Abstract
Dyslipidemia is a major risk factor for cardiovascular complications in people with diabetes. Lowering low-density lipoprotein cholesterol (LDL-C) levels is effective in the primary and secondary prevention of diabetic vascular complications. However, LDL-C levels do not reflect all aspects of diabetic dyslipidemia, which is characterized by hypertriglyceridemia and low high-density lipoprotein cholesterol (HDL-C). Statins, nicotinic acid, and fibrates play a role in treating diabetic dyslipidemia. Atherosclerosis is a major disorder of the blood vessel wall in patients with diabetes. A number of antihyperlipidemic agents may be beneficial and exhibit effects at the actual site of vascular disease and not only on plasma lipoprotein concentrations. Several novel therapeutic compounds are currently being developed. These include additional therapeutics for LDL-C, triglycerides, HDL-C, and modulators of inflammation that can be used as possible synergic agents for the treatment of atherosclerosis and irregularities in plasma lipoprotein concentrations.
Keywords: type 2 diabetes, dyslipidemia, lipoprotein, triglyceride, fibrate, statin
Abbreviations: ACCORD - Action to Control Cardiovascular Risk in Diabetes study; ApoB100 - apolipoprotein B100; ApoA-I - apolipoprotein A-I; ATP - adenosine triphosphate; DGAT-2 - diacylglycerol acyl transferase-2; CARDS - Collaborative Atorvastatin Diabetes Study; CVD - cardiovascular disease; HDL-C - high-density lipoprotein cholesterol; HR - hazard ratio; IDEAL - Incremental Decrease in Endpoints through Aggressive Lipid Lowering study; J-PREDICT - Japan Prevention Trial of Diabetes by Pitavastatin in Patients with Impaired Glucose Tolerance; LDL-C - low-density lipoprotein cholesterol; MTP - microsomal triglyceride transfer protein; NO - nitric oxide; NOD - new-onset diabetes; OR - odds ratio; PCSK9 - pre-protein convertase subtilisin kexin-9 inhibitors; PPAR - peroxisomal proliferator-activating receptor; TG - triglyceride; TNT - Treating to New Targets; VLDL - very low-density lipoprotein
1. Introduction
The diabetic population is at high risk of cardiovascular disease (CVD). It is estimated that patients with diabetes have a 2- to 4-fold higher risk of ischemic disease, including coronary heart disease, stroke, and peripheral vascular disease, than non-diabetic people [1]. In patients with diabetes, an alteration in the distribution of lipids increases the risk of atherosclerosis. Specifically, insulin resistance and insulin deficiency have been identified as causes of dyslipidemia in patients with diabetes mellitus [2]. They are caused by high levels of triglycerides (TGs) and low-density lipoprotein cholesterol (LDL-C) and low levels of high-density lipoprotein cholesterol (HDL-C) [3].
LDL-C is vital for the assessment of lipoprotein-associated risk. An elevated LDL-C level is an established risk factor for CVD and may play a crucial role in diabetes. Current guidelines suggest that the level of LDL-C is the primary metric of cardiovascular risk in people with diabetes [4]. However, LDL-C levels do not reflect the classic features of diabetic dyslipidemia, namely hypertriglyceridemia and low HDL-C. Measurements of plasma apolipoprotein B100 (ApoB100) concentrations and non-HDL-C may improve the definition of dyslipidemia [5].
Dyslipidemia is a major risk factor for macrovascular complications in patients with type 2 diabetes [6]. The management of LDL-C is the primary treatment goal for diabetic dyslipidemia [7]. In previous studies, a 1% reduction in LDL-C levels was associated with a 1% reduction in cardiovascular events, while a 1% increase in HDL-C levels was connected with a 3% reduction in cardiovascular events [8]. Statins are the first-line drugs for most lipid disorders. However, they cannot be used to treat all aspects of dyslipidemia. Numerous novel therapeutic compounds are currently being developed. These include additional therapeutics for LDL-C, TGs, and HDL-C. This review focuses on potential new drugs for treating diabetic dyslipidemia.
2. Current approaches to diabetic dyslipidemia
An elevated LDL-C level is an established risk factor for CVD in people with diabetes. However, LDL-C levels do not reflect all aspects of diabetic dyslipidemia, which is characterized by an elevation in TG levels and low levels of HDL-C. Measuring plasma apolipoprotein B100 (ApoB100) concentrations may improve the definition of risk. Only one ApoB100 molecule is present on each LDL, intermediate-density lipoprotein, and very low-density lipoprotein (VLDL) particle. Thus, the concentration of ApoB100 can reflect the combined molecular concentrations of these atherogenic particle classes [9].
Increased LDL-C levels add to overall cardiovascular risk in patients with diabetes [10]. Aggressive lipid treatments have been recommended for patients with type 2 diabetes. The current treatment targets for people with diabetes who are considered to have ‘high’ or ‘very high’ vascular disease risk are summarized as follows:
- The target value of LDL-C is <70 mg/dl (1.81 mmol/l) for patients with the highest risk and <100 mg/dl (2.58 mmol/l) for those with high risk.
- The respective target values for non-HDL-C are <100 mg/dl (2.58 mmol/l) and <130 mg/dl (3.36 mmol/l).
