Abstract
Background:
One of the most important challenges in health care system is prevention of preterm birth. The present study was aimed to investigate the relation between interleukins 6 and 8 (IL-6 and IL-8) with preterm labor and response to tocolytic therapy.
Materials and Methods:
In the year 2012, 75 women with the symptoms of preterm labor (cases) in compare with 75 term women (controls) were randomly selected and evaluated. Baseline data and serum levels of IL-6 and IL-8 (using immunoassay method) recorded. Hence, tocolysis in women in case group was performed with the use of magnesium sulfate and then they were followed until delivery time to assess the response to treatment.
Results:
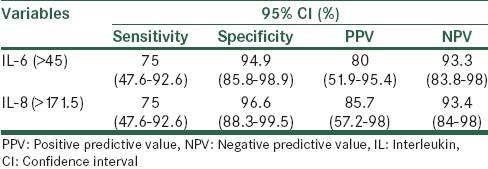
In case group, 59 women response to tocolytic treatment and delivered at term but 16 of them delivered prematurely. The curve constructed cut-off value for IL-6 was >37.9 (area under the curve [AUC], 0.674; standard error [SE], 0.043; P < 0.0001), and > 9.5 for IL-8 (AUC, 0.773; SE, 0.038; P < 0.0001), indicating a significant relationship with preterm labor. Furthermore, there was a significant relationship between serum IL-6 and IL-8 levels with the response to the treatment in cut-off >45 for IL-6 (AUC, 0.894; SE, 0.042; P < 0.0001) and >171 for IL-8 (AUC, 0.864; SE, 0.059; P < 0.0001).
Conclusion:
In summary, our results suggest that the assessment of maternal serum concentrations of IL-6 and IL-8 can be used as appropriate biomarkers for predicting preterm labor and response to tocolytic therapy in these women. However, further studies needs to be done.
Keywords: Cytokines, interleukin, preterm labor, tocolytic
INTRODUCTION
Preterm birth by World Health Organization defined as childbirth occurring at <259 days of gestation or 37 completed weeks since the 1st day of the women's last menstrual period (LMP) and its frequency ranges is from 5% to 13% in high income countries and the incidence is increasing.[1,2] Higher morbidity and mortality rates was observed in infants born prematurely compared with term-birth infants.[3] Furthermore, in almost all high-income and middle-income countries is the leading cause of child deaths and perinatal morbidity and mortality world-wide.[4] The rate of annual neonatal deaths is reported 3.1 million in the world, which, preterm birth complications are estimated to be answerable for 35% of them.[4] Either, in children under 5 years old, after pneumonia, is the second most common cause of death[5] and has lifelong effects on increased risk of cerebral palsy and an increased risk of chronic disease in adulthood.[6] Across the globe, preterm birth is an important perinatal health problem with respect to short- and long-term morbidity and financial implications and has high economic and social cost in terms of neonatal intensive care, for families and health care systems.[7,8]
Spontaneous preterm birth is known as the cause of around half the preterm births, for which there are no known effective prevention measures.[1] Early detection of preterm labor is hard because primary symptoms are often mild and later symptoms often occur too late to intervene.[9] Obstetrical history or symptoms and epidemiological risk factors, as usually used methods to predict preterm delivery, are neither sensitive nor specific.[10,11] Prevent unnecessary and potentially risky interventions and reduce treatment costs may occur in the presence of a method to rapidly and accurately differentiate women who are probable to deliver preterm infants from those who have apparent preterm labor and are unlikely to deliver prematurely.[10]
Cytokines have been explored as biomarkers of impending preterm labor but there is a paucity of data in the current literature concerning the association between maternal serum concentrations of interleukins 6 and 8 (IL-6 and IL-8) and preterm delivery.[12] The presence of increased concentration of IL-6 and IL-8 in a variety of biological fluids including maternal and/or fetal blood, amniotic fluid, urine, cervical and/or vaginal secretions and placental tissue is an independent risk factor for preterm labor.[13,14,15,16,17,18] Some studies observed that elevated maternal serum IL-6 concentration is a risk factor for preterm birth <32 weeks;[7,9,19] conversely, other study stated that maternal serum levels of IL-6 and IL-8 were not increased in preterm labor compared to normal control women.[20]
