Abstract
Duchenne muscular dystrophy (DMD) is a devastating and ultimately fatal disease characterized by progressive muscle wasting and weakness. DMD is caused by the absence of a functional dystrophin protein, which in turn leads to reduced expression and mislocalization of dystrophin-associated proteins including neuronal nitric oxide (NO) synthase mu (nNOSμ). Disruption of nNOSμ signaling results in muscle fatigue and unopposed sympathetic vasoconstriction during exercise, thereby increasing contraction-induced damage in dystrophin-deficient muscles. The loss of normal nNOSμ signaling during exercise is central to the vascular dysfunction proposed over 40 years ago to be an important pathogenic mechanism in DMD. Recent preclinical studies focused on circumventing defective nNOSμ signaling in dystrophic skeletal and cardiac muscle by inhibiting phosphodiesterase 5A (PDE5A) have shown promising results. This review addresses nNOS signaling in normal and dystrophin-deficient muscles and the potential of PDE5A inhibition as a therapeutic approach for the treatment of cardiovascular deficits in DMD.
Keywords: Cardiac muscle, Cardiomyopathy, cGMP, Duchenne Muscular Dystrophy, Dystrophin, Mdx, Nitric oxide, nNOS, Neuronal nitric oxide synthase, PDE5, PDE5 inhibitors, Sildenafil, Skeletal muscle
1 Introduction
The absence of normal neuronal nitric oxide (NO) synthase mu (nNOSμ) signaling in the muscles of humans and mice is a well-described consequence of the loss of dystrophin, the primary cause of Duchenne Muscular Dystrophy (DMD). The absence of nNOSμ signaling impairs blood supply to contracting skeletal muscles, exposing working muscles to continuous damaging ischemic insult (Thomas et al. 1998; Asai et al. 2007). At present, it is not possible to selectively increase nNOS expression or activity using a pharmacological approach, but it is possible to mimic some of the effects of increased nNOS activity, which increases synthesis of cGMP and cGMP signaling (or circumvent aberrant upstream nNOS signaling) by inhibiting the activity of downstream cGMP-hydrolyzing phosphodiesterases (PDEs).
Recent efforts to pharmacologically enhance nNOS-cGMP signaling in dystrophic muscles have involved the targeted inhibition of PDE5A with sildenafil (Viagra®, Revatio®) or tadalafil (Cialis®), commonly used to treat erectile dysfunction and pulmonary hypertension. In the presence of a stimulus of cGMP synthesis such as NO, PDE5A inhibitors block cGMP breakdown, thereby raising cellular cGMP concentrations in many tissues such as smooth muscle. Inhibition of PDE5A is a highly attractive therapeutic approach for treating DMD for at least five reasons. First, there is strong evidence for pronounced vascular dysfunction in DMD, particularly reduced blood delivery to active muscle (Mendell et al. 1971; Thomas et al. 1998; Sander et al. 2000). Second, in several animal models, PDE5A inhibition has been shown to provide beneficial effects on skeletal, smooth, and cardiac muscle tissues (Asai et al. 2007; Khairallah et al. 2008; Reffelmann and Kloner 2009). Third, the ability to treat all muscle tissues is very important since therapeutic approaches that correct only skeletal muscle dysfunction increase cardiac workload, which is damaging to the weakened dystrophic heart (Townsend et al. 2008). Those approaches that fail to address vascular dysfunction resulting from impaired nNOS signaling in smooth muscle result in significant unopposed sympathetic vasoconstriction known to exacerbate skeletal muscle damage (Ito et al. 2006). Fourth, increases in cGMP levels or PDE5A inhibition confer substantial cardioprotective effects in several animal models including improved diastolic dysfunction (Takimoto et al. 2005; Das et al. 2008; Reffelmann and Kloner 2009). This is an important consideration since existing treatments for DMD-associated cardiomyopathy, including angiotensin-converting enzyme (ACE) inhibitors and β-adrenergic blockade, predominantly address systolic, but not the diastolic dysfunction in DMD patients (Markham et al. 2006). Fifth, an obvious practical advantage is the availability of FDA-approved, potent and highly selective PDE5A inhibitors that include Viagra®/ Revatio® (sildenafil), Levitra® (vardenafil), and Cialis® (tadalafil). These drugs have already been extensively tested in normal adults, while sildenafil has also been tested in children. Thus, testing of safety and efficacy in DMD and other muscular dystrophies in humans could occur quite quickly, which is an important consideration for a rapidly progressing and invariably fatal disease. Before discussing studies of the impact of PDE5A inhibition on the dystrophic pathology of the mdx mouse model of DMD, we first briefly outline what is known about the function of nNOS-derived NO-signaling pathways in normal and dystrophin-deficient muscles.
2 nNOS Signaling in Skeletal Muscle
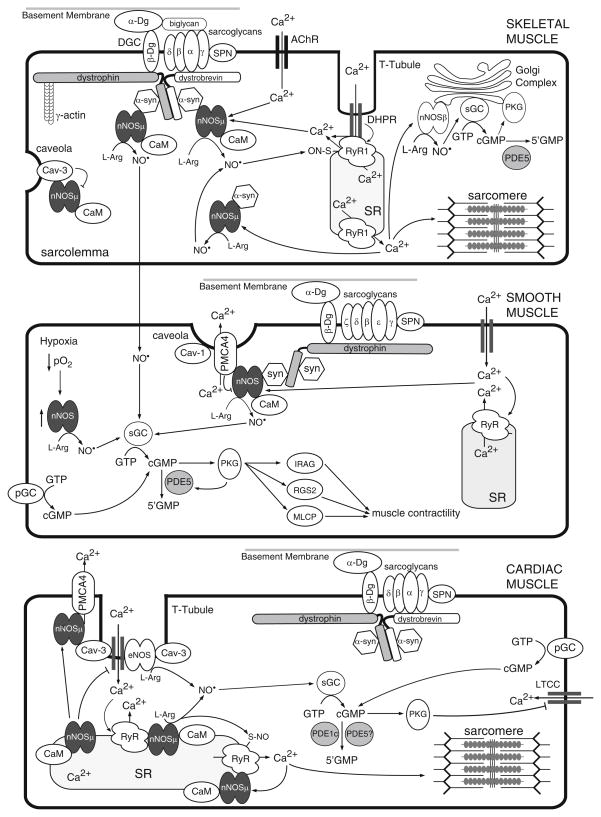
The free radical gas NO is indispensable for normal muscle health and exercise performance. In skeletal muscle, neuronal nitric oxide synthase (nNOS) isozymes are the predominant sources of NO. nNOS isozymes are Ca2+/calmodulin-regulated, heme-containing flavoproteins that synthesize gaseous NO from L-arginine, in an NADPH and O2-dependent manner (Bredt and Snyder 1990; Stuehr et al. 2004). Skeletal muscles express at least two alternatively spliced forms of nNOS called nNOSμ and nNOSβ (Silvagno et al. 1996; Percival et al. 2010; Fig. 1). nNOSμ is localized to the sarcolemmal and to an undefined cytosolic compartment, whereas nNOSβ is localized to the Golgi complex (Brenman et al. 1995, 1996; Thomas et al. 2003; Percival et al. 2010; Fig. 1). nNOSμ contains an amino terminal PDZ (PSD-95, discs-large, ZO-1) domain and an internal short sequence (mu insert) of unknown function, while nNOSβ lacks both the PDZ domain and the mu insert. Instead, nNOSβ contains a short unique amino terminal sequence that is a putative Golgi-targeting motif. Thus, exon choice at the amino terminus appears to regulate the differential targeting of these two nNOS isozymes. Sarcolemmal localization of nNOSμ requires the correct expression and localization of dystrophin, α-syntrophin, and α-dystrobrevin (Brenman et al. 1995, 1996; Adams et al. 2000), all of which are members of the dystrophin glycoprotein complex (DGC). Thus, skeletal muscle has at least two NO-signaling compartments defined by the localization of the two NOS isoenzymes to either the subsarcolemmal space or Golgi membranes.
Fig. 1.
Propagation of NO-cGMP signals in skeletal, smooth, and cardiac muscle. Nitric oxide synthase enzymes (nNOS and eNOS) regulate, and are regulated by, Ca2+ fluxes in muscle cells. Ca2+/CaM activation of nNOS (or eNOS) leads to synthesis of NO, which in turn binds and activates sGC. cGMP produced by sGC then modulates downstream effector activity (see text) Abbreviations: α-Dg, α-dystroglycan; β-Dg, β-dystroglycan; α-syn, α-syntrophin; AChR, nicotinic acetylcholine receptor; CaM, calmodulin; Cav-1, Caveolin-1; Cav-3, caveolin-3; DHPR, dihyropyridine receptor; IRAG, inositol 1,4,5-triphosphate receptor I-associated cGMP kinase substrate; PKG, protein kinase G (cGK); L-arg, L-arginine; LTCC, L-type calcium channel; MLCP, myosin light chain phosphatase; NO, nitric oxide; PDE, phosphodiesterase; pGD, particulate guanylyl cyclase; PMCA4, plasma membrane calcium ATPase 4; RGS2, regulator of G protein signaling 2; RyR, ryanodine receptor; sGC, soluble guanylyl cyclase; SPN, sarcospan; SR, sarcoplasmic reticulum
While nNOSμ and nNOSβ are differentially localized, they both synthesize NO, which exerts its regulatory effects through cGMP-dependent and cGMP-independent pathways. cGMP (guanosine 3′:5′-cyclic monophosphate) is an important second messenger produced by the NO receptor, soluble guanylyl cyclase (sGC) (Mergia et al. 2009). NO binds at multiple sites including the critical heme group within sGC, stimulating it to convert guanosine triphosphate (GTP) into cGMP. In turn, cGMP binds and activates downstream effectors including: cGMP-dependent protein kinases (PKG, also known as cGK), cyclic nucleotide-gated (CNG) channels, and cGMP-regulated PDEs (Hofmann et al. 2009; Craven and Zagotta 2006; Bender and Beavo 2006). In skeletal muscle, sGC and PKG isoforms are localized to the Golgi complex (Percival et al. 2010; Fig. 1). PKG is also localized to the neuromuscular junction (Chao et al. 1997). NO can also act through cGMP-independent pathways by directly reacting with thiol residues of cysteine groups. This NO-based posttranslational modification, known as S-nitrosylation, is also an important signal transduction mechanism. For example, the activity of skeletal muscle RyR1, the ryanodine Ca2+ release channel, is positively regulated by nitrosylation (Eu et al. 2000, Fig. 1). Therefore, in skeletal muscle, nNOS-synthesized NO signals can be propagated through both cGMP-dependent and cGMP-independent mechanisms.
