Abstract
Objective
To test the hypothesis that restoration of antithrombin plasma concentrations attenuates vascular leakage by inhibiting neutrophil activation through syndecan-4 receptor inhibition in an established ovine model of acute lung injury.
Design
Randomized controlled laboratory experiment.
Setting
University animal research facility.
Subjects
Eighteen chronically instrumented sheep.
Interventions
Following combined burn and smoke inhalation injury (40% of total body surface area, third-degree flame burn; 4 × 12 breaths of cold cotton smoke), chronically instrumented sheep were randomly assigned to receive an IV infusion of 6 IU/kg/hr recombinant human antithrombin III or normal saline (n = 6 each) during the 48-hour study period. In addition, six sham animals (not injured, continuous infusion of vehicle) were used to obtain reference values for histological and immunohistochemical analyses.
Measurements and Main Results
Compared to control animals, recombinant human antithrombin III reduced the number of neutrophils per hour in the pulmonary lymph (p < 0.01 at 24 and 48 hr), alveolar neutrophil infiltration (p = 0.04), and pulmonary myeloperoxidase activity (p = 0.026). Flow cytometric analysis revealed a significant reduction of syndecan-4-positive neutrophils (p = 0.002 vs control at 24 hr). Treatment with recombinant human antithrombin III resulted in a reduction of pulmonary nitrosative stress (p = 0.002), airway obstruction (bronchi: p = 0.001, bronchioli: p = 0.013), parenchymal edema (p = 0.044), and lung bloodless wet-to-dry-weight ratio (p = 0.015). Clinically, recombinant human antithrombin III attenuated the increased pulmonary transvascular fluid flux (12–48 hr: p ≤ 0.001 vs control each) and the deteriorated pulmonary gas exchange (12–48 hr: p < 0.05 vs control each) without increasing the risk of bleeding.
Conclusions
The present study provides evidence for the interaction between antithrombin and neutrophils in vivo, its pathophysiological role in vascular leakage, and the therapeutic potential of recombinant human antithrombin III in a large animal model of acute lung injury.
Keywords: acute respiratory distress syndrome, burn and smoke inhalation injury, neutrophil migration, syndecan-4 receptor
Introduction
Pulmonary vascular leakage represents a central pathomechanism of burn and smoke inhalation injury (1), resulting in interstitial edema, impairment of microcirculation, organ dysfunction, and eventually multiple organ failure. The development of acute lung injury (ALI) due to severe pulmonary edema increases the mortality of burn patients by ~40% (2). Therefore, therapeutic strategies to limit this fatal pathophysiological cascade are urgently warranted. However, the underlying mechanistic aspects of the pulmonary hyperpermeability are not completely understood.
Combined burn and smoke inhalation injury is characterized by a hypercoagulable state especially within the first 24–48 hours after the injury (3) and a decrease in plasma concentrations of antithrombin III (ATIII) by 50% (4). The central role of ATIII in the pathogenesis of burn and smoke inhalation injury is emphasized by the fact that ATIII deficiency represents an independent predictor of length of hospital stay and mortality (5, 6). In addition to coagulation disorders, there is a marked activation of the inflammation cascade in ALI (1). Notably, ATIII not only represents the most potent endogenous anticoagulant (7) but also provides multiple anti-inflammatory properties independent from its anticoagulant effects (8). These anti-inflammatory effects are based on an interaction with neutrophils mediated via the syndecan-4 receptor (9), a G-protein-coupled transmembrane proteoglycan receptor on endothelial cells and neutrophils (10). Whereas there are some in vitro studies demonstrating the inhibition of neutrophil activation by ATIII (9–11), the clinical relevance and the therapeutic potential of ATIII for the treatment of vascular leakage remain to be determined.
We hypothesized that an IV infusion of recombinant human ATIII (rhATIII) attenuates vascular leakage by inhibiting neutrophil activation and their transendothelial migration via the syndecan-4 receptor in a clinically relevant, established ovine model of ALI (12–14). Therefore, the present prospective, randomized, controlled laboratory experiment was designed to elucidate the effects of rhATIII on neutrophil activation and their transendothelial migration as well as on pulmonary transvascular fluid flux, pulmonary edema, gas exchange, nitrosative stress, and lung histology changes (15).
Methods
Instrumentation and Surgical Procedures
After approval by the Local Animal Research Committee, 18 female sheep were anesthetized and instrumented for chronic hemodynamic monitoring using an established protocol. Details on the instrumentation are provided in the supplemental data.
Experimental Protocol
After 5 days of recovery, baseline (BL) measurements were performed. Following tracheostomy and placement of an urinary bladder catheter, sheep were subjected to a 40% of total body surface area, third-degree flame burn and 4 × 12 breaths of cold cotton smoke under deep anesthesia using an established protocol (13, 14, 16). The sheep were then randomly assigned to the study groups (n = 6 each): control (injured, continuous infusion of vehicle [NaCl, 0.9%]) or rhATIII (injured, continuous infusion of 6 IU/kg/hr rhATIII [GTC Biotherapeutics, Framingham, MI] from 1 hr postinjury until the end of the study period). The investigators were unaware of the animals’ group assignment during the experiment. In addition, six sham animals (not injured, continuous infusion of vehicle) were used to obtain reference values for histological and immunohistochemical analyses. During the experiment, all animals were deprived from drinking water and equally resuscitated with IV lactated Ringer’s solution according to the formula: 4 mL/kg/% burned body surface area within 24 hours (17). To compensate for the burn-induced hypovolemia, sheep received 50% of the fluid amount calculated for 24 hours within the first 8 hours after injury. After 48 hours, sheep were deeply anesthetized with ketamine and euthanized by injection of 60 mL of saturated potassium chloride.
Pulmonary Hemodynamics, Gas Exchange, and Plasma and Lymph Analyses
Hemodynamic measurements, analyses of plasma and lymph samples for determination of neutrophil counts, nitric oxide, protein concentration, variables of plasmatic coagulation, and flow cytometric analysis for syndecan-4 receptor activation were performed at specific time points. Details on these measurements are provided in the supplemental data.