Even though tocolytics have not been shown to improve neonatal outcomes, they are an important intervention in obstetrics to delay preterm delivery.[21,22] The aim of tocolysis is to delay preterm delivery long enough for antenatal corticosteroids to be administered or for the mother to be transported to a tertiary care center, thereby reducing neonatal morbidity and mortality.[23,24] The use of tocolytic agents in contemporary obstetric practice should be customized and based on the available evidence base for efficacy and fetomaternal safety, gestational age, the maternal condition and potential side-effects of the drug.[25] Magnesium sulfate is one of tocolytic agents which the administration of this to women at risk of preterm birth helps to protect the baby's brain, reduce rates of cerebral palsy and improve long-term neonatal health outcomes and despite maternal side effects, magnesium sulfate is commonly used for tocolysis.[21,26]
The present study was designed to hypothesized two purposes; first, evaluation of maternal serum concentrations of IL-6 and IL-8 in the prediction of women at risk of preterm and second; the evaluation of maternal serum concentrations of IL-6 and IL-8 in the prediction of response to tocolytic treatment in these women.
MATERIALS AND METHODS
Between May, 2012 and October, 2012 a total of 150 singleton pregnant women who had been referred to “Shahid Beheshti” hospital in Isfahan, Iran, were included in the present investigation. The Institutional Review Board at the Isfahan University of Medical Sciences approved the study and all participants provided signatures for informed consent. Seventy five women with the diagnosis of new-onset preterm labor made up the case group. Preterm labor was defined as painful contractions at least every 10 min, documented, despite hydration, for 1 h on an external tocodynamometer or regular labor detected by cardiotocography resulting in a cervical change. None of the women in case group showed clinical signs of infection or of any other maternal or fetal complications. The control group consisted of 75 healthy women in term labor, with uncomplicated gestation.
Criteria for study inclusion in the review were age range between 18 and 35 years old, singleton pregnancy, gestational age of 24-34 complete weeks (was proved by LMP and sonography in the first trimester of pregnancy), less than five parturition, no history of type 1 or 2 diabetes mellitus, hypertension, cardiovascular disease and infectious diseases. Exclusion criteria were: Contraindication by using tocolytic drugs, fetus or amniotic fluid anomaly, uterian or cervical abnormality, emerging some undesirable conditions during parturition such as preeclampsia and abruption.
Baseline data were collected to assess comparability of the study groups, also, serum that was collected at gestational age of 24 and 34 weeks was analyzed for determined the levels of IL-6 and IL-8 using immunoassay method. After that tocolysis in women in case group was performed with the use of magnesium sulfate as follow; first with an infusion of 4 g magnesium sulfate 20% and then 2 g/h continued, then they were followed till delivery time to assess the response to treatment. Term and preterm delivery as an outcome of tocolytic therapy was the gold standard assess the response to treatment.
The sample size was calculated using the comparison of proportions formula with two-sided log-rank test, α = 0.05 and 80% power. Statistical analysis was carried out with the SPSS version 20 (SPSS IBM, New York, U.S.A). Demographic and biological measures were analyzed descriptively using means ± standard deviation for continuous variables and number (%) for categorical variables. Chi-square test was used to compare categorical variables and independent sample t-test was used to compare continuous variables. For serum IL-6 and IL-8, a receiver operating characteristic (ROC) curve analysis was used to establish the cut-off values that optimized the prediction of preterm labor or response to tocolytic therapy. Sensitivity, specificity, positive predictive value (PPV) and negative predictive values (NPV) were then calculated. In addition, areas under the curves (AUC) were evaluated for all study tests. A two-sided P < 0.05 was considered to indicate statistical significance.