Rapid modulation of NO-cGMP signaling is mediated both by the rate of cGMP synthesis and by cGMP degradation by cGMP-PDEs. Several different PDEs are able to hydrolyze cGMP including PDEs 1, 2, 3, 5, 6, 9, and 11. cGMP-specific PDEs, such as PDE5A, hydrolyze only cGMP, thereby decreasing the cellular levels of cGMP (Bender and Beavo 2006; Fig. 1). Inhibition of these cGMP-hydrolyzing PDEs can raise cGMP levels and effectively amplify the upstream NO signal. One of the most studied cGMP-PDEs is PDE5A, which is predominantly expressed in the vascular smooth muscle cells (VSMCs) of most vascular beds, fibroblasts, and myofibroblasts (Wallis et al. 1999). PDE5A-mediated cGMP degradation promotes smooth muscle contraction with concomitant blood vessel constriction. Active PDE5A is also expressed in skeletal muscle homogenates and cell lines including C2C12 myoblasts and myotubes (Bloom 2002; unpublished observations). Although PDE5A expression in cardiomyocytes has been reported, this issue is contentious since others contend there is no significant PDE5A expression or activity in these cells (Senzaki et al. 2001; Takimoto et al. 2005; Reffelmann and Kloner 2009; Lukowski et al. 2010). Thus, PDE5A is expressed in skeletal and smooth muscles and perhaps at very low levels in cardiomyocytes. nNOS is also expressed in VSMCs and cardiomyocytes (Xu et al. 1999; Ward et al. 2005). In VSMCs, nNOS promotes smooth muscle relaxation and blood vessel dilation, particularly during chronic hypoxia (Ward et al. 2005). It is clear that skeletal, cardiac, and smooth muscle cells possess the necessary molecular machinery for localized nNOS-cGMP signaling (Fig. 1). Importantly, inhibition of PDE5 activity provides a general approach to amplify nNOS-mediated signal transduction, or to broadly enhance NO-cGMP signaling activity, particularly in smooth muscle cells.
Recent studies of NO signaling in skeletal muscle have provided new insights into nNOS function. nNOSμ participates in pathways that regulate (1) contraction-induced glucose uptake and glucose homeostasis, (2) muscle mass and atrophy (3) mitochondrial integrity (4) susceptibility to fatigue (5) postexercise strength (6) exaggerated exercise-induced inactivity, (7) and blood delivery during exercise (Thomas et al. 1998; Firestein and Bredt 1999; McConell and Wadley 2008; Suzuki et al. 2007; Percival et al. 2008, 2010; Kobayashi et al. 2008; Wehling-Henricks et al. 2009). Therefore, nNOSμ appears to control physiological pathways that collectively regulate metabolic energy flux, particularly during muscle contraction. These roles also support the proposition that muscle nNOSμ function is most important under conditions of physiological stress, particularly prolonged inactivity or exercise. In agreement with this proposition, the muscles of trained athletes express higher levels of nNOSμ, while nNOSμ levels are lower in less active or sedentary muscles and often absent in myopathic muscles; therefore, establishing a close relationship between nNOSμ expression and muscle activity (Brenman et al. 1995; Chang et al. 1996; Chao et al. 1996; Crosbie et al. 2002; McConell et al. 2007; Suzuki et al. 2007; Kobayashi et al. 2008).
The exercise performance of muscle is highly dependent on oxygen supply. Perhaps, the best studied function of nNOSμ is its ability to attenuate resistance vessel vasoconstriction, matching oxygen delivery with demand during muscle contraction (Thomas and Victor 1998; Thomas et al. 1998, 2003). The localization of nNOSμ to the sarcolemma is critical for this vasomodulatory function and cannot be compensated for by cytoplasmic nNOSμ or Golgi nNOSβ (Thomas et al. 2003; Percival et al. 2010). Taken together, these data demonstrate a role for nNOSμ in regulating oxygen delivery during muscle contraction and strongly support a role for nNOSμ in regulating the exercise performance of skeletal muscle.
3 nNOS Signaling in Cardiac Muscle
As in skeletal muscle, nNOSμ-synthesized NO in the heart has emerged as an important autocrine regulator of cardiomyocyte contractility and coronary blood flow (Barouch et al. 2002; Sears et al. 2003; Zhang et al. 2008; Seddon et al. 2009, Fig. 1). Cardiac nNOSμ plays an essential role in promoting relaxation of the myocardium and may do so via the regulation of Ca2+ flux. For example, nNOSμ-derived NO decreases inward Ca2+ movement (thereby reducing basal contractility) by negatively regulating the activity of the L-type Ca2+ channel (Sears et al. 2003). However, in contrast to its distribution in skeletal muscle, nNOSμ is primarily localized to the sarcoplasmic reticulum in cardiac myocytes in a complex with the ryanodine receptor Ca2+-release channel (RyR2), suggesting tissue-specific differences in nNOSμ function in excitation-contraction coupling (Xu et al. 1999; Sears et al. 2003; Fig. 1). Cardiac nNOSμ is thought to serve a cardioprotective role under conditions of pathophysiological stress. For example, nNOSμ translocation to the sarcolemma occurs during myocardial infarction and heart failure, where it blunts β-adrenergic signaling and reduces cardiac contractility (Bendall et al. 2004). Additional support for a cardioprotective role comes from findings that nNOSμ depletion exacerbates maladaptive cardiac remodeling following myocardial infarction (Saraiva et al. 2005). These data strongly support an important role for nNOSμ in the regulation of cardiomyocyte contractility and Ca2+ flux, functions that are protective in a pathophysiologically distressed heart.
4 Skeletal Muscle Pathogenesis in Duchenne Muscular Dystrophy
Skeletal muscle nNOSμ expression, localization, and signaling are severely disrupted in DMD, an X-linked muscle wasting disease that occurs in 1 in every 3,600–6,000 live male births (Davies and Nowak 2006; Bushby et al. 2009). DMD patients exhibit elevated serum creatine kinase activity levels (due to increased sarcolemmal permeability) and progressive muscle wasting and weakness leading to loss of ambulation by 12 years of age (Davies and Nowak 2006). Voluntary limb and trunk muscles are initially affected, followed by respiratory and cardiac muscle involvement. DMD results predominantly from frame shift mutations in the gene encoding dystrophin (Hoffman et al. 1987).
Dystrophin is a 427 kDa rod-shaped actin-binding protein that resides at the cytoplasmic face of the sarcolemma (Hoffman et al. 1987; Koenig et al. 1988). It is the namesake of the DGC (dystrophin glycoprotein complex), a multiprotein complex that links the extracellular basal lamina with the intracellular γ-actin microfilament system (Ervasti and Campbell 1993). Dystrophin stabilizes myofibers against mechanical forces generated during muscle contraction (Ervasti 2007). The DGC appears to have a mechanotransduction role whereby dystrophin is necessary for inhibition of stretch-activated Ca2+ channel activity (Vandebrouck et al. 2001). As mentioned above, dystrophin also serves as a scaffold on which signaling molecules are localized primarily by the adaptor protein α-syntrophin (Percival et al. 2006). nNOSμ is the best characterized ligand of α-syntrophin (Brenman et al. 1996; Kameya et al. 1999; Adams et al. 2000). The mode of nNOSμ binding to α-syntrophin leaves the PDZ domain of nNOSμ free to bind other proteins including phosphofructokinase (Brenman et al. 1996; Hillier et al. 1999; Firestein and Bredt 1999; Adams et al. 2001). Dystrophin deficiency leads to the loss of α-syntrophin and nNOSμ from the DGC. Mislocalized nNOSμ fails to override exercise-induced sympathetic vasoconstriction (Thomas et al. 2003). Thus, the loss of dystrophin simultaneously impacts muscle structural integrity and uncouples contraction-induced signaling, including NO-mediated signal transduction.
