J Biol Chem. 1994 Mar 25;269(12):9298–9303. [PubMed] [Google Scholar]
- Barr P. J., Mason O. B., Landsberg K. E., Wong P. A., Kiefer M. C., Brake A. J. cDNA and gene structure for a human subtilisin-like protease with cleavage specificity for paired basic amino acid residues. DNA Cell Biol. 1991 Jun;10(5):319–328. doi: 10.1089/dna.1991.10.319. [DOI] [PubMed] [Google Scholar]
- Bauer K. A. Coumarin-induced skin necrosis. Arch Dermatol. 1993 Jun;129(6):766–768. [PubMed] [Google Scholar]
- Brennan S. O., Peach R. J. The processing of human proinsulin and chicken proalbumin by rat hepatic vesicles suggests a convertase specific for X-Y-Arg-Arg or Arg-X-Y-Arg sequences. J Biol Chem. 1991 Nov 15;266(32):21504–21508. [PubMed] [Google Scholar]
- Bresnahan P. A., Leduc R., Thomas L., Thorner J., Gibson H. L., Brake A. J., Barr P. J., Thomas G. Human fur gene encodes a yeast KEX2-like endoprotease that cleaves pro-beta-NGF in vivo. J Cell Biol. 1990 Dec;111(6 Pt 2):2851–2859. doi: 10.1083/jcb.111.6.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon T., Wall R. J., Shamay A., Smith G. H., Hennighausen L. Over-expression of an endogenous milk protein gene in transgenic mice is associated with impaired mammary alveolar development and a milchlos phenotype. Mech Dev. 1991 Dec;36(1-2):67–74. doi: 10.1016/0925-4773(91)90073-f. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Derian C. K., VanDusen W., Przysiecki C. T., Walsh P. N., Berkner K. L., Kaufman R. J., Friedman P. A. Inhibitors of 2-ketoglutarate-dependent dioxygenases block aspartyl beta-hydroxylation of recombinant human factor IX in several mammalian expression systems. J Biol Chem. 1989 Apr 25;264(12):6615–6618. [PubMed] [Google Scholar]
- Dreyfus M., Magny J. F., Bridey F., Schwarz H. P., Planché C., Dehan M., Tchernia G. Treatment of homozygous protein C deficiency and neonatal purpura fulminans with a purified protein C concentrate. N Engl J Med. 1991 Nov 28;325(22):1565–1568. doi: 10.1056/NEJM199111283252207. [DOI] [PubMed] [Google Scholar]
- Drohan W. N., Zhang D. W., Paleyanda R. K., Chang R., Wroble M., Velander W., Lubon H. Inefficient processing of human protein C in the mouse mammary gland. Transgenic Res. 1994 Nov;3(6):355–364. doi: 10.1007/BF01976767. [DOI] [PubMed] [Google Scholar]
- Dubois C. M., Laprise M. H., Blanchette F., Gentry L. E., Leduc R. Processing of transforming growth factor beta 1 precursor by human furin convertase. J Biol Chem. 1995 May 5;270(18):10618–10624. doi: 10.1074/jbc.270.18.10618. [DOI] [PubMed] [Google Scholar]
- Esmon C. T. The roles of protein C and thrombomodulin in the regulation of blood coagulation. J Biol Chem. 1989 Mar 25;264(9):4743–4746. [PubMed] [Google Scholar]
- Foster D. C., Holly R. D., Sprecher C. A., Walker K. M., Kumar A. A. Endoproteolytic processing of the human protein C precursor by the yeast Kex2 endopeptidase coexpressed in mammalian cells. Biochemistry. 1991 Jan 15;30(2):367–372. doi: 10.1021/bi00216a009. [DOI] [PubMed] [Google Scholar]
- Foster D. C., Yoshitake S., Davie E. W. The nucleotide sequence of the gene for human protein C. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4673–4677. doi: 10.1073/pnas.82.14.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. H. Clinical studies of protein C. Semin Thromb Hemost. 1984 Apr;10(2):162–166. doi: 10.1055/s-2007-1004419. [DOI] [PubMed] [Google Scholar]
- Griffin J. H., Evatt B., Zimmerman T. S., Kleiss A. J., Wideman C. Deficiency of protein C in congenital thrombotic disease. J Clin Invest. 1981 Nov;68(5):1370–1373. doi: 10.1172/JCI110385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsuzawa K., Hosaka M., Nakagawa T., Nagase M., Shoda A., Murakami K., Nakayama K. Structure and expression of mouse furin, a yeast Kex2-related protease. Lack of processing of coexpressed prorenin in GH4C1 cells. J Biol Chem. 1990 Dec 25;265(36):22075–22078. [PubMed] [Google Scholar]