Histological, Western Blot, and Immunohistochemical Analyses
Histological analyses and assessment of bloodless lung wet-to-dry-weight ratio were performed as reported in detail earlier (18, 19). 3-Nitrotyrosine protein expression in lung tissue was determined using anti-3-nitrotyrosine monoclonal antibody (Cayman Chemicals, Ann Arbor, MI; Catalog No. 10006966) with streptavidin secondary antibody as described previously (20). Myeloperoxidase activity was determined using a commercially available assay (Myeloperoxidase Activity Assay, Northwest Life Science Specialties, Vancouver, Canada) according to the manufacturer’s protocol.
Statistical Analyses
Sigma Stat 3.1 software (Systat Software, San Jose, CA) was used for statistical analyses. Analysis of variance (ANOVA) methodologies appropriate for two factor experiments with repeated measures across time for each animal were used. Each variable was analyzed separately for differences among groups, across time, and for group by time interaction. After confirming the significance of different group effects over time, post hoc pairwise comparisons among groups were performed using the Student-Newman-Keuls procedure to adjust for the elevated false-positive rate found otherwise in multiple testing. Wet-to-dry-weight ratio, histological scores, tissue concentrations of 3-nitrotyrosine, and myeloperoxidase activity were compared with one-way ANOVA and Holm-Sidak post hoc analysis. Data are presented as mean ± SEM. Differences were considered as statistically significant, when p was less than 0.05.
Results
BL Characteristics
There were no differences among study groups in any of the investigated variables at BL. Mean body weight (control: 39 kg ± 2 kg vs rhATIII: 34 kg ± 2 kg; p = 0.126) and carboxyhemoglobin values after the smoke inhalation, as an index of the severity of injury, did not differ among groups (control: 66% ± 6% vs rhATIII: 69% ± 7%; p = 0.78).
Laboratory Analyses
In control animals, combined burn and smoke inhalation injury was associated with an immediate decrease of ATIII plasma concentrations resulting in significantly lower values compared with BL over the whole study period (p < 0.05 each). The continuous infusion of rhATIII prevented this decrease and kept ATIII plasma concentrations not only higher than in control animals (p < 0.05 each) but also at BL level.
There was an increase in prothrombin time in both groups as compared to BL at 12 hours (p < 0.01 each). However, no statistical differences in variables of plasmatic coagulation, that is, prothrombin time, activated clotting time, and activated partial thromboplastin time, could be shown between the two study groups. Laboratory variables are depicted in detail in Table 1.
Table 1.
Pulmonary arterial pressure, gas exchange, ventilatory parameters, variables of acid base balance as well as coagulation and rhATIII levels
| Variable | Group | BL | 6h | 12 h | 24 h | 48 h |
|---|---|---|---|---|---|---|
| MPAP | control | 20 ± 1 | 26 ± 1* | 30 ± 1* | 31 ± 1* | 34 ± 4* |
| (mm Hg) | rhATIII | 19 ± 1 | 24 ± 1* | 26 ± 1*† | 25 ± 1*† | 28 ± 2* |