- The target values for ApoB100 are <80 mg/dl (1.60 mmol/l) and <90 mg/dl (1.81 mmol/l), respectively (Table 1) [11].
Table 1. Target values for LDL-C, non-HDL-C, and ApoB100 in diabetic patients.
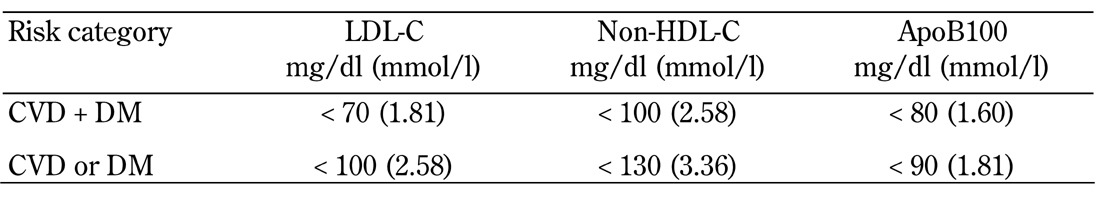
Legend: 1. In very high-risk patients with overt CVD, a lower LDL-C goal of <70 mg/dl (1.8 mmol/l) is an option. If drug-treated patients do not reach the above target, additional American Diabetes Association (ADA) recommendations are the reduction of LDL-C by 30-40% from baseline. Lowering TG to <150 mg/dl (1.7 mmol/l), and raising HDL-C to >40 mg/dl in men and >50 mg/dl in women are desirable [58]. 2. The primary therapeutic goal in relation to LDL-C is < 100 mg/dl (2.6 mmol/l) for those with DM without overt CVD [58]. 3. Non-HDL-C is a secondary target of therapy in patients with high serum triglycerides (≥ 200 mg/dl) [59]. 4. The target values for ApoB100 are <80 mg/dl (1.60 mmol/l) in DM patients with overt CVD and <90 mg/dl (1.8 mmol/l) in those without overt CVD [11].
Although dyslipidemia may be corrected over a few years, the cardiovascular risk cannot be eliminated and cardiovascular health cannot be restored because several factors contribute to lipoprotein-related residual risk in patients with diabetes. Residual risk indicates that serum lipoprotein abnormalities persist after conventional treatment goals have been achieved [12]. The risk factors include altered lipoprotein particle size distribution, a modified apolipoprotein component, and altered activity of lipoprotein-associated enzymes [13]. These risk factors are the targets of new medications.
3. New lipid-lowering drugs based on old drug classes
3.1 Statins
The benefits of statin treatment in the primary and secondary prevention of cardiovascular disease have been observed in diabetic patients [14]. A primary prevention study, the Collaborative Atorvastatin Diabetes Study (CARDS), showed a relative risk reduction of 37% over 5 years of statin treatment in patients with type 2 diabetes [15].
Recent data showed that statin therapy is associated with an increased risk of developing diabetes [16]. A meta-analysis of previous studies indicated that statin use is associated with a 9% increased risk for diabetes [17]. A recent study compared the incidence of new-onset diabetes (NOD) among 15,056 patients with coronary disease (without diabetes at baseline) in the Treating to New Targets (TNT) and Incremental Decrease in Endpoints through Aggressive Lipid Lowering (IDEAL) trials. The researchers looked for differences in patients with 0-1 risk factors for NOD at baseline compared with those who had 2-4 risk factors (i.e., fasting blood glucose >100 mg/dl, history of hypertension, body mass index >30 kg/m2, and fasting triglycerides). Compared with low-dose statin therapy, high-dose statin therapy did not increase the incidence of NOD in patients with 0 to 1 NOD risk factors (3.22% vs. 3.35%; HR: 0.97; 95% CI: 0.77-1.22), but it did cause an increase among patients with 2 to 4 NOD risk factors (14.3% vs. 11.9%; HR: 1.24; 95% CI: 1.08-1.42; p = 0.0027). The number of cardiovascular events was considerably decreased with high-dose statin treatment in both NOD risk groups [18]. The J-PREDICT study was an open-label randomized controlled study of a population with impaired glucose tolerance conducted to evaluate the effect of a statin (pitavastatin) on NOD in patients. This was the first study to evaluate the effect of a statin on the onset of diabetes [19].
Most available data suggest that statins are effective and safe drugs that can produce a maximal reduction of LDL levels, parallel reductions in TG levels, and a modest rise in HDL levels [20]. The primary disadvantage is that double-dose titration results in a 5-7% additional reduction in LDL levels but increases side effects, especially myalgia and myositis [21]. Statins that are currently in development include NCX-6560 and PPD-10558. NCX-6560 (NicOx; Sophia-Antipolis, France), a nitric oxide-releasing derivative of atorvastatin, inhibits cholesterol biosynthesis exhibits anti-inflammatory and anti-thrombotic properties, and reduces LDL-C levels by 57% [22]. PPD-10558 (Furiex; Morrisville, NC), which enhances liver extraction by decreasing blood and muscle tissue exposure, was used in a clinical trial in patients with statin-associated myalgia [23]. However, Furiex Pharmaceuticals ceased the development of PPD-10558 in December 2011 because the drug did not meet its primary efficacy endpoint in a phase 2 proof-of-concept study.