RESULTS
The mean age for the samples was 27.8 ± 4.5 years old. All women from the control group delivered at term and 59 women from the case group after successful tocolytic treatment delivered at term, but 16 of them despite tocolytic therapy delivered prematurely.
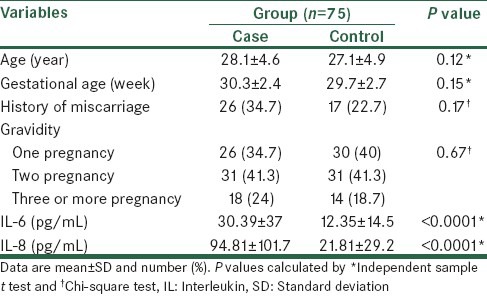
Table 1 shows the comparison of sample characteristics and the concentration of IL-6 and IL-8 between study group and control group. As shown, age, gestational age, history of miscarriage and gravidity in both groups were similar and there were no significant differences between case and control groups for these variables. The concentrations of maternal serum of IL-6 and IL-8 levels in women with symptoms of preterm labor were significantly higher than women with term labor.
Table 1.
Comparison of characteristics, and the concentration of IL-6 and IL-8 between study groups
Relation between gestational age and IL-6 and IL-8 levels was assessed by Pearson correlation coefficient in all patients and case group. The results showed that Pearson correlation in all patients was−0.038, P=0.64 for IL-8 and 0.024, P = 0.77 for IL-6; also, Pearson correlation in case group was−0.178, P = 0.13 for IL-8 and−0.082, P = 0.48 for IL-6.
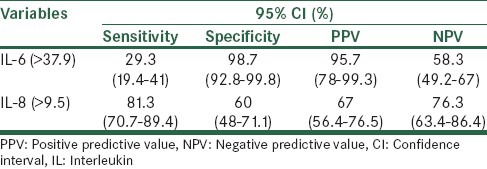
To determine the critical values that could predict preterm labor, ROC curves were generated for IL-6 and IL-8. The curve constructed for IL-6 was >37.9 (AUC, 0.674; standard error [SE], 0.043; P < 0.0001) and >9.5 for IL-8 (AUC, 0.773; SE, 0.038; P < 0.0001), indicating a significant relationship with preterm labor [Figure 1]. Cut-off values and other predictive values for IL-6 and IL-8 to predict preterm labor are shown in Table 2.
Figure 1.
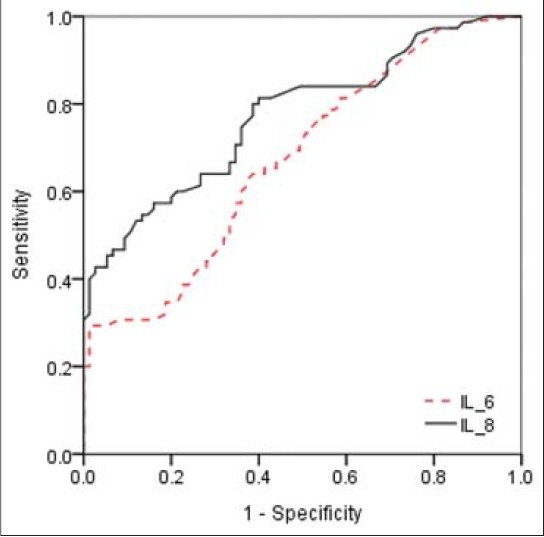
Receiver operating characteristic curves for maternal serum interleukin (IL)-6 (area under the curve [AUC], 0.674; standard error [SE], 0.043; P< 0.0001) and IL-8 (AUC, 0.773; SE, 0.038; P< 0.0001) levels for predicting preterm labor in pregnant women
Table 2.
The prognostic value of evaluation of maternal serum IL-6 and IL-8 for prediction of preterm labor
Based on results of tocolytic therapy women in case group were assessed in two groups; women with successful treatment (n=59) with women with unsuccessful tocolytic treatment (n = 16). Age, gestational age, history of miscarriage, gravidity and serum IL-6 and IL-8 levels were compared between these women, there were no significant differences for age, gestational age, history of miscarriage and gravidity. In women with unsuccessful tocolytic treatment levels of IL-6 and IL-8 were significantly higher than women with successful treatment [Table 3].