| PaO2/FiO2 | control | 506 ± 16 | 503 ± 45 | 431 ± 34 | 160 ± 28* | 83 ± 6* |
| (mmHg) | rhATIII | 540 ± 18 | 554 ± 61 | 574 ± 71† | 358 ± 65*† | 199 ± 48*† |
| Peak pressure | control | 19 ± 1 | 19 ± 1 | 21 ± 1 | 31 ± 2* | 40 ± 2* |
| (cmH2O) | rhATIII | 20 ± 1 | 19 ± 1 | 21 ± 1 | 24 ± 3† | 31 ± 4*† |
| Plateau pressure | control | 17 ± 1 | 16 ± 0 | 18 ± 1 | 28 ± 2* | 39 ± 3* |
| (cmH2O) | rhATIII | 17 ± 0 | 16 ± 1 | 18 ± 1 | 19 ± 2† | 29 ± 4*† |
| Respiratory rate | control | 20 ± 0 | 21 ± 0 | 19 ± 1 | 23 ± 2 | 38 ± 3* |
| (breaths·min−1) | rhATIII | 20 ± 0 | 20 ± 1 | 20 ± 0 | 20 ± 0 | 28 ± 5*† |
| ACT | control | 141 ± 6 | / | 157 ± 8 | 143 ± 10 | 136 ± 8 |
| (sec) | rhATIII | 142 ± 9 | / | 145 ± 8 | 152 ± 10 | 143 ± 11 |
| PT | control | 78 ± 4 | / | 94 ± 5* | 86 ± 5 | 99 ± 7* |
| (sec) | rhATIII | 75 ± 7 | / | 93 ± 5* | 86 ± 5 | 86 ± 6 |
| aPTT | control | 110 ± 4 | / | 117 ± 5 | 128 ± 8 | 122 ± 7 |
| (sec) | rhATIII | 117 ± 3 | / | 121 ± 7 | 119 ± 5 | 117 ± 4 |
| Protein (plasma) | control | 6.2 ± 0.2 | 4.8 ± 0.1* | 4.5 ± 0.1* | 4.0 ± 0.0* | 3.7 ± 0.2* |
| g·dL−1 | rhATIII | 6.1 ± 0.2 | 5.1 ± 0.2* | 4.9 ± 0.1*† | 4.6 ± 0.1*† | 4.9 ± 0.2*† |
| Protein (lymph) | control | 4.1 ± 0.2 | 2.7 ± 0.2* | 2.6 ± 0.2* | 2.2 ± 0.1* | 1.8 ± 0.2* |
| g·dL−1 | rhATIII | 4.5 ± 0.2 | 3.1 ± 0.1* | 3.3 ± 0.2*† | 2.9 ± 0.1*† | 2.9 ± 0.4*† |
| NOx plasma | control | 2.4 ± 0.4 | 8.0 ± 2.0* | 10.9±1.7* | 5.2 ± 1.3 | 10.9±1.9* |
| (µM) | rhATIII | 2.6 ± 0.4 | 4.7 ± 0.5 | 5.3 ± 0.6† | 2.0 ± 0.8 | 1.8 ± 0.6† |
| AT plasma | control | 100 ± 0 | 83 ± 6* | 72 ± 4* | 72 ± 13* | 71 ± 8* |
| (% of BL) | rhATIII | 100 ± 0 | 113 ± 15† | 110 ± 4† | 101 ± 5† | 106 ± 5† |
, p < .05 vs. BL;
, p < .05 vs. control; each group n = 6
ACT, activated clotting time; aPTT, activated partial thromboplastin time; AT, antithrombin; BE, base excess; BL, baseline; FiO2, inspiratory oxygen fraction; MPAP, mean pulmonary arterial pressure; PaCO2, partial pressure of carbon dioxide; PaO2, partial pressure of oxygen; pH, potentia hydrogenii; PT, prothrombin time; rhATIII, recombinant human antithrombin III
Neutrophil Activation and Transendothelial Migration
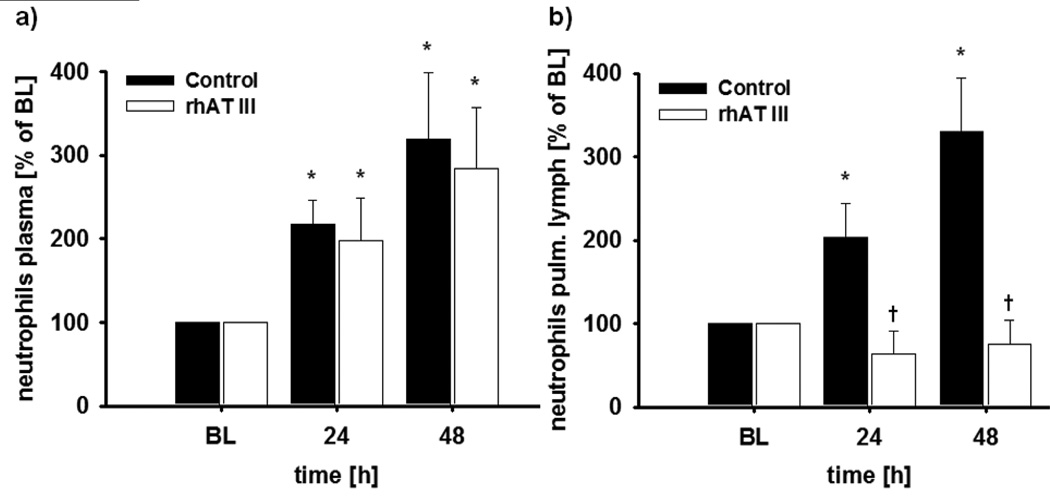
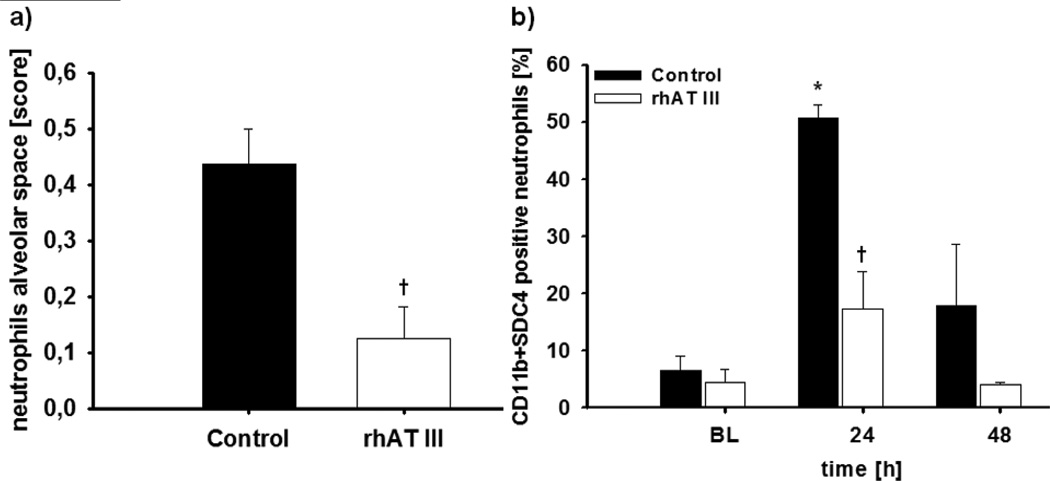
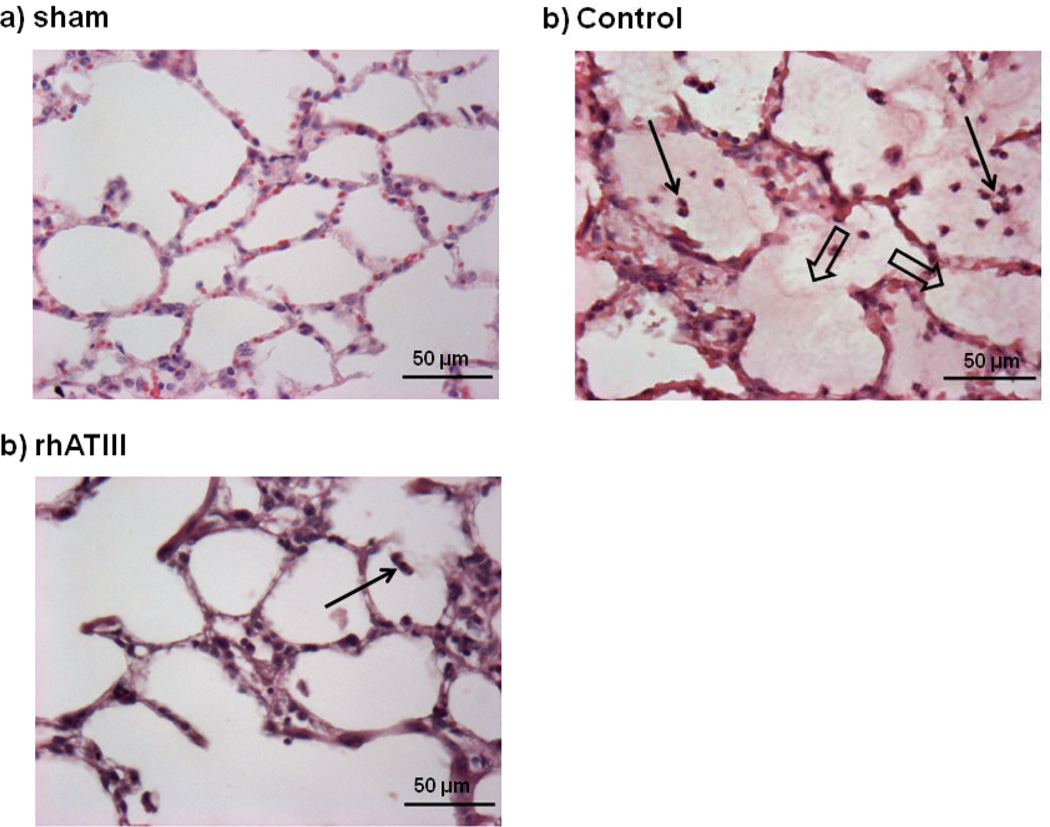
In both study groups, burn and smoke inhalation injury was characterized by an increase of neutrophils in the peripheral blood up to about 300% as compared to BL (p < 0.01 each) (Fig. 1A). Interestingly, there was a corresponding increase in the number of neutrophils per hour in the pulmonary lymph fluid in control animals (p = 0.014 vs BL at 48 hr), but no increase at all in animals treated with rhATIII (p < 0.01 vs control at 24 and 48 hr) (Fig. 1B). In addition, histological analyses revealed significantly less neutrophils in the alveolar space in the rhATIII group as compared to the control group (p = 0.004) (Figs. 2A and 3). These changes were associated with a significant decrease in myeloperoxidase activity, an index of leukocyte accumulation including neutrophils (21), in the rhATIII group as compared to the control group (p = 0.026) (Table 2).