Disruption of nNOSμ expression and signaling occurs not only in DMD, but also in other myopathies, including: Becker Muscular Dystrophy (also from less pathogenic mutations of dystrophin), Limb Girdle Muscular Dystrophies 2C, 2D, and 2E (resulting from mutations of γ-, α-, and β-sarcoglycan, respectively), and Ulrich Congenital Muscular Dystrophy (collagen VI mutation) (Brenman et al. 1995; Chang et al. 1996; Chao et al. 1996; Crosbie et al. 2002; Kobayashi et al. 2008). These myopathies are all characterized by the absence of sarcolemmal nNOSμ protein expression, whereas both cytosolic and sarcolemmal nNOSμ expression are substantially reduced (≥80%) in dystrophin-deficient muscles (Chang et al. 1996). Thus, dystrophin is necessary for the normal expression and localization of nNOSμ. The mechanisms by which nNOSμ signaling is disrupted remain to be determined in other myopathies. In summary, it is clear that nNOS signaling abnormalities are common to a broad spectrum of muscle diseases.
In addition to the dysregulation of nNOSμ, many other proteins and pathways are deregulated in dystrophin-deficient muscle. The loss of dystrophin increases muscle instability and permeability, reflected by excessive Ca2+ influx. In turn, Ca2+ overload leads to activation of proteases and mitochondrial dysfunction causing muscle necrosis and cycles of muscle cell degeneration and regeneration (Davies and Nowak 2006). Regeneration is easily observed histologically as clusters of centrally nucleated fibers. Muscle breakdown is accompanied by infiltration of inflammatory cells, particularly macrophages. Initially, the regenerative capacity of dystrophin-deficient muscle keeps pace with degeneration, but is soon exhausted and myofibers are gradually replaced by adipose and fibrous connective tissue (Davies and Nowak 2006).
Corticosteroid treatment, despite significant side effects and limited efficacy, is the mainstay therapy for the preservation of skeletal muscle function in DMD (Manzur et al. 2008). Death typically ensues in the third decade of life with 75% of DMD patients dying from respiratory failure while the remainder succumbs to heart failure (Finsterer and Stöllberger 2003). However, the incidence and severity of cardiac dysfunction are on the rise because of improvements in noninvasive ventilatory support (Eagle et al. 2007). Better understanding of the cascade of pathological changes in dystrophin-deficient muscles is necessary to identify new targets for therapeutic pharmacological intervention in the short term.
The effects of dystrophin-deficiency have been extensively studied in the mdx mouse, an animal model for DMD. As is the case in humans, mdx mice exhibit significant skeletal muscle weakness, susceptibility to fatigue, exercise intolerance, shorter lifespan, elevated serum creatine kinase activity, widespread muscle degeneration and regeneration, muscle necrosis, Ca2+ overload, fibrosis, and inflammation (Stedman et al. 1991; Davies and Nowak 2006; Chamberlain et al. 2007; Willmann et al. 2009). Dystrophin-deficient mdx myofibers exhibit a characteristic susceptibility to lengthening contraction that may result from inappropriate stretch-activated Ca2+ channel activity (Petrof et al. 1993; Dellorusso et al. 2001; Whitehead et al. 2006). The diaphragm muscle in mdx mice best recapitulates the pathology observed in the skeletal muscles from DMD patients (Stedman et al. 1991). However, mice are less affected by the absence of dystrophin than humans and exhibit a slower and milder disease progression. Highly effective compensatory mechanisms are clearly at play in dystrophin-deficient tissues of mdx mice. Increased expression of utrophin, a functional paralogue of dystrophin, is thought to play a significant compensatory role (Davies and Nowak 2006). Overall, however, mdx mice represent a useful model for investigating dystrophic pathology and for evaluating the efficacy of experimental treatments.
5 Cardiac Muscle Pathogenesis in Duchenne Muscular Dystrophy
Like skeletal muscle, cardiac muscle is also severely affected by the loss of dystrophin with cardiomyopathy beginning early in DMD patients. Left ventricle (LV) dysfunction is readily detectable in patients in their teens. By 18 years of age, clinically relevant symptoms of cardiomyopathy are evident in 90% of patients (Chenard et al. 1993; de Kermadec et al. 1994; Finsterer and Stöllberger 2003). Substantial improvements in noninvasive respiratory support have extended patient longevity, but are accompanied by increased incidence of severe cardiac dysfunction (Eagle et al. 2007). This has necessarily led to an upturn in the management of cardiac dysfunction that now predominates in the late stages of DMD.
In contrast to skeletal muscle, the impact of dystrophin-deficiency on cardiac function in humans has received much less attention. Over time, a DMD-associated dilated cardiomyopathy (DCM) can develops as a consequence of the absence of dystrophin from the heart (Finsterer and Stöllberger 2003). Similar to skeletal muscle, the absence of dystrophin in the cardiomyocyte enhances membrane permeability leading to Ca2+ overload. Pathologically, high intracellular Ca2+ concentrations result in excessive protease activity and impair mitochondrial oxidative phosphorylation, causing widespread cardiomyocyte necrosis. Cardiomyocyte death results in inflammation and extensive fibrosis, particularly of the LV wall, reducing ventricular compliance and diastolic function (Moriuchi et al. 1993; Frankel and Rosser 1976). Atrial and ventricular chamber walls stretch and thin out due to cardiomyocyte death, leading to chamber dilation. As a result, both systolic and diastolic function of the dystrophin-deficient heart is impaired. Eventually, DCM develops into congestive heart failure. DCM may be associated with arrhythmias and other electrical impulse conduction defects that also contribute to cardiac dysfunction (Finsterer and Stöllberger 2003; Thrush et al. 2009). Sinus tachycardia (abnormally fast heart rate) is a prevalent arrhythmia in DMD. Short PR intervals, tall R waves, and prominent Q waves are also common impulse conduction abnormalities (Finsterer and Stöllberger 2003; Thrush et al. 2009).
In the clinic, ACE inhibitors and β-adrenergic blockade provide the mainstay of therapeutic intervention in DMD (Finsterer and Stöllberger 2003). While these interventions are often effective for enhancing systolic function in DMD hearts, diastolic dysfunction is left largely unaddressed. This is an important point relevant to the potential utility of PDE5A inhibitors in treating DMD-associated DCM since sildenafil, currently used to treat erectile dysfunction and pulmonary hypertension, will be tested in large clinical study for efficacy in treating diastolic dysfunction in heart failure (ClinicalTrials.gov Identifier: NCT00781508). Despite current interventions, DMD deaths from cardiac failure are on the rise and according to some estimates, death from congestive heart failure and/or arrhythmias can account for 20–50% of deaths in DMD (Finsterer and Stöllberger 2003; Ishikawa et al. 1999). Thus, dystrophin-deficiency has serious negative consequences for cardiac as well as skeletal muscle function.
Unlike skeletal muscle, the role of nNOS in the dystrophic heart has not been investigated in humans. Also, studies of NO-cGMP signaling in mdx mouse hearts are limited. Knowledge of the NOS-signaling pathway activity in dystrophy is very important from a therapeutic standpoint, since NO is required for many of the cardioprotective effects of sildenafil (Nagayama et al. 2008). In the absence of NO, sGC may not be sufficiently active and, consequently, fails to produce physiologically sufficient amounts of cGMP, rendering the inhibition of cGMP-hydrolyzing PDEs therapeutically ineffective (Fernhoff et al. 2009).
nNOS activity, but not nNOS protein expression, is inhibited in dystrophin-deficient cardiac muscle. Cardiac nNOSμ levels appear unaffected by dystrophin-deficiency, despite a significant reduction in nNOSμ activity (Bia et al. 1999; Wehling-Henricks et al. 2005). The activity of endothelial NO synthase (eNOS), the other NO-generating enzyme in cardiomyocytes, is unaffected by the absence of dystrophin (Bia et al. 1999). Elevated expression of atrial natriuretic peptide (the ligand for the transmembrane guanylyl cyclase ANP receptor A [NPR-A]) mRNA in mdx hearts is consistent with deficits in cGMP signaling in dystrophic cardiac tissue (Khairallah et al. 2007) and suggests that increased ANP pathway activity may be a compensatory mechanism in the mdx heart.
Although the impact of dystrophin-deficiency on cardiac function has received less attention than in skeletal muscles, it is clear that mdx mice develop a less severe, but pronounced cardiomyopathy compared with humans. Two important distinctions between mdx and DMD hearts are that ventricular fibrosis is less extensive and chamber dilation is not pronounced in murine mdx hearts (Quinlan et al. 2004; Wehling-Henricks et al. 2005; Spurney et al. 2008). The reasons for these differences are unknown. However, as in humans, the absence of dystrophin initiates a similar cascade of pathological events that leads to increased membrane permeability and Ca2+ overload, culminating in cardiomyocyte necrosis and death. Increased cardiomyocyte death likely results in part from an increased susceptibility to contraction-induced damage (Danialou et al. 2001). The predisposition of dystrophin-deficient cardiomyocytes to necrosis and mechanical stress results in significant contractile dysfunction.
Early mdx mouse studies suggested that cardiomyopathy could only be detected around 8 months of age (Quinlan et al. 2004). However, recent studies have demonstrated cardiac dysfunction including LV systolic and diastolic dysfunction in mice as young as 8–10 weeks of age (Danialou et al. 2001; Wu et al. 2008; Khairallah et al. 2007). Cardiac dysfunction is not evident from noninvasive in vivo analysis at these young ages, suggesting compensatory mechanisms in vivo. These findings are consistent with the progression of DCM in DMD patients, where early cardiomyopathy goes largely unnoticed, only becoming clinically symptomatic in the second decade of life. Hearts from 9- to 10-month-old mdx mice exhibit marked systolic dysfunction and pathological LV remodeling (Quinlan et al. 2004; Spurney et al. 2008). Also, myocardial performance index (MPI) is significantly increased, indicative of increased cardiac dysfunction (Spurney et al. 2008). Thus, collectively, these data indicate pronounced left ventricular dysfunction in mdx hearts.