- Jallat S., Perraud F., Dalemans W., Balland A., Dieterle A., Faure T., Meulien P., Pavirani A. Characterization of recombinant human factor IX expressed in transgenic mice and in derived trans-immortalized hepatic cell lines. EMBO J. 1990 Oct;9(10):3295–3301. doi: 10.1002/j.1460-2075.1990.tb07529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhappan C., Geiser A. G., Kordon E. C., Bagheri D., Hennighausen L., Roberts A. B., Smith G. H., Merlino G. Targeting expression of a transforming growth factor beta 1 transgene to the pregnant mammary gland inhibits alveolar development and lactation. EMBO J. 1993 May;12(5):1835–1845. doi: 10.1002/j.1460-2075.1993.tb05832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman R. J., Wasley L. C., Furie B. C., Furie B., Shoemaker C. B. Expression, purification, and characterization of recombinant gamma-carboxylated factor IX synthesized in Chinese hamster ovary cells. J Biol Chem. 1986 Jul 25;261(21):9622–9628. [PubMed] [Google Scholar]
- Lin S. W., Smith K. J., Welsch D., Stafford D. W. Expression and characterization of human factor IX and factor IX-factor X chimeras in mouse C127 cells. J Biol Chem. 1990 Jan 5;265(1):144–150. [PubMed] [Google Scholar]
- Misumi Y., Ohkubo K., Sohda M., Takami N., Oda K., Ikehara Y. Intracellular processing of complement pro-C3 and proalbumin is inhibited by rat alpha 1-protease inhibitor variant (Met352----Arg) in transfected cells. Biochem Biophys Res Commun. 1990 Aug 31;171(1):236–242. doi: 10.1016/0006-291x(90)91382-3. [DOI] [PubMed] [Google Scholar]
- Paleyanda R. K., Zhang D. W., Hennighausen L., McKnight R. A., Lubon H. Regulation of human protein C gene expression by the mouse WAP promoter. Transgenic Res. 1994 Nov;3(6):335–343. doi: 10.1007/BF01976765. [DOI] [PubMed] [Google Scholar]
- Rehemtulla A., Kaufman R. J. Preferred sequence requirements for cleavage of pro-von Willebrand factor by propeptide-processing enzymes. Blood. 1992 May 1;79(9):2349–2355. [PubMed] [Google Scholar]
- Stanton C., Taylor R., Wallin R. Processing of prothrombin in the secretory pathway. Biochem J. 1991 Jul 1;277(Pt 1):59–65. doi: 10.1042/bj2770059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton C., Wallin R. Processing and trafficking of clotting factor X in the secretory pathway. Effects of warfarin. Biochem J. 1992 May 15;284(Pt 1):25–31. doi: 10.1042/bj2840025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttie J. W. Report of Workshop on expression of vitamin K-dependent proteins in bacterial and mammalian cells, Madison, Wisconsin, USA, April 1986. Thromb Res. 1986 Oct 1;44(1):129–134. doi: 10.1016/0049-3848(86)90189-1. [DOI] [PubMed] [Google Scholar]
- Velander W. H., Johnson J. L., Page R. L., Russell C. G., Subramanian A., Wilkins T. D., Gwazdauskas F. C., Pittius C., Drohan W. N. High-level expression of a heterologous protein in the milk of transgenic swine using the cDNA encoding human protein C. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12003–12007. doi: 10.1073/pnas.89.24.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velander W. H., Page R. L., Morcöl T., Russell C. G., Canseco R., Young J. M., Drohan W. N., Gwazdauskas F. C., Wilkins T. D., Johnson J. L. Production of biologically active human protein C in the milk of transgenic mice. Ann N Y Acad Sci. 1992 Oct 13;665:391–403. doi: 10.1111/j.1749-6632.1992.tb42602.x. [DOI] [PubMed] [Google Scholar]
- Wasley L. C., Rehemtulla A., Bristol J. A., Kaufman R. J. PACE/furin can process the vitamin K-dependent pro-factor IX precursor within the secretory pathway. J Biol Chem. 1993 Apr 25;268(12):8458–8465. [PubMed] [Google Scholar]
- Whitelaw C. B., Archibald A. L., Harris S., McClenaghan M., Simons J. P., Clark A. J. Targeting expression to the mammary gland: intronic sequences can enhance the efficiency of gene expression in transgenic mice. Transgenic Res. 1991 Dec;1(1):3–13. doi: 10.1007/BF02512991. [DOI] [PubMed] [Google Scholar]
- Yan S. C., Grinnell B. W., Wold F. Post-translational modifications of proteins: some problems left to solve. Trends Biochem Sci. 1989 Jul;14(7):264–268. doi: 10.1016/0968-0004(89)90060-1. [DOI] [PubMed] [Google Scholar]
- van den Ouweland A. M., van Duijnhoven H. L., Keizer G. D., Dorssers L. C., Van de Ven W. J. Structural homology between the human fur gene product and the subtilisin-like protease encoded by yeast KEX2. Nucleic Acids Res. 1990 Feb 11;18(3):664–664. doi: 10.1093/nar/18.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]