Figure 1.
Neutrophil counts in the plasma (A) and the pulmonary (pulm.) lymph (B). *p < 0.05 versus baseline; †p < 0.05 versus control; n = 6 per group. BL = baseline, rhAT III = recombinant human antithrombin III.
Figure 2.
Neutrophils in the alveolar space (A) and flow cytometric analysis of CD11b and syndecan-4 (SDC4)-positive neutrophils in the plasma (B). *p < 0.05 versus baseline; †p < 0.05 versus control; n = 6 per group. BL = baseline, rhAT III = recombinant human antithrombin III.
Figure 3.
Representative histological slides of sham (A), control (B), and recombinant human antithrombin III (rhATIII) (C) animals. Thin arrows, intra-alveolar polymorphonuclear cells; wide arrows, intra-alveolar edema; n = 6 per group.
Table 2.
Wet-to-dry-weight ratio, histological and immunohistochemical analyses
| Variable | control | rhATIII | sham |
|---|---|---|---|
| Wet-to-dry-weight ratio | 6.0 ± 0.3# | 4.7 ± 0.3† | 4.5 ± 0.1 |
| Obstruction bronchi (score) | 13.7 ± 1.9# | 4.8 ± 0.7† | 3.2 ± 1.2 |
| Obstruction bronchioli (score) | 3.5 ± 0.8# | 0.8 ± 0.2† | 0.9 ± 0.1 |
| Alveolar edema (score) | 1.1 ± 0.3# | 0.3 ± 0.2† | 0.0 ± 0.0 |
| Pulmonary hemorrhage (score) | 0.25 ± 0.09# | 0.04 ± 0.04 | 0.0 ± 0.0 |
| Myeloperoxidase activity (U·mL−1) | 12.2 ± 1.5# | 8.4 ± 0.4#† | 4.0 ± 0.7 |
, p< .05 vs. sham;
, p< .05 vs. control; each group n = 6
rhATIII, recombinant human antithrombin III; w/d-weight, wet-to-dry-weight ratio
Flow cytometric analysis of CD11b and syndecan-4-positive neutrophils in the peripheral blood showed a peak after 24 hours in control animals (p = 0.001 vs BL) (Fig. 2B). This increase was prevented in animals treated with rhATIII (p = 0.002 vs control).
Nitrosative Stress
Plasma concentrations of nitrites and nitrates (NOx) significantly increased in control animals as compared to BL (p < 0.05 at 24 and 48 hr) (Table 1). This increase was significantly attenuated in the rhATIII group (p < 0.05 vs control at 24 and 48 hr).
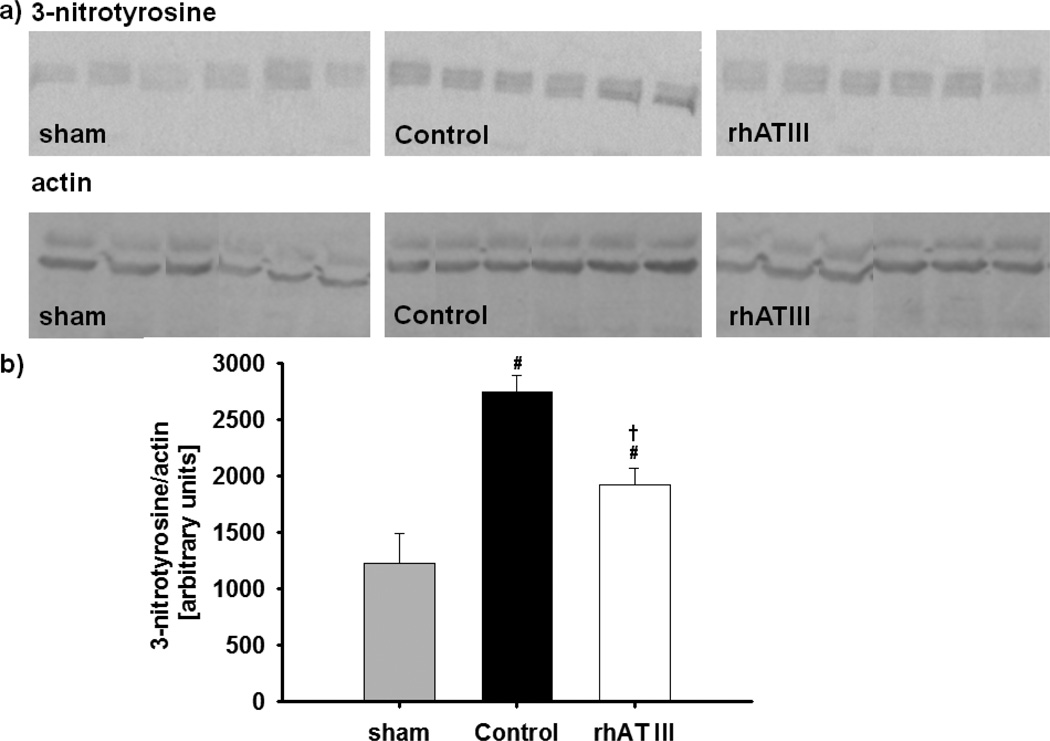
Western blot analysis of lung tissue revealed an increase in 3-nitrotyrosine concentrations in both injured groups as compared to sham animals (p < 0.05 each). However, infusion of rhATIII reduced the pulmonary 3-nitrotyrosine level as compared to the control group (p = 0.002). The Western blot analyses are depicted in Figure 4.