As observed in humans, contractile dysfunction is often accompanied by arrhythmias and abnormal electrical impulse conduction. Electrocardiographic studies reveal that mdx and DMD hearts exhibit similar aberrant impulse interval characteristics including deep Q waves, a decreased S:R wave ratio, polyphasic R waves, shortened PR interval and QTc intervals and cardiac arrhythmias, such as premature ventricular contractions (Chu et al. 2002; Wehling-Henricks et al. 2005; Bostick et al. 2009). In summary, dystrophin-deficiency negatively affects cardiomyocyte survival and function in mdx mice. Furthermore, mdx hearts recapitulate key features of cardiomyopathy in DMD hearts including cardiomyocyte necrosis, susceptibility to mechanical stress, diastolic dysfunction, systolic dysfunction, and impulse propagation defects.
6 Vascular Dysfunction Contributes to the Pathogenesis of DMD
While research in DMD has primarily focused on dystrophin function in skeletal muscle and, to a lesser extent, cardiac muscle, smooth muscle dysfunction may also play a role in the dystrophic phenotype. Both dystrophin and nNOSμ are expressed in VSMCs (Ward et al. 2005; Ito et al. 2006; Fig. 1). Indeed, abnormalities in smooth muscle in mdx mice appear to contribute to the dystrophic phenotype, for example, by impairing blood supply during exercise (Ito et al. 2006). This is particularly relevant to the therapeutic utility of PDE5 inhibitors in dystrophy because PDE5A is highly expressed in VSMCs in the vascular beds of the circulatory system; thus, any consideration of the effects of systemic PDE5A inhibition on mdx pathology must also consider any potential impact on VSMC function (Wallis et al. 1999). Furthermore, vascular dysfunction has long been suspected to contribute to the dystrophic pathology of DMD (Mendell et al. 1971).
Evidence of vascular dysfunction, specifically small clusters of necrotic fibers, was first observed in skeletal muscle biopsies from DMD patients over 40 years ago. This muscle pathology could be recapitulated by vascular obstruction, suggesting that muscle necrosis could result from defects in a shared blood vessel. This reasoning formed the basis for the vascular hypothesis proposed by Engel and coworkers that stated that skeletal muscle microcirculation dysfunction could account for the pathogenesis of DMD (Mendell et al. 1971). This hypothesis was largely abandoned when no structural abnormalities of the vasculature were found (Jerusalem et al. 1974). However, the vascular theory was revisited when Victor and coworkers demonstrated that α-adrenergic receptor-mediated sympathetic vasoconstriction was unopposed in the exercising hind limbs of mdx and KN1 (nNOS knockout 1) mice and contracting forearms of DMD patients (Thomas et al. 1998; Sander et al. 2000). Thus, during exercise, dystrophin-deficient muscles lacking sarcolemmal nNOSμ would be subjected to repeated rounds of ischemia (functional ischemia) causing myofiber damage in dystrophin-deficient muscles and contributing to the profound exercise intolerance observed in DMD patients. The loss of nNOSμ targeting provides a mechanism for the vascular dysfunction observed in dystrophin-deficient muscle.
Direct evidence that reduced blood flow in postcontraction muscles played a primary role in the disease pathogenesis came from in vivo microscopy studies. Pretreatment of dystrophic mdx sternomastoid muscle with the NO donor SNAP (S-nitroso-N-acetylpenicillamine), or the cGMP analog 8-CPT-cGMP (8-chlorophenylthio-cGMP) reversed the ischemic effects of primary arteriole constriction and prevented contraction-induced myofiber damage (Asai et al. 2007). This study provided proof of principle that pharmacological augmentation of NO-cGMP signaling to increase blood supply to active muscles can reduce postexercise muscle damage. The key role of sarcolemmal nNOSμ in preventing ischemic damage in dystrophic muscle was later confirmed by a study of the therapeutic capability of a ΔH2-R15 minidystrophin cDNA that could restore sarcolemmal nNOSμ expression in mdx mice (Lai et al. 2009). Sarcolemmal nNOSμ expression restored the ability of mdx muscle to oppose exercise-induced sympathetic vasoconstriction, prevented contraction-induced fiber degeneration and macrophage infiltration and improved exercise performance (Lai et al. 2009). Thus, contraction-induced sarcolemmal nNOSμ-derived NO signaling to the VSMC of adjacent resistance vessels plays an important role in preserving skeletal muscle integrity.
While the importance of contraction-triggered paracrine signaling from skeletal muscle nNOSμ-synthesized NO to the adjacent vasculature is apparent, dystrophin-associated nNOS in VSMC of blood vessels may also facilitate opposition to sympathetic vasoconstriction during exercise (Fig. 1). In mdx mice, the loss of dystrophin leads to the reduction of nNOSμ isoenzyme expression in both skeletal and vascular smooth muscle (Ito et al. 2006). It is not clear whether the nNOS isozyme in question is nNOSμ or nNOSα since both may be expressed in VSMCs (Boulanger et al. 1998; Ward et al. 2005). Nevertheless, increased smooth muscle specific-expression of dystrophin in mdx mice restored nNOS protein expression and provided an intermediate level of inhibition of vasoconstriction during contraction (Ito et al. 2006). Smooth muscle-specific dystrophin expression in mdx mice reduced serum CK levels, indicating decreased myofiber permeability (Ito et al. 2006). Unlike the paracrine action of sarcolemmal nNOSμ-derived NO on adjacent blood vessels, VSMC nNOS-derived NO acts in an autocrine fashion to promote smooth muscle cGMP-dependent relaxation and vasodilation. Together, these data suggest that aberrant nNOS signaling in VSMCs can also contribute to the microvascular dysfunction and dystrophic pathology by increasing myofiber permeability and susceptibility to ischemic damage during exercise. Thus, the vascular dysfunction in mdx mice likely results from simultaneous disruption of nNOS isozyme expression and signaling in both skeletal and smooth muscle.
7 Augmentation of Nitric Oxide Signaling in mdx Mice
The observations that nNOSμ signaling is disrupted in dystrophin-deficient skeletal, cardiac, and smooth muscle cells, combined with abnormal nNOS and cGMP signaling in dystrophin-deficient cardiac muscles, provides a compelling rationale for the use of approaches that enhance nNOS signaling to treat dystrophinopathies. To date, such approaches have used exogenous sources of NO to enhance NO signaling, such as NO synthase transgenes or NO donors. Other approaches have focused on modulating downstream effector activity, specifically by inhibiting PDE5A activity to enhance cGMP levels that normally result from NO-mediated activation of sGC.
Targeting nNOS signaling pathways has proved efficacious for reducing muscle damage and improving exercise performance in the mdx mouse model of DMD. Reengineering of conventional minidystrophin gene therapy cassettes to restore sarcolemmal nNOSμ expression provides significant additional improvements over conventional microdystrophin cassettes, including enhanced vasomodulation, exercise performance, and resistance to exercise-induced muscle damage (Lai et al. 2009). Thus, the ability to restore sarcolemmal nNOSμ expression substantially improves gene therapy-based intervention in mdx mice. These data are consistent with reduced contraction-induced myofiber damage observed after the application of NO donors (Asai et al. 2007). Cytosolic expression of an nNOSα transgene reduced muscle damage without affecting membrane permeability in adult mdx mice (Wehling et al. 2001). Substantial reductions in macrophages densities and cytolytic activity were observed, suggesting that the anti-inflammatory properties of NO were responsible for the reduction in muscle damage (Wehling et al. 2001). In agreement with this proposal, a compensatory increase in utrophin expression was not observed (Tidball and Wehling-Henricks 2004). Transduction of mdx skeletal muscles by adenovirus carrying a constitutively active eNOS gene also improved dystrophic pathology and increased myofiber size by upregulating follistatin expression (Colussi et al. 2008). Histone deacetylase 2 (HDAC 2) inhibition by NO-dependent S-nitrosylation is responsible for the anti-dystrophic impact of increased cytosolic NO, highlighting a novel link between NO signaling and chromatin remodeling in dystrophic skeletal muscle (Colussi et al. 2008). These findings provide evidence that NO can also exert beneficial effects on dystrophic muscle through cGMP-independent pathways. Taken together, these studies provide a compelling rationale for the potential therapeutic utility of increasing NO-cGMP signaling in dystrophin-deficient tissues.
As in skeletal muscle, cardioprotective effects of increased NO concentrations have been reported in mdx cardiac muscle. Cardiomyocyte-specific expression of a nNOSα transgene in mdx mice significantly reduced interstitial fibrosis (Wehling-Henricks et al. 2005). Interestingly, ectopic expression of nNOSα in mdx hearts (nNOSα is not normally expressed in cardiomyocytes) corrected common impulse conduction defects including deep Q waves, a decreased S:R wave ratio, polyphasic R waves and shortened PR interval, as well as preventing cardiac arrhythmias, such as premature ventricular contractions (Wehling-Henricks et al. 2005). Thus, increased cardiomyocyte NO levels can improve prominent features of cardiac dystrophic pathology in mdx mice.