3.2 Fibrates
Fibrates are activators of peroxisomal proliferator-activating receptor alpha (PPAR-α). Their chief actions are lowering TG levels and slightly raising HDL-C levels. High-dose fibrates can reduce TG levels by 70%, raise HDL levels by 20%, and reduce LDL levels by 10-25% [24]. Fibrates exhibit differential specificity for tissue peroxisome proliferator-activated receptors (PPARs) with a predominant PPAR-α action. PPAR-α is a ligand-activated transcriptional factor that belongs to the family of nuclear receptors. PPAR-α regulates the expression of genes involved in fatty acid β-oxidation, and is a major regulator of energy homeostasis. It may also have an effect on microvascular and macrovascular disorders [25]. More specific PPAR-α agonists have been developed, and multiple PPAR agonists are currently assessed.
3.3 Niacin/nicotinic acid
Nicotinic acid is also known as water-soluble vitamin B3 (niacin). It is the oldest hypolipidemic agent, and has been used since 1955 [26]. Nicotinic acid is the most effective agent available for raising HDL-C levels and lowering TG levels [27]. However, its lipid-modifying mechanism remains unclear. The common view is that nicotinic acid inhibits the synthesis of TGs and VLDLs in the liver by inhibiting diacylglycerol acyl transferase-2 (DGAT-2), which contains a crucial enzyme for hepatic TG synthesis [28, 29]. Nicotinic acid decreases LDL levels by 5-25% and TG levels by 20-50% [30]. Moreover, niacin is the most effective agent currently used for raising HDL-C levels by increasing ApoA1 production and inhibiting the expression of the ATP-synthase β-chain receptor. It is also one of the few agents that can reduce lipoprotein (a) concentrations by 28-40% [31, 32].
A meta-regression study found that niacin can reduce all CVD events (OR: 0.66; p = 0.007) and major coronary heart disease events (OR: 0.75; p = 0.02) [33]. Its widespread use is limited by the flushing that is mediated by the activation of prostaglandin E synthesis and its hepatotoxicity. In a new trial of a non-flush niacin derivative, ARI-3037MO (Arisaph Pharmaceuticals; Boston, MA) was well tolerated and did not provoke flushing in 58 healthy men and women [34].
4. New classes of drugs: therapies for reducing LDL-cholesterol levels
4.1 Squalene synthase inhibitors
Statins have been extensively used to reduce LDL-C levels, and are effective in preventing heart disease. However, statins are associated with adverse side effects in some patients, and do not work effectively in others. Squalene synthase is a critical enzyme in the cholesterol biosynthesis pathway. Clinical studies have shown that squalene synthase inhibitors are effective in lowering plasma levels of total cholesterol and LDL-C through a reduction in cholesterol synthesis [35].
The process by which statins produce myalgia and muscle injury is unclear. Statins reduce the production of isoprenoids such as ubiquinone. Ubiquinone (coenzyme Q10) participates in electron transport during oxidative phosphorylation in mitochondria [36]. Statin treatment reduces serum ubiquinone levels, possibly because ubiquinone is transported in LDL-C particles. Reducing the level of ubiquinone alters the mitochondrial function in muscle, leading to muscle injury and myopathy. Inhibition of squalene synthase leads to a reduction in cholesterol synthesis without affecting the synthesis of ubiquinone. Therefore, it has the potential to reduce myalgia [37]. Lapaquistat acetate (TAK-475; Takeda; Osaka, Japan), alone or in combination with statins, effectively reduces levels of LDL-C and other cardiovascular risk markers such as C-reactive protein. However, the problem of hepatic dysfunction limits the development of this drug [38].
4.2 Microsomal transfer protein inhibitors
Microsomal triglyceride transfer protein (MTP) is a protein in the lumen of the endoplasmic reticulum. It is responsible for the transfer of triglyceride and other lipids from their site of synthesis in the endoplasmic reticulum into the lumen during the assembly of VLDLs [39]. VLDLs produced by the liver are the major sources of LDLs in plasma. MTP inhibitors have the potential to be used as a drug to lower plasma lipids (Figure 1). Specific inhibitors of MTP were shown to reduce LDL levels by 70%-80 and TG levels by 30%-40% [40].
Figure 1. Relationship of the different proteins in the hepatocytes involved in lipid modification.