Table 3.
Comparison of characteristics, and the concentration of IL-6 and IL-8 between cases regard to response to tocolytic therapy
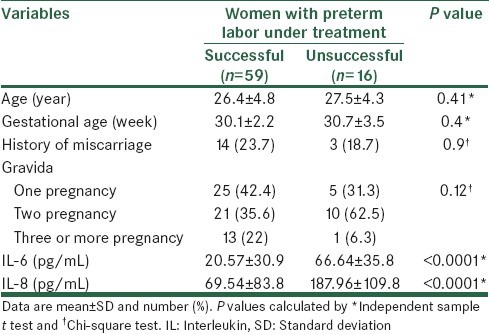
Figure 2 is a ROC curve describing the values of the serum IL-6 and IL-8 levels in predicting response to tocolytic therapy in women with symptoms of preterm labor. There was a significant relationship between serum IL-6 and IL-8 levels with the response to the treatment in cut-off >45 for IL-6 (AUC, 0.894; SE, 0.042; P < 0.0001) and >171 for IL-8 (AUC, 0.864; SE, 0.059; P < 0.0001). Furthermore, the sensitivity, specificity, PPV and NPV of an elevated serum IL-6 and IL-8 levels to predict response to tocolytic therapy in preterm women are shown in Table 4.
Figure 2.
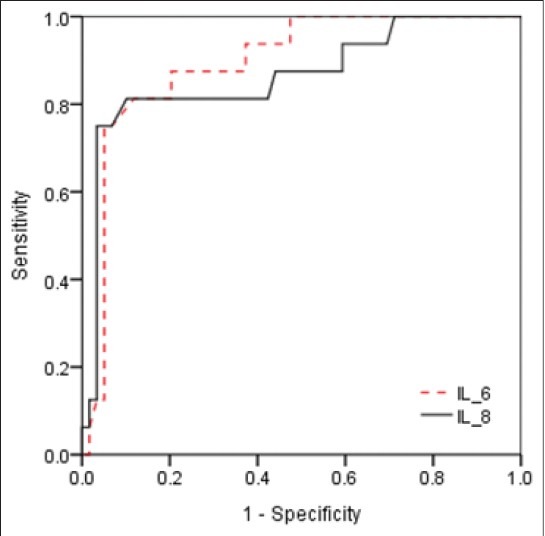
Receiver operating characteristic curves for maternal serum interleukin (IL)-6 (area under the curve [AUC], 0.894; standard error [SE], 0.042; P< 0.0001) and IL-8 (AUC, 0.864; SE, 0.059; P< 0.0001) levels for predicting response to treatment in women with symptoms of preterm labor
Table 4.
The prognostic value of evaluation of maternal serum IL-6 and IL-8 in preterm women for prediction of response to tocolytic therapy
DISCUSSION
One of the most important challenges in modern maternity care is the prevention of preterm birth.[2] In the presented study, several cytokines were detected in the serum of pregnant women to assess the correlation the concentrations with preterm labor and response to treatment in pregnant women. In this study, a marked correlation of elevated IL-6 and IL-8 levels in women with preterm labor was observed when compared with healthy pregnant women, whereas the best cut-off values of IL-6 > 37.9 and IL-8 > 9.5 with a sensitivity of 29.3, 81.3% and specificity of 98.7 and 60%, respectively, were the best values in the prediction of preterm labor. In addition, elevated serum IL-6 and IL-8 levels in preterm women with successful tocolytic therapy were markedly higher than preterm women with unsuccessful tocolytic therapy. We find that cut-off values of IL-6 > 45 and IL-8 > 171.5 with a sensitivity of 75% and specificity of 94.9 and 96.6%, respectively, were the best values in the prognosis of successful tocolytic therapy in preterm women.