Not only are increases in NO concentrations cardioprotective, but increases in cGMP concentrations are also cardioprotective in mdx hearts (Khairallah et al. 2008). Indeed, cardiomyocyte-specific expression of constitutively active NPR-A (atrial natriuretic peptide receptor A guanylyl cyclase), a plasma membrane-associated guanylyl cyclase, reduced cardiomyopathy in mdx hearts (Khairallah et al. 2008). The NPR-A transgene-mediated an increase in cGMP in dystrophin-deficient cardiomyocytes improved cardiomyocyte viability, blunted the progressive increase in LV end diastolic pressure (preload), and increased the cardiac power two-fold, but did not protect mdx hearts against contraction-induced damage (Khairallah et al. 2008). These data demonstrated that chronic increases in cardiomyocyte cGMP levels could reduce cardiac dysfunction in mdx mice. In summary, as observed in skeletal muscle, several different approaches indicate that enhanced NO-cGMP signaling has potent antidystrophic effects in dystrophin-deficient hearts.
8 Use of PDE5A Inhibitors to Amplify cGMP Signaling in mdx Mice
As described above, one of the consequences of NO signaling is an increase in sGC activity, with a concomitant increase in cytosolic cGMP. While it is not possible to selectively increase nNOS expression or activity using a pharmacological approach, it is possible to enhance NO signal transduction by inhibiting the activity of cGMP-hydrolyzing PDEs, such as PDE5A, thus raising cytosolic cGMP. Three studies of the impact of PDE5A inhibition on the dystrophic pathology of cardiac and skeletal muscle in the mdx mouse model of DMD have been reported and will now be summarized.
In one published study of the cardiac effects of sildenafil-mediated inhibition of PDE5A in mdx mice, Khairallah and coworkers reported that sildenafil administered daily by intraperitoneal injection over 6 weeks (0.7 mg/kg/day) enabled mdx hearts to sustain a higher heart rate in response to increased workload (Khairallah et al. 2008). Sildenafil significantly reduced Evan’s Blue dye uptake in mdx cardiomyocytes, indicative of reduced membrane permeability and suggestive of decreased susceptibility to contraction-induced damage (Khairallah et al. 2008). Sildenafil also decreased sgca1 (α1 subunit of sGC) and Anf (atrial natriuretic factor) transcript expression, suggesting that inhibition of PDE5A improved upstream cGMP signaling and decreased early pathological remodeling in mdx hearts, respectively. Cardioprotective effects were not due to utrophin upregulation.
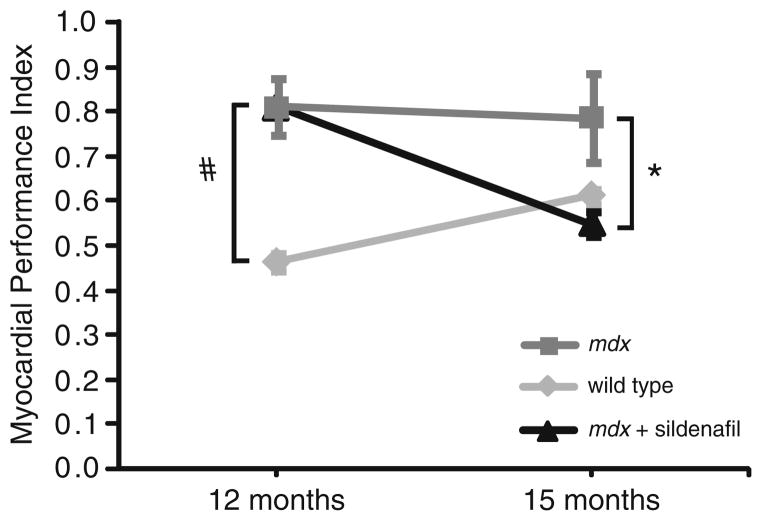
In parallel independent studies, we have found that sildenafil confers significant cardioprotection to old mdx hearts (Adamo et al. 2010). Twelve-month-old mdx mice exhibit significant LV dysfunction, as indicated by a higher than normal MPI measured by echocardiography (Spurney et al. 2008; Adamo et al. 2010, Fig. 2). Treatment of 12-month-old mdx mice for 3 months with sildenafil in their drinking water significantly reduces MPI to wild-type levels (Adamo et al. 2010, Fig. 2). Thus, sildenafil can reverse established left ventricle dysfunction, even in aged mdx mice. These data suggest that older DMD patients with established cardiomyopathy may benefit from PDE5A inhibition.
Fig. 2.
Sildenafil treatment reverses cardiomyopathy in old mdx mice. Twelve-month-old mdx mice exhibit significant left ventricle dysfunction as indicated by a high myocardial performance index compared with wild-type mice (#p < 0.05). Twelve-month-old mdx mice treated for 3 months with the PDE5 inhibitor sildenafil ad libitum exhibited a significantly reduced (p < 0.05) myocardial performance index comparable to wild-type controls. Sildenafil treatment can reverse established global left ventricle dysfunction in old mdx mice (Adamo et al. 2010)
Since cardiomyocytes express very little or no PDE5A, the question remains as to how PDE5A inhibition reduces cardiac pathology and dysfunction in the mdx heart (Takimoto et al. 2005; Lukowski et al. 2010). PDE5A protein expression may be higher in mdx cardiomyocytes, but at present it is unknown whether PDE5A levels or action are affected by the loss of dystrophin. Sildenafil may exert some of its cardioprotective effects by inhibiting PDE5A activity in VSMC of the systemic or cardiac vasculature. In addition, off-target effects of sildenafil on PDE1C could contribute to cardiomyocyte-specific effects of sildenafil. Like PDE5A, PDE1C can hydrolyze cGMP. But unlike PDE5A, PDE1C is found in abundance in cardiomyocytes (Bender and Beavo 2006). High nanomolar concentrations of sildenafil can inhibit PDE1C activity; therefore, inhibition of PDE1C could also account for some of the cardioprotective effects of sildenafil (Lukowski et al. 2010; Vandeput et al. 2009). Nonetheless, it is clear that increasing cGMP levels sildenafil treatment reduces cardiac pathology and dysfunction in mdx hearts (Khairallah et al. 2008). On the basis of these findings, Khairallah and coworkers proposed that upregulation of cGMP by PDE5 inhibition should be explored as a new therapeutic approach to treating DMD.
The impact of PDE5 inhibition on contraction-induced muscle damage during ischemic exercise has also been investigated in mdx mice (Asai et al. 2007). Restoration of postcontraction blood flow to the sternomastoid muscle in situ was achieved by acute application of the PDE5 inhibitor, tadalafil. Tadalafil treatment reversed the ischemia and reduced contraction-induced lesions and myofiber death. Pregnant mice were administered tadalafil in their drinking water (1 mg tadalafil per 100 mL); thus, pups had received tadalafil in utero and then also received tadalafil in their drinking water until 4 weeks of age (Asai et al. 2007). Treated hind limb and respiratory skeletal muscles exhibited reduced muscle necrosis and fibrosis (Asai et al. 2007). In addition, treated mdx muscles also exhibited decreased variability in myofiber size and a reduction in regenerating centrally nucleated myofibers, suggesting that tadalafil decreased muscle degeneration.
Taken together, these data suggest that tadalafil-mediated inhibition of PDE5A was sufficient to restore blood supply to muscles after exercise and significantly reduced contraction-induced damage in the dystrophin-deficient skeletal muscles. Like the heart, PDE5A inhibition resulted in less contraction-induced damage in mdx muscles. The work of Asai and coworkers supports the proposition that vascular therapy with PDE5A inhibitors may be of therapeutic benefit to DMD patients.
Consistent with findings that mdx myofibers in situ experience excessive contraction-induced damage under ischemic conditions, mdx mice exhibit marked cage inactivity after mild treadmill exercise (Kobayashi et al. 2008). KN1 (nNOS knockout 1) mice also exhibited the same postexercise inactivity, suggesting to the authors that loss of skeletal muscle sarcolemmal nNOSμ was responsible. This postexercise decrease in cage activity is thought to be analogous to the exaggerated fatigue response to mild exercise observed in patients with neuromuscular diseases such as DMD.
To test the impact of improved postcontraction blood flow on postexercise cage activity, Kobayashi and coworkers treated mdx and KN1 mice acutely with tadalafil or sildenafil. Sildenafil (300 mg/kg/day) was administered in the food, while tadalafil (300 mg/kg/day) was administered directly by gavage. Both inhibitors were administered the day before and on the day of exercise testing. PDE5A inhibition substantially increased perfusion of the mdx hind limb with blood after exercise. Interestingly, postexercise inactivity was reduced 30–40% by PDE5A inhibition in mdx mice, but was unaffected in KN1 mice, demonstrating that nNOS expression (nNOS-2, nNOSα, nNOSμ) is required for the postexercise benefits of PDE5A inhibition. This is consistent with findings that NO is required for both basal and maximal activation of sGC activity and for many of the physiological effects of sildenafil (Nagayama et al. 2008; Fernhoff et al. 2009). This may be an important consideration since nNOS levels may be substantially lower or absent in DMD patients, thus lowering the potential efficacy of sildenafil (Chang et al. 1996). Treated mdx mice ran twice the distance of untreated mdx mice. Despite increased exercise performance, serum CK activity was significantly reduced, suggesting reduced membrane permeability in agreement with results reported by Asai et al. (2007). Together, these data suggest that in dystrophin-deficient muscle tissues, the loss of the vasomodulatory function of nNOSμ may be compensated for, to some degree, by PDE5A inhibition.