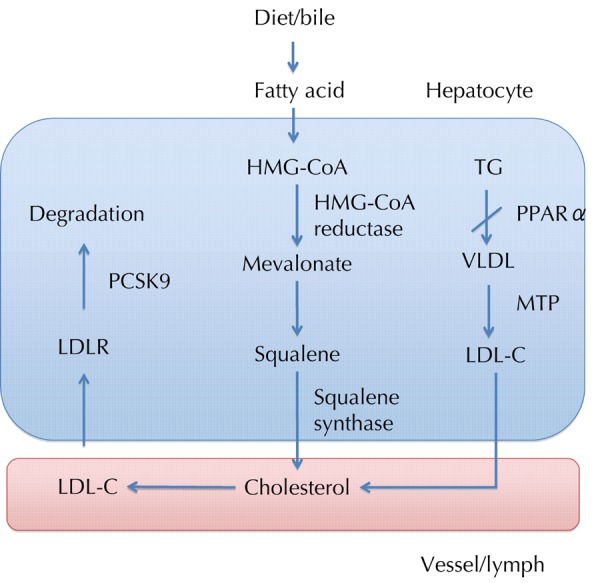
In hepatocytes, fatty acid is synthesized to cholesterol by the enzymes, HMG-CoA reductase and squalene synthase. Activating PPARα reduces TG accumulation in the liver, thus decreasing VLDL turnover. Microsomal TG transfer protein (MTP) is essential for the assembly and secretion of VLDL. Another alternative approach to lowering LDL-C is to limit hepatic assembly of VLDL, mediated by MTP. LDL-C is degraded via the LDL receptor (LDLR); PCSK9 promotes LDLR catabolism. The above proteins have been the potential targets for hypolipidemic agents.
Lomitapide (AEGR-733; Aegerion Pharmaceuticals) was proven effective in reducing LDL-C levels by 50.9% and ApoB levels by 55.6% in patients with homozygous familial hypercholesterolemia. The major adverse events were the elevation of liver aminotransferase levels and the accumulation of hepatic fat [41]. SLx-4090 (Nano Terra, Brighton, MA, USA) is an orally administered MTP inhibitor which was designed to act selectively in the enterocytes lining the GI tract. It prevents the formation of chylomicrons, which are used to transport triglyceride and cholesterol into the systemic circulation. SLx-4090 can reduce LDL-C and postprandial TG levels [42].
4.3 Thyroid hormone mimetics
Selective thyroid hormone, a β-selective mimetic, markedly reduced serum cholesterol levels, and produced weight loss without causing tachycardia [43]. However, the exact mechanisms remain unclear. One mechanism may involve the activation of the LDL-receptor pathway [44]. Eprotirome (KB-2115; Karo Bio AB), sobetirome (GC-1; QRX-431; QuatRx Inc, Ann Arbor, MI, USA), and T-0681 (Kissei Pharmaceutical Co.) are novel thyroid β-receptor agonists. In a 12-week trial of eprotirome, a thyroid hormone analogue, LDL-C levels were reduced by 7-32% in males and similar reductions were observed in levels of serum ApoB, TGs, and lipoprotein(a). No substantial hepatic or muscle dysfunction was observed [45].
4.4 Preprotein convertase subtilisin kexin-9 inhibitors (PCSK9)
PCSK9 has been recently identified as the third gene involved in intracellular and extracellular regulation of LDL receptor expression. PCSK9 plays a crucial role in post-translationally regulating the degradation of the LDL receptor (Figure 1). Inhibiting PCSK9 can potentially treat hypercholesterolemia [46]. PCSK-9 activity is associated with fasting insulinemia, postprandial lipid metabolism, and the hormonal control of lipids by estrogens, androgens, and growth hormone [47]. Numerous monoclonal antibodies to PCSK9 are currently in development, including REGN-727/SAR-236553 (Regeneron, Tarrytown, NY/SanofiAventis, Paris, France), AMG-145 (Amgen; Thousand Oaks, CA, USA), and NVP-LGT-209 (Novartis, Basel, Switzerland). In a recent phase 2 trial of REGN-727 in patients with heterozygous familial hypercholesterolemia and LDL-C concentrations of at least 2.6 mmol/l on a stable diet, REGN-727 was well tolerated and reduced LDL-C by 28.9-67.9% in various doses [48].
5. Therapies for raising HDL cholesterol
5.1 HDL-derived proteins and peptides
ApoA1 is a protein that is encoded by the APOA1 gene in humans and has a specific role in lipid metabolism. ApoA1 is the major protein component of HDL in plasma, and helps to clear cholesterol from arteries. ApoA1 is an excellent predictor for ischemic heart disease mortality. Serum ApoA1 is a more effective risk marker than ApoB, the ApoB/ApoA1 ratio, HDL-C, and LDL-C for CVD and mortality in elderly men [49]. CSL-112 (CSL Ltd, Parkville, Victoria, Australia), a human ApoA1 formulation, was designed to increase HDL levels. CSL-112 was infused into the blood of 18 healthy volunteers, and rapidly increased serum cholesterol efflux and the level of HDL-C. No changes were observed in TG or ApoB levels. CSL-112 is currently in phase 2 clinical development for the indication of acute coronary syndrome [50].
5.2 Combined peroxisomal proliferator activating receptor alpha-gamma agonists
Peroxisome proliferator-activated receptor (PPAR) plays a crucial role in lipid homeostasis and inflammatory responses (Figure 2). PPAR-α agonists (fibrates) can reduce levels of TG by 70% and LDL-C by 10-25% and raise HDL-C levels by 20%. However, it has a negligible effect on HbA1c levels. In the ACCORD study, fenofibrate added to simvastatin therapy for diabetes had slight beneficial effects on patients with moderate hypertriglyceridemia (>2.3 mmol/l) and low HDL-C levels (<0.88 mmol/l) [24].
Figure 2.