Figure 4.
3-Nitrotyrosine analysis: western blots (A) and densitometry calculations (B). #p < 0.05 versus sham; †p < 0.05 versus control; n = 6 per group. rhAT III = recombinant human antithrombin III.
Histological Analyses
Lung tissues of control animals were characterized by an increased obstruction of bronchi and bronchioli as well as higher histological scores for alveolar edema and hemorrhage as compared to sham animal (p < 0.05 each). Obstruction of bronchi and bronchioli was attenuated in animals treated with rhATIII as compared to control animals (p ≤ 0.01 each). In addition, alveolar edema score was significantly lower in the rhATIII group than in the control group (p = 0.044). No statistical differences in alveolar hemorrhage could be shown between injured groups. However, there was a trend to less hemorrhage in samples from treated animals (p = 0.065). The individual scores and data are presented in detail in Table 2. Representative slides are shown in Figure 3.
Ventilatory Variables
Beginning at 24 hours after the injury, peak and plateau pressures increased in control animals, reaching the upper limit at 48 hours (p < 0.001 vs BL each). In parallel, respiratory rate in control animals almost doubled during the study period (p < 0.001 vs BL).
In the rhATIII group, peak and plateau pressures were significantly lower than in the control group from 24 to 48 hours (p < 0.05 each). In addition, respiratory rate was lower as compared to control animals at the end of the study period (p = 0.007).
Pulmonary Hemodynamics, Gas Exchange, Transvascular Fluid Flux, and Bloodless Lung Wet-to-Dry-Weight Ratio
Mean pulmonary arterial pressure (MPAP) progressively increased over the study period in both groups (p < 0.001 vs BL at 48 hr each) (Table 1). However, treatment with rhATIII attenuated this increase as compared to control animals (p < 0.05 at 12 and 24 hr). Combined burn and smoke inhalation injury was associated with a severe deterioration of pulmonary gas exchange as represented by the significantly lower PaO2/FIO2 ratios in both study groups at 24 and 48 hours as compared to BL (p < 0.001 each). However, compared to the control group, PaO2/FIO2 ratios were significantly higher in the rhATIII group from 12 hours until 48 hours postinjury (p < 0.05 each) (Table 1). Notably, two animals of the control group had to be killed before the end of the study period (at 36 hr and 42 hr, respectively) because of fulminant pulmonary failure (PaO2 < 50 mm Hg at FIO2 of 1.0).
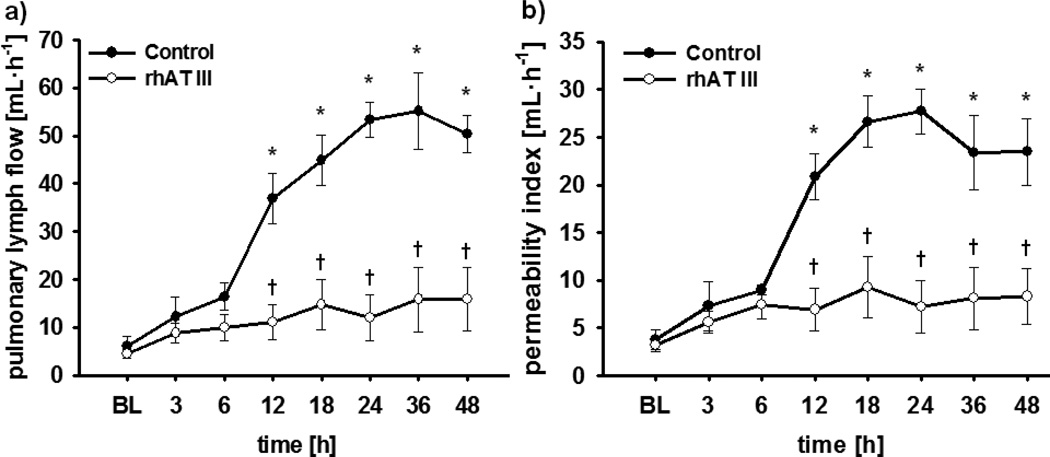
Pulmonary vascular leakage was evidenced by a marked increase in transvascular fluid flux (Fig. 5A). In control animals, pulmonary lymph flow increased more than 10-fold within 24 hours and stayed at this level until the end of the study period (p < 0.001 vs BL each). Similar changes were seen in pulmonary permeability index (Fig. 5B), that is, a significant increase starting at 12 hours and persisting until 48 hours (p < 0.001 vs BL each). The continuous infusion of rhATIII reversed these increases in pulmonary transvascular fluid flux (12–48 hr: ≤ 0.001 vs control each) and permeability index (12–48 hr: p ≤ 0.001 vs control each). In addition, bloodless lung wet-to-dry-weight ratio was significantly lower in animals treated with rhATIII than in control animals (p = 0.015) (Table 2).
Figure 5.
Pulmonary lymph flow (A) and permeability index (B). *p < 0.05 versus baseline; †p < 0.05 versus control; n = 6 per group. BL = baseline, rhAT III = recombinant human antithrombin III.