The relationship between skeletal muscle performance, blood supply, and nNOSμ is complex. Kobayashi and coworkers concluded that the absence of contraction-induced sarcolemmal nNOSμ signaling to the adjacent VSMC was responsible for postexercise inactivity. While the beneficial effects of PDE5A inhibition are clear, the mechanisms by which they occur are not. Although it remains to be determined whether augmentation of nNOSμ signaling from skeletal to VSMC is the sole pathway responsible for these beneficial effects, it seems unlikely for several reasons. First, augmentation of smooth muscle NO-cGMP signaling alone in mdx mice can enhance blood supply and reduce membrane permeability (Ito et al. 2006). Since PDE5A expression is high in VSMC, inhibition of PDE5A may promote smooth muscle relaxation independently of NO produced by skeletal muscle. Second, PDE5A inhibition affects vascular bed function throughout the cardiovascular system and likely enhances systemic hemodynamics during exercise. Third, additional, recently identified nNOS-sGC-PKG signaling pathways in skeletal muscle control postexercise muscle strength that could be affected by PDE5A inhibition in skeletal muscle (Percival et al. 2010). Fourth, nNOS knockout mice exhibit pronounced muscle fatigue and weakness during exercise that could contribute to the observed postexercise weakness (Percival et al. 2008). Finally, acute sildenafil treatment enhances cardiac function in mdx mice and could assist cardiovascular recovery (Khairallah et al. 2008). Thus, the factors that contribute to the postexercise inactivity after mild exercise in mdx mice are many and likely include muscle weakness during and/or after exercise. The beneficial effects of PDE5A inhibition likely reflect effects at many sites of action, making mechanistic interpretation of these whole animal studies very difficult. Nonetheless, while the molecular mechanisms responsible are debatable, Kobayashi et al. have clearly shown that PDE5A inhibition in mdx mice provides nNOS-dependent enhancement of activity during and after exercise as well as reducing exercise-induced muscle damage.
9 Conclusion
Although the examples of therapeutic benefit are few and the mechanisms are poorly understood, the evidence that PDE5A inhibition reduces skeletal and cardiac muscle damage, particularly contraction-induced myofiber damage during exercise, is compelling. PDE5A inhibition also enhances exercise performance, reduces the negative effects of mild exercise, and enhances the workload capacity of dystrophin-deficient hearts. Thus, further studies are required to flesh out this promising therapeutic approach. However, mice are not men and ultimately, any efficacy of PDE5A inhibitors in preclinical studies must be validated in a clinical setting. Efficacy in both skeletal muscle and cardiac tissue makes PDE5A inhibition particularly attractive as a therapeutic approach and warrants further research into the potential utility of PDE5A inhibition in the treatment of cardiovascular disease in DMD.
Acknowledgments
The authors thanks members of the Froehner lab and Dr Kimberley Craven for helpful discussions and suggestions. Research related to the role of nNOS and PDE5A inhibitors in our laboratories is supported by the Muscular Dystrophy Association (JMP), Charlie’s Fund (SCF and JAB), Parent Project Muscular Dystrophy (JMP and SCF), NIH grants NS33145 (SCF), NS59514 (SCF and JAB), and AR056221 (SCF and JAB).
Contributor Information
Justin M. Percival, Email: justinp2@u.washington.edu, Department of Physiology and Biophysics, University of Washington, 357290, 98195-7290 Seattle, WA, USA
Candace M. Adamo, Department of Pharmacology, University of Washington, 357290, 98195-7290 Seattle, WA, USA
Joseph A. Beavo, Department of Pharmacology, University of Washington, 357290, 98195-7290 Seattle, WA, USA
Stanley C. Froehner, Department of Physiology and Biophysics, University of Washington, 357290, 98195-7290 Seattle, WA, USA
References
- Adamo CM, Dai DF, Percival JM, Minami E, Willis MS, Patrucco E, Froehner SC, Beavo JA. Sildenafil reverses cardiac dysfunction in the mdx mouse model of Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2010;107:19079–19083. doi: 10.1073/pnas.1013077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams ME, Kramarcy N, Krall SP, Rossi SG, Rotundo RL, Sealock R, Froehner SC. Absence of alpha-syntrophin leads to structurally aberrant neuromuscular synapses deficient in utrophin. J Cell Biol. 2000;150:1385–1398. doi: 10.1083/jcb.150.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams ME, Mueller HA, Froehner SC. In vivo requirement of the alpha-syntrophin PDZ domain for the sarcolemmal localization of nNOS and aquaporin-4. J Cell Biol. 2001;155:113–122. doi: 10.1083/jcb.200106158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai A, Sahani N, Kaneki M, Ouchi Y, Martyn JA, Yasuhara SE. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phospho-diesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS One. 2007;2:e806. doi: 10.1371/journal.pone.0000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, Hobai IA, Lemmon CA, Burnett AL, O’Rourke B, Rodriguez ER, Huang PL, Lima JA, Berkowitz DE, Hare JM. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–339. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- Bendall JK, Damy T, Ratajczak P, Loyer X, Monceau V, Marty I, Milliez P, Robidel E, Marotte F, Samuel JL, Heymes C. Role of myocardial neuronal nitric oxide synthase-derived nitric oxide in beta-adrenergic hyporesponsiveness after myocardial infarction-induced heart failure in rat. Circulation. 2004;110:2368–2375. doi: 10.1161/01.CIR.0000145160.04084.AC. [DOI] [PubMed] [Google Scholar]
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- Bia BL, Cassidy PJ, Young ME, Rafael JA, Leighton B, Davies KE, Radda GK, Clarke K. Decreased myocardial nNOS, increased iNOS and abnormal ECGs in mouse models of Duchenne muscular dystrophy. J Mol Cell Cardiol. 1999;31:1857–1862. doi: 10.1006/jmcc.1999.1018. [DOI] [PubMed] [Google Scholar]
- Bloom TJ. Cyclic nucleotide phosphodiesterase isozymes expressed in mouse skeletal muscle. Can J Physiol Pharmacol. 2002;80:1132–1135. doi: 10.1139/y02-149. [DOI] [PubMed] [Google Scholar]
- Bostick B, Yue Y, Long C, Marschalk N, Fine DM, Chen J, Duan D. Cardiac expression of a mini-dystrophin that normalizes skeletal muscle force only partially restores heart function in aged mdx mice. Mol Ther. 2009;17:253–261. doi: 10.1038/mt.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger CM, Heymes C, Benessiano J, Geske RS, Lévy BI, Vanhoutte PM. Neuronal nitric oxide synthase is expressed in rat vascular smooth muscle cells: activation by angiotensin II in hypertension. Circ Res. 1998;83:1271–1278. doi: 10.1161/01.res.83.12.1271. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Xia H, Aldape K, Bredt DS. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Bushby K, Finkel R, Birnkrant DJ, Case LE, Clemens PR, Cripe L, Kaul A, Kinnett K, McDonald C, Pandya S, Poysky J, Shapiro F, Tomezsko J, Constantin C DMD Care Considerations Working Group. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2009;9:77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- Chamberlain JS, Metzger J, Reyes M, Townsend D, Faulkner JA. Dystrophin-deficient mdx mice display a reduced life span and are susceptible to spontaneous rhabdomyosarcoma. FASEB J. 2007;21:2195–2204. doi: 10.1096/fj.06-7353com. [DOI] [PubMed] [Google Scholar]
- Chang WJ, Iannaccone ST, Lau KS, Masters BS, McCabe TJ, McMillan K, Padre RC, Spencer MJ, Tidball JG, Stull JT. Neuronal nitric oxide synthase and dystrophin-deficient muscular dystrophy. Proc Natl Acad Sci USA. 1996;93:9142–9147. doi: 10.1073/pnas.93.17.9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao DS, Gorospe JR, Brenman JE, Rafael JA, Peters MF, Froehner SC, Hoffman EP, Chamberlain JS, Bredt DS. Selective loss of sarcolemmal nitric oxide synthase in Becker muscular dystrophy. J Exp Med. 1996;184:609–618. doi: 10.1084/jem.184.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao DS, Silvagno F, Xia H, Cornwell TL, Lincoln TM, Bredt DS. Nitric oxide synthase and cyclic GMP-dependent protein kinase concentrated at the neuromuscular endplate. Neuroscience. 1997;76:665–672. doi: 10.1016/s0306-4522(96)00367-3. [DOI] [PubMed] [Google Scholar]