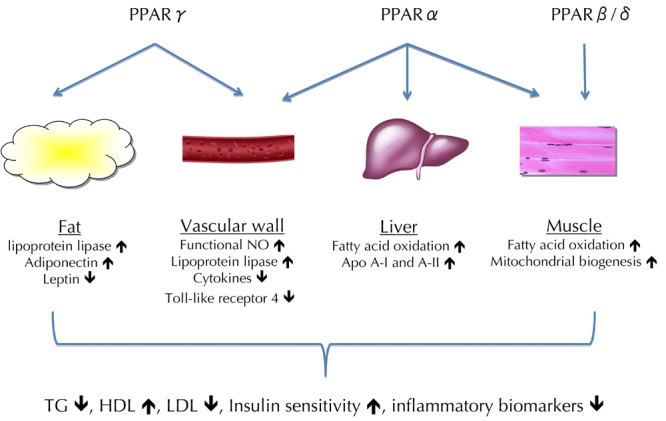
The three peroxisome proliferator-activated receptor (PPAR) subtypes have their unique and overlapping ligand specificity. Activating PPARβ/δ in muscle tissue leads to mitochondrial biogenesis and increased fatty acid oxidation. While PPARα and γ are activated in the liver, vascular wall, and adipose tissue, apolipoprotein A-I (ApoA-I) and A-II, nitric oxide (NO), lipoprotein lipase, and adiponectin are increased, and toll-like receptor 4, leptin, and inflammatory cytokines repressed. These changes in combination contribute to improved insulin sensitivity and a normalized lipid profile with decreased TG, increased HDL, and decreased small dense LDL levels.
PPAR-γ agonists (thiazolidinediones; glitazones) yielded a 0.5-1% reduction in HbA1c levels, a 5-15% reduction in TG levels, and a 0-4% increase in HDL levels [51]. Because PPARs are structurally homologous, it is possible to synthesize PPAR α-γ co-agonists. Clinical trials were conducted on several drugs (muraglitazar, tesaglitazar, and ragaglitazar). However, a number of studies were discontinued because of concerns regarding the development of bladder cancer in animal models (naveglitazar, MK-0767, and ragaglitazar). Only aleglitazar (R1439; Hoffman LaRoche, Basel, Switzerland) is still being studied in an ongoing phase 3 clinical trial.
Aleglitazar improves lipid profile in patients and decreases levels of the cardiovascular markers of inflammation and clotting [52]. In a phase 2 study involving 322 patients with type 2 diabetes, the drug reduced HbA1c levels by 0.36-1.35%, TG levels by 12-38%, and LDL-C levels by 3-22%, while raising HDL-C levels by 12-27%. No increased heart failure, weight gain, or fluid retention was observed at lower doses [53].
5.3 Peroxisomal proliferator-activating receptor (PPAR) delta agonists
Because of the limited clinical efficacy of PPAR-α and PPAR-γ agonists, attention has been turned to PPAR-β and PPAR-δ receptors. PPAR-α receptors affect liver tissue and PPAR-γ affects adipose tissue. In mouse studies, the PPAR-β/δ agonist improved lipid and glycemic profiles. PPAR-β/δ may be responsible for lipid and glucose management in the muscle [54]. GW501516 is a highly selective PPAR-δ agonist. In a 2-week study, 6 healthy moderately overweight men were treated with GW501516 and exhibited reductions in fasting plasma TG (-30%), ApoB (-26%), LDL-C (-23%), and insulin (-11%) levels, whereas HDL-C levels were unchanged [55]. A lipoprotein turnover study involving 13 dyslipidemic men with central obesity found that GW501516 decreased plasma TG, fatty acid, ApoB-100, and ApoB-48 concentrations. GW501516 increased the hepatic removal of VLDL particles through the ApoB-100 LDL receptor and by decreasing ApoC-III concentrations [56].
In a recent study involving 94 patients with mixed hyperlipidemia and 47 patients with prediabetes, a new PPAR α-δ co-agonist GFT-505 (Genfit, Loos, France) improved insulin resistance, reduced TG levels by 16.7% and 24.8%, and raised HDL-C levels by 7.8% and 9.2%, respectively [57].
6. Conclusions
This paper presents novel compounds for the treatment of hyperlipidemia and the lipid-related risk factors of cardiovascular disease. Statins are the most effective and safest drugs for reducing LDL levels. They exhibit parallel reductions in TG levels and a modest increase in HDL levels. Statins are available as monotherapy or as a part of combination products.
New agents with higher efficacy, safety, and tolerability than current agents are being tested for use as monotherapy. These agents also have the potential to be used in combination therapy. In current clinical trials, efficacy and safety are critical issues in demonstrating the benefits of novel drug combinations.
Disclosures: The authors report no conflict of interests.