Discussion
The major findings of the present study are that a continuous infusion of 6 IU/kg/hr rhATIII restored ATIII plasma concentrations and reduced neutrophil activation, adhesion, as well as transendothelial migration and thereby attenuated pulmonary microvascular endothelial hyperpermeability and severity of ALI following burn and smoke inhalation in a clinically relevant ovine model. Notably, the interaction of rhATIII with the syndecan-4 receptor represents a potential mechanism of action.
The present study provides in vivo evidence that the restoration of endogenous ATIII plasma levels significantly attenuated the increased adhesion of activated neutrophils and their subsequent transendothelial migration to the pulmonary interstitium that was evident in control animals. This statement is supported by the results of three independent methods: the decrease in lung tissue myeloperoxidase activity, an index of neutrophil accumulation (21); the lower histological scores for alveolar as well as interstitial neutrophils; and the reduced number of neutrophils in the lung lymph. Neutrophil adherence to the endothelium can directly induce endothelial injury resulting in vascular leakage and eventually pulmonary edema (22, 23). The clinical relevance of neutrophils in the pathogenesis of inhalation injury was confirmed by Basadre et al (24), who demonstrated that in leukocyte-depleted sheep the characteristic changes, such as a fall in PaO2/FIO2 ratio and an increase in MPAP and transvascular fluid flux, were markedly attenuated.
Activated neutrophils also directly damage the lung by producing reactive oxygen species (25). These reactive oxygen species react with nitric oxide (NO) to form reactive nitrogen species, such as the highly cytotoxic peroxynitrite. Treatment with rhATIII reduced pulmonary nitrosative stress following burn and smoke inhalation as indicated by a reduction of pulmonary 3-nitrotyrosine levels, which represent a stable in vivo biomarker of peroxynitrite (26), and excessive NO production (plasma NOx) as compared to control animals. The critical role of nitrosative stress in the pathogenesis of ALI is supported by several experimental studies (25).
A potential mechanism for the reduced neutrophil activation and transendothelial migration represents the interaction of rhATIII with the syndecan-4 receptor on endothelial cells and neutrophils (10). Binding of antithrombin to the syndecan-4 receptor leads to an inhibition of the intracellular signal transduction cascade initiated by inflammatory stimuli (27). In the present study, this hypothesis was supported by flow cytometric analysis that revealed a peak in CD11b/syndecan-4-positive neutrophils in control animals in accordance with the maximum of transvascular fluid flux at 24 hours postinjury. Contrary, the continuous infusion of rhATIII blocked the syndecan-4 receptor, thereby reducing the number of syndecan-4 receptor-activated neutrophils and the subsequent increase in transendothelial migration. Critically, a causal relationship between the reduction in syndecan-4 receptor-activated neutrophils and the attenuated vascular leakage cannot be proven based on the present results. However, the present in vivo findings are supported by numerous in vitro studies demonstrating that ATIII regulates neutrophil migration by interacting with the syndecan-4 receptor (9–11, 28), making this hypothesis very likely. Future research may focus on the effects of ATIII on actin cytoskeletal elements, intercellular adhesion molecules, and cell migration to further elucidate the detailed mechanism.
Clinically, the inhibition of neutrophil activation and transendothelial migration by rhATIII almost completely prevented the fulminant vascular leakage seen in the control group, as evidenced by values for transvascular fluid flux and pulmonary permeability index that were close to BL level. As a consequence, pulmonary edema represented by the bloodless lung wet-to-dry-weight ratio and the histological edema score was significantly attenuated in rhATIII-treated animals. These results strongly suggest that rhATIII reduced pulmonary endothelial permeability to water, protein, and neutrophils via an attenuated neutrophil activation. This hypothesis is supported by studies in endotoxin-induced ALI in rats that received a prophylactic bolus of 250 IU ATIII (29, 30). The reduction in pulmonary vascular leakage was elegantly demonstrated by measuring the content of 125I-labeled bovine serum albumin in the lung versus the blood. In line with the present findings on syndecan-4, the authors proposed an interaction with heparin-like substances on the endothelial surface as a mechanism for the inhibition of leukocyte activation. Similarly, in crushed injury-induced ALI in rats, ATIII (therapeutic bolus of 250 IU/kg) reduced the pulmonary accumulation of inflammatory cells and the expression of vascular cell adhesion molecule-1 (31). The superiority of ATIII versus other anticoagulants, such as heparin, activated protein C, or tissue factor pathway inhibitor, in attenuating ALI-associated inflammation was reported in two rodent models of pneumonia (32, 33). Whereas all anticoagulants attenuated pulmonary coagulopathy, only ATIII reduced bacterial outgrowth and histological changes.
Another hallmark of smoke inhalation injury is the progressive airway obstruction resulting from reactive bronchoconstriction, airway mucosal edema, fibrin deposition, as well as obstructive cast formation (34). In the present study, rhATIII-treated animals suffered from less airway obstruction than control animals, as evidenced by the reduced histological airway obstruction scores. In this context, it is important to consider that ATIII has potent anticoagulant effects by inhibiting the formation of thrombin (35). The reduction in airway pressures and an improvement in pulmonary gas exchange due to the prevention (12) or lyses (36) of fibrin, respectively, by using aerolized anticoagulants have been reported in the literature. In addition, the inhibition of neutrophil activation by rhATIII probably contributed to the reduced cast formation, since neutrophils not only promote the coagulation cascade but are also constituents of these airway casts (18). This assumption is supported by Murakami et al, who reported that IV administration of rhATIII resulted in increased ATIII concentrations in the bronchoalveolar fluid. Because the difference between peak and plateau pressures was unchanged in the rhATIII group, a reduction in elastance due to attenuation of pulmonary edema (as opposed to airways resistance) probably represented the main reason for the reduced airway pressures.
Combined burn and smoke inhalation injury resulted in a fulminant pulmonary failure in untreated animals fulfilling the criteria for acute respiratory distress syndrome (PaO2/FIO2 ratio < 200 mm Hg) already after 24 hours. Two animals of the control group had to be killed before the end of the study period because sufficient oxygenation (PaO2/FIO2 ratio > 50 mm Hg) could not be achieved despite increasing FIO2 and ventilator pressures. Contrary, in animals treated with rhATIII, pulmonary dysfunction was significantly attenuated with PaO2/FIO2 ratios more than twice as high than in the control group at 24 and 48 hours. The reduction of vascular leakage, pulmonary edema, nitrosative stress, and airway obstruction probably all contributed to this finding.