Similar to our findings previous studies[1,17,20,27,28,29] found that significant increase in the serum levels of IL-6 in patients with preterm labor was observed compared to the control group. Furthermore, von Minckwitz et al.[14] showed that maternal serum IL-8 concentration in patients with preterm labor was significantly higher than term women. Either Alvarez-de-la-Rosa et al.[30] noted that the concentration of IL-6 and IL-8 in preterm women were higher in comparison to those who were not in labor, although this difference was not statistically significant. Dissimilarity to our findings in another study Bahar et al.[20] reported that between women in preterm labor compared to term women there was no statistically significant difference in maternal serum cytokine concentrations measured. The differences in assay methods or differences in the sensitivity of different kits may be clarified the variations between results of these studies, however, most of these studies in agreement with the present study showed high levels of IL-6 and IL-8 in preterm labor compared with term women.
The association between maternal serum concentrations of IL-6 and IL-8 with early preterm delivery, have explored by few studies in women that are at increased risk for preterm delivery. In a nested case control study, authors reported that for preterm birth before 35 weeks, maternal serum concentration of IL-6 was not a significant marker.[31] Moreover, this was in disagreement with our results. Another study suggested that elevated maternal serum concentration of IL-6 is a risk factor for early preterm delivery before 32 weeks.[19] A study done by Vogel et al.[15] found that before 35 weeks, high maternal serum concentration of IL-6 was associated with an increased risk of spontaneous preterm birth. Either it is reported that serum IL-6 seems to be a biomarker for the identification of women with preterm.[32] In agreement with these studies results of the present study showed that serum concentration of IL-6 is a risk factor for predict preterm labor before 34 weeks. Whereas at cut-off value of IL-6 >37.9 with specificity of 98.7%, PPV is 95.7% and investigated that this value can be used as a suitable predicting marker for preterm labor.
IL-6 and IL-8 can be measured in serum using enzyme-linked immunosorbent assay techniques which gives the opportunity to detect these cytokines easily in routine practice. And evaluate their impact on preterm treatment results can be useful. Therefore, in this study we hypothesized that response to tocolytic therapy in women that are at increased risk for preterm labor can be predict by evaluation of maternal serum concentrations of IL-6 and IL-8. Our results showed that serum concentration of IL-6 and IL-8 are good marker to predict response to tocolytic therapy in preterm women before 34 weeks. And both of these markers have good specificity and PPV at and reveled that cut-off values of >45 for IL-6 and >171.5 for IL-8 can be used as an appropriate biomarkers values to predicting response to tocolytic therapy. Due to deficiency of research in the evaluation of the probably role of cytokines in prediction of response to tocolytic therapy in preterm women and importance of prevention of preterm labor, results of the present study can be noted as basement for more researches to finding possible relations.