- Chenard AA, Becane HM, Tertrain F, de Kermadec JM, Weiss YA. Ventricular arrhythmia in Duchenne muscular dystrophy: prevalence, significance and prognosis. Neuromuscul Disord. 1993;3:201–206. doi: 10.1016/0960-8966(93)90060-w. [DOI] [PubMed] [Google Scholar]
- Chu V, Otero JM, Lopez O, Sullivan MF, Morgan JP, Amende I, Hampton TG. Electrocardiographic findings in mdx mice: a cardiac phenotype of Duchenne muscular dystrophy. Muscle Nerve. 2002;26:513–519. doi: 10.1002/mus.10223. [DOI] [PubMed] [Google Scholar]
- Colussi C, Mozzetta C, Gurtner A, Illi B, Rosati J, Straino S, Ragone G, Pescatori M, Zaccagnini G, Antonini A, Minetti G, Martelli F, Piaggio G, Gallinari P, Steinkuhler C, Clementi E, Dell’Aversana C, Altucci L, Mai A, Capogrossi MC, Puri PL, Gaetano C. HDAC2 blockade by nitric oxide and histone deacetylase inhibitors reveals a common target in Duchenne muscular dystrophy treatment. Proc Natl Acad Sci USA. 2008;105:19183–19187. doi: 10.1073/pnas.0805514105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven KB, Zagotta WN. CNG and HCN channels: two peas, one pod. Annu Rev Physiol. 2006;68:375–401. doi: 10.1146/annurev.physiol.68.040104.134728. [DOI] [PubMed] [Google Scholar]
- Crosbie RH, Barresi R, Campbell KP. Loss of sarcolemma nNOS in sarcoglycan-deficient muscle. FASEB J. 2002;16:1786–1791. doi: 10.1096/fj.02-0519com. [DOI] [PubMed] [Google Scholar]
- Danialou G, Comtois AS, Dudley R, Karpati G, Vincent G, Des Rosiers C, Petrof BJ. Dystrophin-deficient cardiomyocytes are abnormally vulnerable to mechanical stress-induced contractile failure and injury. FASEB J. 2001;15:1655–1667. doi: 10.1096/fj.01-0030fje. [DOI] [PubMed] [Google Scholar]
- Das A, Xi L, Kukreja RC. Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem. 2008;283:29572–29585. doi: 10.1074/jbc.M801547200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KE, Nowak KJ. Molecular mechanisms of muscular dystrophies: old and new players. Nat Rev Mol Cell Biol. 2006;7:762–773. doi: 10.1038/nrm2024. [DOI] [PubMed] [Google Scholar]
- de Kermadec JM, Bécane HM, Chénard A, Tertrain F, Weiss Y. Prevalence of left ventricular systolic dysfunction in Duchenne muscular dystrophy: an echocardiographic study. Am Heart J. 1994;127:618–623. doi: 10.1016/0002-8703(94)90672-6. [DOI] [PubMed] [Google Scholar]
- Dellorusso C, Crawford RW, Chamberlain JS, Brooks SV. Tibialis anterior muscles in mdx mice are highly susceptible to contraction-induced injury. J Muscle Res Cell Motil. 2001;22:467–475. doi: 10.1023/a:1014587918367. [DOI] [PubMed] [Google Scholar]
- Eagle M, Bourke J, Bullock R, Gibson M, Mehta J, Giddings D, Straub V, Bushby K. Managing Duchenne muscular dystrophy-the additive effect of spinal surgery and home nocturnal ventilation in improving survival. Neuromuscul Disord. 2007;17:470–475. doi: 10.1016/j.nmd.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Ervasti JM. Dystrophin, its interactions with other proteins, and implications for muscular dystrophy. Biochim Biophys Acta. 2007;1772:108–117. doi: 10.1016/j.bbadis.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Ervasti JM, Campbell KP. Dystrophin and the membrane skeleton. Curr Opin Cell Biol. 1993;5:82–87. doi: 10.1016/s0955-0674(05)80012-2. [DOI] [PubMed] [Google Scholar]
- Eu JP, Sun J, Xu L, Stamler JS, Meissner G. The skeletal muscle calcium release channel: coupled O2 sensor and NO signaling functions. Cell. 2000;102:499–509. doi: 10.1016/s0092-8674(00)00054-4. [DOI] [PubMed] [Google Scholar]
- Fernhoff NB, Derbyshire ER, Marletta MA. A nitric oxide/cysteine interaction mediates the activation of soluble guanylate cyclase. Proc Natl Acad Sci USA. 2009;106:21602–21607. doi: 10.1073/pnas.0911083106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J, Stöllberger C. The heart in human dystrophinopathies. Cardiology. 2003;99:1–19. doi: 10.1159/000068446. [DOI] [PubMed] [Google Scholar]
- Firestein BL, Bredt DS. Interaction of neuronal nitric-oxide synthase and phosphofructokinase-M. J Biol Chem. 1999;274:10545–10550. doi: 10.1074/jbc.274.15.10545. [DOI] [PubMed] [Google Scholar]
- Frankel KA, Rosser RJ. The pathology of the heart in progressive muscular dystrophy: epimyocardial fibrosis. Hum Pathol. 1976;7:375–386. doi: 10.1016/s0046-8177(76)80053-6. [DOI] [PubMed] [Google Scholar]
- Hillier BJ, Christopherson KS, Prehoda KE, Bredt DS, Lim WA. Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS-syntrophin complex. Science. 1999;284:812–815. [PubMed] [Google Scholar]
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Hofmann F, Bernhard D, Lukowski R, Weinmeister P. cGMP regulated protein kinases (cGK) Handb Exp Pharmacol. 2009;191:137–162. doi: 10.1007/978-3-540-68964-5_8. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Bach JR, Minami R. Cardioprotection for Duchenne’s muscular dystrophy. Am Heart J. 1999;137:895–902. doi: 10.1016/s0002-8703(99)70414-x. [DOI] [PubMed] [Google Scholar]
- Ito K, Kimura S, Ozasa S, Matsukura M, Ikezawa M, Yoshioka K, Ueno H, Suzuki M, Araki K, Yamamura K, Miwa T, Dickson G, Thomas GD, Miike T. Smooth muscle-specific dystrophin expression improves aberrant vasoregulation in mdx mice. Hum Mol Genet. 2006;15:2266–2275. doi: 10.1093/hmg/ddl151. [DOI] [PubMed] [Google Scholar]
- Jerusalem F, Engel AG, Gomez MR. Duchenne dystrophy I. morphometric study of the muscle microvasculature. Brain. 1974;97:115–122. doi: 10.1093/brain/97.1.115. [DOI] [PubMed] [Google Scholar]
- Kameya S, Miyagoe Y, Nonaka I, Ikemoto T, Endo M, Hanaoka K, Nabeshima Y, Takeda S. alpha1-syntrophin gene disruption results in the absence of neuronal-type nitric-oxide synthase at the sarcolemma but does not induce muscle degeneration. J Biol Chem. 1999;274:2193–2200. doi: 10.1074/jbc.274.4.2193. [DOI] [PubMed] [Google Scholar]
- Khairallah M, Khairallah R, Young ME, Dyck JR, Petrof BJ, Des Rosiers C. Metabolic and signaling alterations in dystrophin-deficient hearts precede overt cardiomyopathy. J Mol Cell Cardiol. 2007;43:119–1129. doi: 10.1016/j.yjmcc.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Khairallah M, Khairallah RJ, Young ME, Allen BG, Gillis MA, Danialou G, Deschepper CF, Petrof BJ, Des Rosiers C. Sildenafil and cardiomyocyte-specific cGMP signaling prevent cardiomyopathic changes associated with dystrophin deficiency. Proc Natl Acad Sci USA. 2008;105:7028–7033. doi: 10.1073/pnas.0710595105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi YM, Rader EP, Crawford RW, Iyengar NK, Thedens DR, Faulkner JA, Parikh SV, Weiss RM, Chamberlain JS, Moore SA, Campbell KP. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature. 2008;456:511–515. doi: 10.1038/nature07414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig M, Monaco AP, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53:219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Lai Y, Thomas GD, Yue Y, Yang HT, Li D, Long C, Judge L, Bostick B, Chamberlain JS, Terjung RL, Duan D. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J Clin Invest. 2009;119:624–635. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowski R, Rybalkin SD, Loga F, Leiss V, Beavo JA, Hofmann F. Cardiac hypertrophy is not amplified by deletion of cGMP-dependent kinase-I in cardiomyocytes. Proc Natl Acad Sci USA. 2010;107:5646–5651. doi: 10.1073/pnas.1001360107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2008;1:CD003725. doi: 10.1002/14651858.CD003725.pub3. [DOI] [PubMed] [Google Scholar]
- Markham LW, Michelfelder EC, Border WL, Khoury PR, Spicer RL, Wong BL, Benson DW, Cripe LH. Abnormalities of diastolic function precede dilated ardiomyopathy associated with Duchenne muscular dystrophy. J Am Soc Echocardiogr. 2006;19:865–871. doi: 10.1016/j.echo.2006.02.003. [DOI] [PubMed] [Google Scholar]
- McConell GK, Wadley GD. Potential role of nitric oxide in contraction-stimulated glucose uptake and mitochondrial biogenesis in skeletal muscle. Clin Exp Pharmacol Physiol. 2008;35:1488–1492. doi: 10.1111/j.1440-1681.2008.05038.x. [DOI] [PubMed] [Google Scholar]
- McConell GK, Bradley SJ, Stephens TJ, Canny BJ, Kingwell BA, Lee-Young RS. Skeletal muscle nNOS mu protein content is increased by exercise training in humans. Am J Physiol Regul Integr Comp Physiol. 2007;293:R821–R828. doi: 10.1152/ajpregu.00796.2006. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Engel WK, Derrer EC. Duchenne muscular dystrophy: functional ischemia reproduces its characteristic lesions. Science. 1971;172:1143–1145. doi: 10.1126/science.172.3988.1143. [DOI] [PubMed] [Google Scholar]