Finally, it is important to note that the treatment with rhATIII was not associated with an increased risk of bleeding, as suggested by no statistical differences in plasmatic coagulation variables (Table 1) and histological analysis of pulmonary hemorrhage (Table 2). This finding is supported by numerous experimental studies, using even higher doses than 6 IU/kg/hr (29, 31). In respect to bleeding complications, the combination with heparin rather than the dose of antithrombin seems to be the critical factor (37). Notably, the prevention of a severe decrease in ATIII plasma levels might represent itself a beneficial effect because mortality of burn patients is associated with the reduction of ATIII plasma levels (5).
This study has some limitations that we want to acknowledge. The present model was associated with a mortality of 33% in the control group. Because there was no causal treatment of the burn, differences in survival time in the current study should not be overestimated. Furthermore, the effects of the investigated therapeutic approach were only analyzed during the acute phase of the injury. As the primary goal of the present study was to investigate the mechanism of action of rhATIII, the study period of 48 hours, however, was appropriate. Nevertheless, future studies are warranted to elucidate the long-term effects. Another limitation is the lack of a rhATIII-treated sham group, especially in the context of the anticoagulant effects of antithrombin. In addition, interpretation of the current data is limited by the use of previously healthy animals, whereas the majority of patients typically suffer from comorbidities. Finally, the risk of false-positive results in a study with numerous outcome variables and time points has to be taken into consideration.
Conclusions
In summary, the present study provides evidence for the interaction between antithrombin and neutrophils in vivo, its pathophysiological role in vascular leakage, and the therapeutic potential of rhATIII in a large animal model of ALI. These findings may promote the performance of clinical studies to confirm these promising experimental results.
Supplementary Material
Acknowledgements
We thank the technicians of the Investigational ICU for expert technical assistance during the study.
Grant Support: Shriners of North America (Grant nos. 86300 and 84050) and T32 GM008256.
References
- 1.Rehberg S, Maybauer MO, Enkhbaatar P, et al. Pathophysiology, management and treatment of smoke inhalation injury. Expert Rev Respir Med. 2009;3:283–297. doi: 10.1586/ERS.09.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suzuki M, Aikawa N, Kobayashi K, et al. Prognostic implications of inhalation injury in burn patients in Tokyo. Burns. 2005;31:331–336. doi: 10.1016/j.burns.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 3.García-Avello A, Lorente JA, Cesar-Perez J, et al. Degree of hypercoagulability and hyperfibrinolysis is related to organ failure and prognosis after burn trauma. Thromb Res. 1998;89:59–64. doi: 10.1016/s0049-3848(97)00291-0. [DOI] [PubMed] [Google Scholar]
- 4.Kowal-Vern A, Walenga JM, McGill V, et al. The impact of antithrombin (H) concentrate infusions on pulmonary function in the acute phase of thermal injury. Burns. 2001;27:52–60. doi: 10.1016/s0305-4179(00)00057-7. [DOI] [PubMed] [Google Scholar]
- 5.Lavrentieva A, Kontakiotis T, Bitzani M, et al. Early coagulation disorders after severe burn injury: Impact on mortality. Intensive Care Med. 2008;34:700–706. doi: 10.1007/s00134-007-0976-5. [DOI] [PubMed] [Google Scholar]
- 6.Niedermayr M, Schramm W, Kamolz L, et al. Antithrombin deficiency and its relationship to severe burns. Burns. 2007;33:173–178. doi: 10.1016/j.burns.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 7.Roemisch J, Gray E, Hoffmann JN, et al. Antithrombin: A new look at the actions of a serine protease inhibitor. Blood Coagul Fibrinolysis. 2002;13:657–670. doi: 10.1097/00001721-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Wiedermann CJ. Clinical review: Molecular mechanisms underlying the role of antithrombin in sepsis. Crit Care. 2006;10:209. doi: 10.1186/cc4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunzendorfer S, Kaneider N, Rabensteiner A, et al. Cell-surface heparin sulfate proteoglycan-mediated regulation of human neutrophil migration by the serpin antithrombin III. Blood. 2001;97:1079–1085. doi: 10.1182/blood.v97.4.1079. [DOI] [PubMed] [Google Scholar]
- 10.Kaneider NC, Förster E, Mosheimer B, et al. Syndecan-4-dependent signaling in the inhibition of endotoxin-induced endothelial adherence of neutrophils by antithrombin. Thromb Haemost. 2003;90:1150–1157. doi: 10.1160/TH03-03-0184. [DOI] [PubMed] [Google Scholar]
- 11.Nevière R, Tournoys A, Mordon S, et al. Antithrombin reduces mesenteric venular leukocyte interactions and small intestine injury in endotoxemic rats. Shock. 2001;15:220–225. doi: 10.1097/00024382-200115030-00010. [DOI] [PubMed] [Google Scholar]
- 12.Enkhbaatar P, Esechie A, Wang J, et al. Combined anticoagulants ameliorate acute lung injury in sheep after burn and smoke inhalation. Clin Sci (Lond) 2008;114:321–329. doi: 10.1042/CS20070254. [DOI] [PubMed] [Google Scholar]
- 13.Westphal M, Enkhbaatar P, Schmalstieg FC, et al. Neuronal nitric oxide synthase inhibition attenuates cardiopulmonary dysfunctions after combined burn and smoke inhalation injury in sheep. Crit Care Med. 2008;36:1196–1204. doi: 10.1097/CCM.0b013e31816a1a0c. [DOI] [PubMed] [Google Scholar]