References
- 1.Solano MP, Goldberg RB. Management of dyslipidemia in diabetes. Cardiol Rev. 2006;14(3):125–135. doi: 10.1097/01.crd.0000188034.76283.5e. [DOI] [PubMed] [Google Scholar]
- 2.Chahil TJ, Ginsberg HN. Diabetic dyslipidemia. Endocrinol Metab Clin North Am. 2006;35(3):491–510. doi: 10.1016/j.ecl.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2009;5(3):150–159. doi: 10.1038/ncpendmet1066. [DOI] [PubMed] [Google Scholar]
- 4.Buse JB, Ginsberg HN, Bakris GL, Clark NG, Costa F, Eckel R, Fonseca V, Gerstein HC, Grundy S, Nesto RW. et al. Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2007;115(1):114–126. doi: 10.1161/CIRCULATIONAHA.106.179294. [DOI] [PubMed] [Google Scholar]
- 5.Wu M, Lyons TJ. Treatment approaches for diabetes and dyslipidemia. Horm Res Paediatr. 2011;76(Suppl 1):76–80. doi: 10.1159/000329180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farmer JA. Diabetic dyslipidemia and atherosclerosis: evidence from clinical trials. Curr Diab Rep. 2008;8(1):71–77. doi: 10.1007/s11892-008-0013-2. [DOI] [PubMed] [Google Scholar]
- 7.Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witztum JL. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008;31(4):811–822. doi: 10.2337/dc08-9018. [DOI] [PubMed] [Google Scholar]
- 8.Sacks FM. The role of high-density lipoprotein (HDL) cholesterol in the prevention and treatment of coronary heart disease: expert group recommendations. Am J Cardiol. 2002;90(2):139–143. doi: 10.1016/s0002-9149(02)02436-0. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins AJ, Best JD, Klein RL, Lyons TJ. Lipoproteins, glycoxidation and diabetic angiopathy. Diabetes Metab Res Rev. 2004;20(5):349–368. doi: 10.1002/dmrr.491. [DOI] [PubMed] [Google Scholar]
- 10.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16(2):434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 11.Brunzell JD, Davidson M, Furberg CD, Goldberg RB, Howard BV, Stein JH, Witztum JL. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008;51(15):1512–1524. doi: 10.1016/j.jacc.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 12.Fruchart JC, Sacks F, Hermans MP, Assmann G, Brown WV, Ceska R, Chapman MJ, Dodson PM, Fioretto P, Ginsberg HN. et al. The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in patients with dyslipidemia. Am J Cardiol. 2008;102(10 Suppl):1K–34K. doi: 10.1016/S0002-9149(08)01833-X. [DOI] [PubMed] [Google Scholar]
- 13.Taskinen MR. Quantitative and qualitative lipoprotein abnormalities in diabetes mellitus. Diabetes. 1992;41(Suppl 2):12–17. doi: 10.2337/diab.41.2.s12. [DOI] [PubMed] [Google Scholar]
- 14.Eldor R, Raz I. American Diabetes Association indications for statins in diabetes: is there evidence? Diabetes Care. 2009;32(Suppl 2):S384–S391. doi: 10.2337/dc09-S345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Fuller JH. Rapid emergence of effect of atorvastatin on cardiovascular outcomes in the Collaborative Atorvastatin Diabetes Study (CARDS) Diabetologia. 2005;48(12):2482–2485. doi: 10.1007/s00125-005-0029-y. [DOI] [PubMed] [Google Scholar]
- 16.Carter AA GT, Camacho X, Juurlink DN, Shah BR, Mamdani MM. Risk of incident diabetes among patients treated with statins: population based study. BMJ. 2013;346:f2610. doi: 10.1136/bmj.f2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, Seshasai SR, McMurray JJ, Freeman DJ, Jukema JW. et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 18.Waters DD, Ho JE, Boekholdt SM, DeMicco DA, Kastelein JJ, Messig M, Breazna A, Pedersen TR. Cardiovascular event reduction versus new-onset diabetes during atorvastatin therapy: effect of baseline risk factors for diabetes. J Am Coll Cardiol. 2013;61(2):148–152. doi: 10.1016/j.jacc.2012.09.042. [DOI] [PubMed] [Google Scholar]
- 19.Kadowaki T. Japan Prevention Trial of Diabetes by Pitavastatin in Patients with Impaired Glucose Tolerance (the J-PREDICT study): rationale, study design, and clinical characteristics of 1269 patients. Diabetol Int. 2011;2(3):134–140. [Google Scholar]
- 20.Wierzbicki AS, Poston R, Ferro A. The lipid and non-lipid effects of statins. Pharmacol Ther. 2003;99(1):95–112. doi: 10.1016/s0163-7258(03)00055-x. [DOI] [PubMed] [Google Scholar]
- 21.Davidson MH, McGarry T, Bettis R, Melani L, Lipka LJ, LeBeaut AP, Suresh R, Sun S, Veltri EP. Ezetimibe coadministered with simvastatin in patients with primary hypercholesterolemia. J Am Coll Cardiol. 2002;40(12):2125–2134. doi: 10.1016/s0735-1097(02)02610-4. [DOI] [PubMed] [Google Scholar]
- 22.Djian JP MR, Guilmin L, Ferreira T, Pfister P. Abstract 14267: Ncx 6560, a novel nitric oxyde donating atorvastatin with a promising safety and efficacy profile: a randomised, double blind placebo and active control study. Circulation. 2010;122:A14267. [Google Scholar]
- 23.Study of the safety and tolerability associated with PPD10558 versus atorvastatin in patients previously intolerant to statins due to statin-associated myalgia (SAM) Furiex Pharamceuticals Inc. http://clinicaltrials.gov/ct2/show/NCT01279590.