- Mergia E, Koesling D, Friebe A. Genetic mouse models of the NO receptor ‘soluble’ guanylyl cyclases. Handb Exp Pharmacol. 2009;191:33–46. doi: 10.1007/978-3-540-68964-5_3. [DOI] [PubMed] [Google Scholar]
- Moriuchi T, Kagawa N, Mukoyama M, Hizawa K. Autopsy analyses of the muscular dystrophies Tokushima. J Exp Med. 1993;40:83–93. [PubMed] [Google Scholar]
- Nagayama T, Zhang M, Hsu S, Takimoto E, Kass DA. Sustained soluble guanylate cyclase stimulation offsets nitric-oxide synthase inhibition to restore acute cardiac modulation by sildenafil. J Pharmacol Exp Ther. 2008;326:380–387. doi: 10.1124/jpet.108.137422. [DOI] [PubMed] [Google Scholar]
- Percival JM, Adams ME, Froehner SC. Syntrophin: a molecular adaptor conferring a signaling role to the dystrophin-associated protein complex. In: Winder SJ, editor. Molecular mechanisms of muscular dystrophies. Landes Bioscience; TX: 2006. [Google Scholar]
- Percival JM, Anderson KN, Gregorevic P, Chamberlain JS, Froehner SC. Functional deficits in nNOSmu-deficient skeletal muscle: myopathy in nNOS knockout mice. PLoS ONE. 2008;3:e3387. doi: 10.1371/journal.pone.0003387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival JM, Anderson KN, Huang PL, Adams ME, Froehner SC. Golgi and sarcolemmal nNOS isozymes differentially regulate contraction-induced fatigue and vasoconstriction in exercising skeletal muscle. J Clin Invest. 2010;120:816–826. doi: 10.1172/JCI40736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci USA. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan JG, Hahn HS, Wong BL, Lorenz JN, Wenisch AS, Levin LS. Evolution of the mdx mouse cardiomyopathy: physiological and morphological findings. Neuromuscul Disord. 2004;14:491–496. doi: 10.1016/j.nmd.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Reffelmann T, Kloner RA. Phosphodiesterase 5 inhibitors: are they cardioprotective? Cardiovasc Res. 2009;83:204–12. doi: 10.1093/cvr/cvp170. [DOI] [PubMed] [Google Scholar]
- Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraiva RM, Minhas KM, Raju SV, Barouch LA, Pitz E, Schuleri KH, Vandegaer K, Li D, Hare JM. Deficiency of neuronal nitric oxide synthase increases mortality and cardiac remodeling after myocardial infarction: role of nitroso-redox equilibrium. Circulation. 2005;112:3415–3422. doi: 10.1161/CIRCULATIONAHA.105.557892. [DOI] [PubMed] [Google Scholar]
- Sears CE, Bryant SM, Ashley EA, Lygate CA, Rakovic S, Wallis HL, Neubauer S, Terrar DA, Casadei B. Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ Res. 2003;92:e52–e59. doi: 10.1161/01.RES.0000064585.95749.6D. [DOI] [PubMed] [Google Scholar]
- Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, Casadei B, Chowienczyk P, Shah AM. Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circulation. 2009;119:2656–2662. doi: 10.1161/CIRCULATIONAHA.108.822205. [DOI] [PubMed] [Google Scholar]
- Senzaki H, Smith CJ, Juang GJ, Isoda T, Mayer SP, Ohler A, Paolocci N, Tomaselli GF, Hare JM, Kass DA. Cardiac phosphodiesterase 5 (cGMP-specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J. 2001;15:1718–1726. doi: 10.1096/fj.00-0538com. [DOI] [PubMed] [Google Scholar]
- Silvagno F, Xia H, Bredt DS. Neuronal nitric-oxide synthase-mu, an alternatively spliced isoform expressed in differentiated skeletal muscle. J Biol Chem. 1996;271:11204–11208. doi: 10.1074/jbc.271.19.11204. [DOI] [PubMed] [Google Scholar]
- Spurney CF, Knoblach S, Pistilli EE, Nagaraju K, Martin GR, Hoffman EP. Dystrophin-deficient cardiomyopathy in mouse: expression of Nox4 and Lox are associated with fibrosis and altered functional parameters in the heart. Neuromuscul Disord. 2008;18:371–381. doi: 10.1016/j.nmd.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, Narusawa M, Leferovich JM, Sladky JT, Kelly AM. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–359. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem. 2004;279:36167–36170. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Motohashi N, Uezumi A, Fukada S, Yoshimura T, Itoyama Y, Aoki M, Miyagoe-Suzuki Y, Takeda S. NO production results in suspension-induced muscle atrophy through dislocation of neuronal NOS. J Clin Invest. 2007;117:2468–2476. doi: 10.1172/JCI30654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol. 1998;506:817–826. doi: 10.1111/j.1469-7793.1998.817bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci USA. 1998;95:15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GD, Shaul PW, Yuhanna IS, Froehner SC, Adams ME. Vasomodulation by skeletal muscle-derived nitric oxide requires alpha-syntrophin-mediated sarcolemmal localization of neuronal nitric oxide synthase. Circ Res. 2003;92:554–560. doi: 10.1161/01.RES.0000061570.83105.52. [DOI] [PubMed] [Google Scholar]
- Thrush PT, Allen HD, Viollet L, Mendell JR. Re-examination of the electrocardiogram in boys with Duchenne muscular dystrophy and correlation with its dilated cardiomyopathy. Am J Cardiol. 2009;103:262–265. doi: 10.1016/j.amjcard.2008.08.064. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Wehling-Henricks M. Expression of a NOS transgene in dystrophin-deficient muscle reduces muscle membrane damage without increasing the expression of membrane-associated cytoskeletal proteins. Mol Genet Metab. 2004;82:312–320. doi: 10.1016/j.ymgme.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Townsend D, Yasuda S, Li S, Chamberlain JS, Metzger JM. Emergent dilated cardiomyopathy caused by targeted repair of dystrophic skeletal muscle. Mol Ther. 2008;16:832–835. doi: 10.1038/mt.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandebrouck C, Duport G, Cognard C, Raymond G. Cationic channels in normal and dystrophic human myotubes. Neuromuscul Disord. 2001;11:72–79. doi: 10.1016/s0960-8966(00)00153-x. [DOI] [PubMed] [Google Scholar]
- Vandeput F, Krall J, Ockaili R, Salloum FN, Florio V, Corbin JD, Francis SH, Kukreja RC, Movsesian MA. cGMP-hydrolytic activity and its inhibition by sildenafil in normal and failing human and mouse myocardium. J Pharmacol Exp Ther. 2009;330:884–891. doi: 10.1124/jpet.109.154468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis RM, Corbin JD, Francis SH, Ellis P. Tissue distribution of phospho-diesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am J Cardiol. 1999;83:3C–12C. doi: 10.1016/s0002-9149(99)00042-9. [DOI] [PubMed] [Google Scholar]
- Ward ME, Toporsian M, Scott JA, Teoh H, Govindaraju V, Quan A, Wener AD, Wang G, Bevan SC, Newton DC, Marsden PA. Hypoxia induces a functionally significant and translationally efficient neuronal NO synthase mRNA variant. J Clin Invest. 2005;115:3128–3139. doi: 10.1172/JCI20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehling-Henricks M, Jordan MC, Roos KP, Deng B, Tidball JG. Cardiomyopathy in dystrophin-deficient hearts is prevented by expression of a neuronal nitric oxide synthase transgene in the myocardium. Hum Mol Genet. 2005;14:1921–1933. doi: 10.1093/hmg/ddi197. [DOI] [PubMed] [Google Scholar]
- Wehling-Henricks M, Oltmann M, Rinaldi C, Myung KH, Tidball JG. Loss of positive allosteric interactions between neuronal nitric oxide synthase and phosphoructokinase contributes to defects in glycolysis and increased fatigability in muscular dystrophy. Hum Mol Genet. 2009;18:3439–3451. doi: 10.1093/hmg/ddp288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead NP, Streamer M, Lusambili LI, Sachs F, Allen DG. Streptomycin reduces stretch-induced membrane permeability in muscles from mdx mice. Neuromuscul Disord. 2006;16:845–854. doi: 10.1016/j.nmd.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Willmann R, Possekel S, Dubach-Powell J, Meier T, Ruegg MA. Mammalian animal models for Duchenne muscular dystrophy. Neuromuscul Disord. 2009;19:241–249. doi: 10.1016/j.nmd.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Wu B, Moulton HM, Iversen PL, Jiang J, Li J, Li J, Spurney CF, Sali A, Guerron AD, Nagaraju K, Doran T, Lu P, Xiao X, Lu QL. Effective rescue of dystrophin improves cardiac function in dystrophin-deficient mice by a modified morpholino oligomer. Proc Natl Acad Sci USA. 2008;105:14814–14819. doi: 10.1073/pnas.0805676105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu KY, Huso DL, Dawson TM, Bredt DS, Becker LC. Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc Natl Acad Sci USA. 1999;96:657–662. doi: 10.1073/pnas.96.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YH, Zhang MH, Sears CE, Emanuel K, Redwood C, El-Armouche A, Kranias EG, Casadei B. Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice. Circ Res. 2008;102:242–249. doi: 10.1161/CIRCRESAHA.107.164798. [DOI] [PubMed] [Google Scholar]