In summary, our observations suggest that the assessment of maternal serum concentrations of IL-6 and IL-8 can be used as suitable biomarkers for predicting preterm labor and response to tocolytic therapy in these women. However, this observation needs further studies to assess more than these biologic markers as predictor.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts CL, Morris JM, Rickard KR, Giles WB, Simpson JM, Kotsiou G, et al. Protocol for a randomised controlled trial of treatment of asymptomatic candidiasis for the prevention of preterm births ACTRN12610000607077. BMC Pregnancy Childbirth. 2011;11:19. doi: 10.1186/1471-2393-11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoyert DL, Mathews TJ, Menacker F, Strobino DM, Guyer B. Annual summary of vital statistics: 2004. Pediatrics. 2006;117:168–83. doi: 10.1542/peds.2005-2587. [DOI] [PubMed] [Google Scholar]
- 4.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–61. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 5.Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, de Bernis L, et al. Evidence-based, cost-effective interventions: How many newborn babies can we save? Lancet. 2005;365:977–88. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- 6.Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: A systematic review. Lancet. 2012;379:445–52. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shahshahan Z, Hashemi M. Crown-rump length discordance in twins in the first trimester and its correlation with perinatal complications. J Res Med Sci. 2011;16:1224–7. [PMC free article] [PubMed] [Google Scholar]
- 8.Green NS, Damus K, Simpson JL, Iams J, Reece EA, Hobel CJ, et al. Research agenda for preterm birth: Recommendations from the March of Dimes. Am J Obstet Gynecol. 2005;193:626–35. doi: 10.1016/j.ajog.2005.02.106. [DOI] [PubMed] [Google Scholar]
- 9.Woodworth A, Moore J, G’Sell C, Verdoes A, Snyder JA, Morris L, et al. Diagnostic accuracy of cervicovaginal interleukin-6 and interleukin-6: Albumin ratio as markers of preterm delivery. Clin Chem. 2007;53:1534–40. doi: 10.1373/clinchem.2006.084798. [DOI] [PubMed] [Google Scholar]
- 10.Lockwood CJ, Kuczynski E. Risk stratification and pathological mechanisms in preterm delivery. Paediatr Perinat Epidemiol. 2001;15(Suppl 2):78–89. doi: 10.1046/j.1365-3016.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- 11.Mercer BM, Goldenberg RL, Das A, Moawad AH, Iams JD, Meis PJ, et al. The preterm prediction study: A clinical risk assessment system. Am J Obstet Gynecol. 1996;174:1885–93. doi: 10.1016/s0002-9378(96)70225-9. [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, Mitchell MD. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins. 1989;37:13–22. doi: 10.1016/0090-6980(89)90028-2. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa K, Furuichi Y, Shimotsu A, Nakamura M, Yoshinaga M, Kamitomo M, et al. Associations between systemic status, periodontal status, serum cytokine levels, and delivery outcomes in pregnant women with a diagnosis of threatened premature labor. J Periodontol. 2003;74:1764–70. doi: 10.1902/jop.2003.74.12.1764. [DOI] [PubMed] [Google Scholar]
- 14.von Minckwitz G, Grischke EM, Schwab S, Hettinger S, Loibl S, Aulmann M, et al. Predictive value of serum interleukin-6 and-8 levels in preterm labor or rupture of the membranes. Acta Obstet Gynecol Scand. 2000;79:667–72. [PubMed] [Google Scholar]
- 15.Vogel I, Goepfert AR, Thorsen P, Skogstrand K, Hougaard DM, Curry AH, et al. Early second-trimester inflammatory markers and short cervical length and the risk of recurrent preterm birth. J Reprod Immunol. 2007;75:133–40. doi: 10.1016/j.jri.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Torbé A, Czajka R, Kordek A, Rzepka R, Kwiatkowski S, Rudnicki J. Maternal serum proinflammatory cytokines in preterm labor with intact membranes: Neonatal outcome and histological associations. Eur Cytokine Netw. 2007;18:102–7. doi: 10.1684/ecn.2007.0092. [DOI] [PubMed] [Google Scholar]