- 24.Wierzbicki AS. Fibrates: no ACCORD on their use in the treatment of dyslipidaemia. Curr Opin Lipidol. 2010;21(4):352–358. doi: 10.1097/MOL.0b013e32833c1e74. [DOI] [PubMed] [Google Scholar]
- 25.Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E. et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370(9600):1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 26.Altschul R, Hoffer A, Stephen JD. Influence of nicotinic acid on serum cholesterol in man. Arch Biochem Biophys. 1955;54(2):558–559. doi: 10.1016/0003-9861(55)90070-9. [DOI] [PubMed] [Google Scholar]
- 27.Dunn FL. Management of dyslipidemia in people with type 2 diabetes mellitus. Rev Endocr Metab Disord. 2010;11(1):41–51. doi: 10.1007/s11154-010-9132-6. [DOI] [PubMed] [Google Scholar]
- 28.Wierzbicki AS. Niacin: the only vitamin that reduces cardiovascular events. Int J Clin Pract. 2011;65(4):379–385. doi: 10.1111/j.1742-1241.2011.02630.x. [DOI] [PubMed] [Google Scholar]
- 29.Kei A. Nicotinic acid: Do we know how it works after 55 years of clinical experience? World J Pharmacol. 2012;1(3):50–54. [Google Scholar]
- 30.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 31.Capuzzi DM, Guyton JR, Morgan JM, Goldberg AC, Kreisberg RA, Brusco OA, Brody J. Efficacy and safety of an extended-release niacin (Niaspan): a long-term study. Am J Cardiol. 1998;82(12A):74U–81U. doi: 10.1016/s0002-9149(98)00731-0. [DOI] [PubMed] [Google Scholar]
- 32.Vogt A, Kassner U, Hostalek U, Steinhagen-Thiessen E. Evaluation of the safety and tolerability of prolonged-release nicotinic acid in a usual care setting: the NAUTILUS study. Curr Med Res Opin. 2006;22(2):417–425. doi: 10.1185/030079906x89766. [DOI] [PubMed] [Google Scholar]
- 33.Lavigne PM, Karas RH. The Current State of Niacin in Cardiovascular Disease Prevention: A Systematic Review and Meta-Regression. J Am Coll Cardiol. 2012 doi: 10.1016/j.jacc.2012.10.030. In press. [DOI] [PubMed] [Google Scholar]
- 34.Claude Benedict JN, William Bachovchin, Christopher Kiritsy. A novel niacin analog (ARI-3037MO) induces favorable plasma lipid changes without flush in single and multiple ascending dose placebo controlled trials in normal healthy volunteers. American heart association 2012; Abstract 9595 (Los Angeles California) [Google Scholar]
- 35.Do R, Kiss RS, Gaudet D, Engert JC. Squalene synthase: a critical enzyme in the cholesterol biosynthesis pathway. Clin Genet. 2009;75(1):19–29. doi: 10.1111/j.1399-0004.2008.01099.x. [DOI] [PubMed] [Google Scholar]
- 36.Ghirlanda G, Oradei A, Manto A, Lippa S, Uccioli L, Caputo S, Greco AV, Littarru GP. Evidence of plasma CoQ10-lowering effect by HMG-CoA reductase inhibitors: a double-blind, placebo-controlled study. J Clin Pharmacol. 1993;33(3):226–229. doi: 10.1002/j.1552-4604.1993.tb03948.x. [DOI] [PubMed] [Google Scholar]
- 37.Seiki S, Frishman WH. Pharmacologic inhibition of squalene synthase and other downstream enzymes of the cholesterol synthesis pathway: a new therapeutic approach to treatment of hypercholesterolemia. Cardiol Rev. 2009;17(2):70–76. doi: 10.1097/CRD.0b013e3181885905. [DOI] [PubMed] [Google Scholar]
- 38.Stein EA, Bays H, O'Brien D, Pedicano J, Piper E, Spezzi A. Lapaquistat acetate: development of a squalene synthase inhibitor for the treatment of hypercholesterolemia. Circulation. 2011;123(18):1974–1985. doi: 10.1161/CIRCULATIONAHA.110.975284. [DOI] [PubMed] [Google Scholar]
- 39.Hussain MM, Iqbal J, Anwar K, Rava P, Dai K. Microsomal triglyceride transfer protein: a multifunctional protein. Front Biosci. 2003;8:s500–s506. doi: 10.2741/1071. [DOI] [PubMed] [Google Scholar]
- 40.Wierzbicki AS, Hardman T, Prince WT. Future challenges for microsomal transport protein inhibitors. Curr Vasc Pharmacol. 2009;7(3):277–286. doi: 10.2174/157016109788340703. [DOI] [PubMed] [Google Scholar]
- 41.Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, Sarkis A, Millar JS, Ikewaki K, Siegelman ES, Gregg RE, Rader DJ. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007;356(2):148–156. doi: 10.1056/NEJMoa061189. [DOI] [PubMed] [Google Scholar]
- 42.Burnett JR, Watts GF. MTP inhibition as a treatment for dyslipidaemias: time to deliver or empty promises? Expert Opin Ther Targets. 2007;11(2):181–189. doi: 10.1517/14728222.11.2.181. [DOI] [PubMed] [Google Scholar]