- 14.Enkhbaatar P, Connelly R, Wang J, et al. Inhibition of neuronal nitric oxide synthase in ovine model of acute lung injury. Crit Care Med. 2009;37:208–214. doi: 10.1097/CCM.0b013e318193226a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rehberg S, Yamamoto Y, Traber LD, et al. Pulmonary vascular leakage after combined burn and smoke inhalation injury is reduced by continuous intravenous infusion of recombinant human antithrombin due to inhibition of neutrophil activation. Intensive Care Med. 2009;35(Suppl 1):S8. [Google Scholar]
- 16.Lange M, Connelly R, Traber DL, et al. Combined neuronal and inducible nitric oxide synthase inhibition in ovine acute lung injury. Crit Care Med. 2009;37:223–229. doi: 10.1097/CCM.0b013e3181926104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baxter CR, Shires T. Physiological response to crystalloid resuscitation of severe burns. Ann N Y Acad Sci. 1968;150:874–894. doi: 10.1111/j.1749-6632.1968.tb14738.x. [DOI] [PubMed] [Google Scholar]
- 18.Cox RA, Burke AS, Soejima K, et al. Airway obstruction in sheep with burn and smoke inhalation injuries. Am J Respir Cell Mol Biol. 2003;29:295–302. doi: 10.1165/rcmb.4860. [DOI] [PubMed] [Google Scholar]
- 19.Pearce ML, Yamashita J, Beazell J. Measurement of pulmonary edema. Circ Res. 1965;16:482–488. doi: 10.1161/01.res.16.5.482. [DOI] [PubMed] [Google Scholar]
- 20.Gillett AM, Wallace MJ, Gillespie MT, et al. Increased expansion of the lung stimulates calmodulin 2 expression in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2002;282:L440–L447. doi: 10.1152/ajplung.00202.2001. [DOI] [PubMed] [Google Scholar]
- 21.Makita H, Nishimura M, Miyamoto K, et al. Effect of anti-macrophage migration inhibitory factor antibody on lipopolysaccharide-induced pulmonary neutrophil accumulation. Am J Respir Crit Care Med. 1998;158:573–579. doi: 10.1164/ajrccm.158.2.9707086. [DOI] [PubMed] [Google Scholar]
- 22.Abdi S, Herndon DN, Traber LD, et al. Lung edema formation following inhalation injury: Role of the bronchial blood flow. J Appl Physiol. 1991;71:727–734. doi: 10.1152/jappl.1991.71.2.727. [DOI] [PubMed] [Google Scholar]
- 23.Kumar P, Shen Q, Pivetti CD, et al. Molecular mechanisms of endothelial hyperpermeability: Implications in inflammation. Expert Rev Mol Med. 2009;11:e19. doi: 10.1017/S1462399409001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basadre JO, Sugi K, Traber DL, et al. The effect of leukocyte depletion on smoke inhalation injury in sheep. Surgery. 1988;104:208–215. [PubMed] [Google Scholar]
- 25.Rehberg S, Maybauer MO, Maybauer DM, et al. The role of nitric oxide and reactive nitrogen species in experimental ARDS. Front Biosci (Schol Ed) 2010;2:18–29. doi: 10.2741/s43. [DOI] [PubMed] [Google Scholar]
- 26.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci U S A. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oelschläger C, Römisch J, Staubitz A, et al. Antithrombin III inhibits nuclear factor kappaB activation in human monocytes and vascular endothelial cells. Blood. 2002;99:4015–4020. doi: 10.1182/blood.v99.11.4015. [DOI] [PubMed] [Google Scholar]
- 28.Friedl J, Puhlmann M, Bartlett DL, et al. Induction of permeability across endothelial cell monolayers by tumor necrosis factor (TNF) occurs via a tissue factor-dependent mechanism: Relationship between the procoagulant and permeability effects of TNF. Blood. 2002;100:1334–1339. [PubMed] [Google Scholar]
- 29.Uchiba M, Okajima K, Murakami K. Effects of various doses of antithrombin III on endotoxin-induced endothelial cell injury and coagulation abnormalities in rats. Thromb Res. 1998;89:233–241. doi: 10.1016/s0049-3848(98)00012-7. [DOI] [PubMed] [Google Scholar]
- 30.Uchiba M, Okajima K, Murakami K, et al. Attenuation of endotoxin induced endothelial cell injury and coagulation abnormalities in rats. Am J Physiol. 1996;270:L921–L930. doi: 10.1152/ajplung.1996.270.6.L921. [DOI] [PubMed] [Google Scholar]
- 31.Sonoi H, Matsumoto N, Ogura H, et al. The effect of antithrombin on pulmonary endothelial damage induced by crush injury. Shock. 2009;32:593–600. doi: 10.1097/SHK.0b013e3181a23ad0. [DOI] [PubMed] [Google Scholar]
- 32.Choi G, Hofstra JJ, Roelofs JJ, et al. Antithrombin inhibits bronchoalveolar activation of coagulation and limits lung injury during Streptococcus pneumoniae pneumonia in rats. Crit Care Med. 2008;36:204–210. doi: 10.1097/01.CCM.0000292012.87482.F4. [DOI] [PubMed] [Google Scholar]
- 33.Hofstra JJ, Cornet AD, de Rooy BF, et al. Nebulized antithrombin limits bacterial outgrowth and lung injury in Streptococcus pneumonia pneumonia in rats. Crit Care. 2009;13:R145. doi: 10.1186/cc8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Enkhbaatar P, Traber DL. Pathophysiology of acute lung injury in combined burn and smoke inhalation injury. Clin Sci (Lond) 2004;107:137–143. doi: 10.1042/CS20040135. [DOI] [PubMed] [Google Scholar]
- 35.MacLaren R, Stringer KA. Emerging role of anticoagulants and fibrinolytics in the treatment of acute respiratory distress syndrome. Pharmacotherapy. 2007;27:860–873. doi: 10.1592/phco.27.6.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Enkhbaatar P, Murakami K, Cox R, et al. Aerosolized tissue plasminogen inhibitor improves pulmonary function in sheep with burn and smoke inhalation. Shock. 2004;22:70–75. doi: 10.1097/01.shk.0000129201.38588.85. [DOI] [PubMed] [Google Scholar]
- 37.Rehberg S, Traber DL, Enkhbaatar P. Update on antithrombin for the treatment of burn and smoke inhalation injury. In: Vincent JL, editor. Yearbook of Intensive Care and Emergency Medicine. Berlin, Heidelberg, New York: Springer; 2010. pp. 285–296. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.