- 17.Murtha AP, Greig PC, Jimmerson CE, Herbert WN. Maternal serum interleukin-6 concentration as a marker for impending preterm delivery. Obstet Gynecol. 1998;91:161–4. doi: 10.1016/s0029-7844(97)00602-9. [DOI] [PubMed] [Google Scholar]
- 18.Murtha AP, Greig PC, Jimmerson CE, Roitman-Johnson B, Allen J, Herbert WN. Maternal serum interleukin-6 concentrations in patients with preterm premature rupture of membranes and evidence of infection. Am J Obstet Gynecol. 1996;175:966–9. doi: 10.1016/s0002-9378(96)80033-0. [DOI] [PubMed] [Google Scholar]
- 19.Sorokin Y, Romero R, Mele L, Wapner RJ, Iams JD, Dudley DJ, et al. Maternal serum interleukin-6, C-reactive protein, and matrix metalloproteinase-9 concentrations as risk factors for preterm birth <32 weeks and adverse neonatal outcomes. Am J Perinatol. 2010;27:631–40. doi: 10.1055/s-0030-1249366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahar AM, Ghalib HW, Moosa RA, Zaki ZM, Thomas C, Nabri OA. Maternal serum interleukin-6, interleukin-8, tumor necrosis factor-alpha and interferon-gamma in preterm labor. Acta Obstet Gynecol Scand. 2003;82:543–9. [PubMed] [Google Scholar]
- 21.Kawagoe Y, Sameshima H, Ikenoue T, Yasuhi I, Kawarabayashi T. Magnesium sulfate as a second-line tocolytic agent for preterm labor: A randomized controlled trial in Kyushu Island. J Pregnancy 2011. 2011:1–6. doi: 10.1155/2011/965060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haas DM, Imperiale TF, Kirkpatrick PR, Klein RW, Zollinger TW, Golichowski AM. Tocolytic therapy: A meta-analysis and decision analysis. Obstet Gynecol. 2009;113:585–94. doi: 10.1097/AOG.0b013e318199924a. [DOI] [PubMed] [Google Scholar]
- 23.Kam KY, Lamont RF. Developments in the pharmacotherapeutic management of spontaneous preterm labor. Expert Opin Pharmacother. 2008;9:1153–68. doi: 10.1517/14656566.9.7.1153. [DOI] [PubMed] [Google Scholar]
- 24.Simhan HN, Caritis SN. Prevention of preterm delivery. N Engl J Med. 2007;357:477–87. doi: 10.1056/NEJMra050435. [DOI] [PubMed] [Google Scholar]
- 25.Tan TC, Devendra K, Tan LK, Tan HK. Tocolytic treatment for the management of preterm labour: A systematic review. Singapore Med J. 2006;47:361–6. [PubMed] [Google Scholar]
- 26.Howson CP, Kinney MV, Lawn JE, editors. Geneva: World Health Organization; 2012. March of Dimes, PMNCH, Save the Children, WHO. Born Too Soon: The Global Action Report on Preterm Birth; p. 52. [Google Scholar]
- 27.Lewis DF, Barrilleaux PS, Wang Y, Adair CD, Baier J, Kruger T. Detection of interleukin-6 in maternal plasma predicts neonatal and infectious complications in preterm premature rupture of membranes. Am J Perinatol. 2001;18:387–91. doi: 10.1055/s-2001-18694. [DOI] [PubMed] [Google Scholar]
- 28.Turhan NO, Karabulut A, Adam B. Maternal serum interleukin 6 levels in preterm labor: Prediction of admission-to-delivery interval. J Perinat Med. 2000;28:133–9. doi: 10.1515/JPM.2000.018. [DOI] [PubMed] [Google Scholar]
- 29.Sozmen S, Mungan T, Micozkadioglu SD, Tapisiz OL. Predictive value of maternal serum and vaginal interleukin-6 levels in preterm labor. J Soc Gynecol Investig. 2005;12:e1–6. doi: 10.1016/j.jsgi.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 30.Alvarez-de-la-Rosa M, Rebollo FJ, Codoceo R, Gonzalez Gonzalez A. Maternal serum interleukin 1, 2, 6, 8 and interleukin-2 receptor levels in preterm labor and delivery. Eur J Obstet Gynecol Reprod Biol. 2000;88:57–60. doi: 10.1016/s0301-2115(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 31.Goldenberg RL, Iams JD, Mercer BM, Meis PJ, Moawad A, Das A, et al. The Preterm Prediction Study: Toward a multiple-marker test for spontaneous preterm birth. Am J Obstet Gynecol. 2001;185:643–51. doi: 10.1067/mob.2001.116752. [DOI] [PubMed] [Google Scholar]
- 32.Murtha AP, Sinclair T, Hauser ER, Swamy GK, Herbert WN, Heine RP. Maternal serum cytokines in preterm premature rupture of membranes. Obstet Gynecol. 2007;109:121–7. doi: 10.1097/01.AOG.0000250474.35369.12. [DOI] [PubMed] [Google Scholar]