- 43.Grover GJ, Egan DM, Sleph PG, Beehler BC, Chiellini G, Nguyen NH, Baxter JD, Scanlan TS. Effects of the thyroid hormone receptor agonist GC-1 on metabolic rate and cholesterol in rats and primates: selective actions relative to 3,5,3’-triiodo-L-thyronine. Endocrinology. 2004;145(4):1656–1661. doi: 10.1210/en.2003-0973. [DOI] [PubMed] [Google Scholar]
- 44.Salter AM, Hayashi R, al-Seeni M, Brown NF, Bruce J, Sorensen O, Atkinson EA, Middleton B, Bleackley RC, Brindley DN. Effects of hypothyroidism and high-fat feeding on mRNA concentrations for the low-density-lipoprotein receptor and on acyl-CoA:cholesterol acyltransferase activities in rat liver. Biochem J. 1991;276(Pt 3):825–832. doi: 10.1042/bj2760825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ladenson PW, Kristensen JD, Ridgway EC, Olsson AG, Carlsson B, Klein I, Baxter JD, Angelin B. Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N Engl J Med. 2010;362(10):906–916. doi: 10.1056/NEJMoa0905633. [DOI] [PubMed] [Google Scholar]
- 46.Tibolla G, Norata GD, Artali R, Meneghetti F, Catapano AL. Proprotein convertase subtilisin/kexin type 9 (PCSK9): from structure-function relation to therapeutic inhibition. Nutr Metab Cardiovasc Dis. 2011;21(11):835–843. doi: 10.1016/j.numecd.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Baass A, Dubuc G, Tremblay M, Delvin EE, O'Loughlin J, Levy E, Davignon J, Lambert M. Plasma PCSK9 is associated with age, sex, and multiple metabolic markers in a population-based sample of children and adolescents. Clin Chem. 2009;55(9):1637–1645. doi: 10.1373/clinchem.2009.126987. [DOI] [PubMed] [Google Scholar]
- 48.Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, Wu R, Pordy R. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380(9836):29–36. doi: 10.1016/S0140-6736(12)60771-5. [DOI] [PubMed] [Google Scholar]
- 49.Florvall G, Basu S, Larsson A. Apolipoprotein A1 is a stronger prognostic marker than are HDL and LDL cholesterol for cardiovascular disease and mortality in elderly men. J Gerontol A Biol Sci Med Sci. 2006;61(12):1262–1266. doi: 10.1093/gerona/61.12.1262. [DOI] [PubMed] [Google Scholar]
- 50.Gille A, Wright S, Shear C. A novel formulation of human apolipoprotein AI, provides sustained increases in biomarkers of cholesterol transport following repeat dosing: A placebocontrolled, randomized multiple ascending dose study in healthy subjects. American heart association 2012; Abstract 11851. [Google Scholar]
- 51.Stumvoll M, Haring HU. Glitazones: clinical effects and molecular mechanisms. Ann Med. 2002;34(3):217–224. [PubMed] [Google Scholar]
- 52.Lecka-Czernik B. Aleglitazar, a dual PPARalpha and PPARgamma agonist for the potential oral treatment of type 2 diabetes mellitus. IDrugs. 2010;13(11):793–801. [PubMed] [Google Scholar]
- 53.Henry RR, Lincoff AM, Mudaliar S, Rabbia M, Chognot C, Herz M. Effect of the dual peroxisome proliferator-activated receptor-alpha/gamma agonist aleglitazar on risk of cardiovascular disease in patients with type 2 diabetes (SYNCHRONY): a phase II, randomised, dose-ranging study. Lancet. 2009;374(9684):126–135. doi: 10.1016/S0140-6736(09)60870-9. [DOI] [PubMed] [Google Scholar]
- 54.Lee CH, Olson P, Evans RM. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003;144(6):2201–2207. doi: 10.1210/en.2003-0288. [DOI] [PubMed] [Google Scholar]
- 55.Riserus U, Sprecher D, Johnson T, Olson E, Hirschberg S, Liu A, Fang Z, Hegde P, Richards D, Sarov-Blat L. et al. Activation of peroxisome proliferator-activated receptor (PPAR)delta promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes. 2008;57(2):332–339. doi: 10.2337/db07-1318. [DOI] [PubMed] [Google Scholar]
- 56.Ooi EM, Watts GF, Sprecher DL, Chan DC, Barrett PH. Mechanism of action of a peroxisome proliferator-activated receptor (PPAR)-delta agonist on lipoprotein metabolism in dyslipidemic subjects with central obesity. J Clin Endocrinol Metab. 2011;96(10):E1568–E1576. doi: 10.1210/jc.2011-1131. [DOI] [PubMed] [Google Scholar]
- 57.Cariou B, Zair Y, Staels B, Bruckert E. Effects of the new dual PPAR alpha/delta agonist GFT505 on lipid and glucose homeostasis in abdominally obese patients with combined dyslipidemia or impaired glucose metabolism. Diabetes Care. 2011;34(9):2008–2014. doi: 10.2337/dc11-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Standards of medical care in diabetes 2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]


