Abstract
Although efficacious in vitro, it is well known that adoptive immunotherapeutic modalities lose their potency when applied in vivo. Furthermore, malignant cell exposure to blood platelets attenuates the anticancer activity of natural killer (NK) cells. We argue that upon contact with redox iron, fibrinogen is converted to a hydrophobic fibrin-like polymer that coats tumor cells and provides protection from immune-mediated destruction.
Keywords: fibrinogen, hydrophobic bonds, iron, NK cells, parafibrin, polyphenols
Introduction
Placke et al.1 have recently presented a view that blood platelets impair natural killer (NK) cells ability to recognize cancer cells by forming a physical barrier around the tumor cells. In connection with this it should be remembered that platelet require for their proper functions the presence of fibrinogen, an essential component of blood coagulation cascade.2 Fibrinogen (FBG) is a large molecular weight (340 kDa) multi-chain plasma protein that is converted with thrombin to fibrin monomer(s) (FM), an essential component of the clotting assembly that spontaneously polymerizes to form fibrin clots. However, prior to the formation of solid clots FMs remain soluble by forming complexes with the intact fibrinogen molecules. Due to the increased hydrophobicity of fibrin monomers3 such complexes readily interact with cellular membranes of cells in the blood system. Thus, in addition to inducing platelet aggregation, FM complexes have been shown to cause red blood cell (RBC) agglutination and increased sedimentation,4 as well as cell aggregation of the bacterium Staphylococci.5 Under normal hemostatic conditions fibrin clots are susceptible to degradation by the blood fibrinolytic system that secures proper wound healing and growth of connective tissue at the site of vessel wall injury. This system is activated by the release of tissue plasminogen activator (tPA) resulting in the generation of active plasmin that under normal conditions effectively degrades fibrin into soluble fragments. However, it is not understood why degradation of fibrin in certain types of cancer is incomplete, despite increased tPA production as in the case of prostate cancer.6
Mechanism of Parafibrin Formation
We have previously reported that the exposure of plasma proteins to disulfide-reducing agents resulted in the formation of huge insoluble aggregates, which when adhered to tumor cell membranes have been proposed to act as a barrier to tumor recognition by the innate immune system.7 Subsequently, it was further documented that similar aggregates could be induced with another non-enzymatic agent, free iron ions.8 In contrast to thrombin-generated fibers, those formed in the presence of iron ions exhibit dramatically different physicochemical properties. The first step in this conversion involves the interaction of trivalent iron ions (Fe3+) with the hydroxyl groups of water to form highly reactive hydroxyl radicals (HO.) according to the following reaction:
HO + Fe3+ → HO. + Fe2+
Of note, our first observance of this reaction took place without the involvement of hydrogen peroxide required for the classic Fenton reaction. The hydroxyl radicals generated by the interaction of hydroxyl and iron cleaves intra-molecular disulfide bonds in the fibrinogen molecules causing their unfolding with the exposure of hydrophobic groups normally buried inside the tridimensional structure of the polypeptide chains. In the absence of specific chaperons the exposed hydrophobic epitopes form scrambled intermolecular linkages resulting in the formation of fibrin-like fibrils (parafibrin). The most important feature of parafibrin is its hydrophobicity and total resistance to proteolytic degradation. This unusual phenomenon is due to the fact that the hydrophobic forces holding together the polypeptide chains are purely physical and do not involve peptide bonds.9 Consequently, once attached to the surfaces of various cells, parafibrin induces a permanent state of inflammation by eliciting the release of cytokines and proteases from macrophages that impairs their normal functions.
The damaging effect of parafibrin has recently been extended to the pathogenesis of cardiovascular10 and Alzheimer disease.11 These pathological conditions share a common mechanism by which native FBG molecules become hydrophobic by a single event of the conversion from the folded to the unfolded state. This phenomenon is very similar to that occurring when native prion protein PrP becomes converted to its toxic form PrPSc simply by unfolding, without any alteration in its primary structure.12 Interestingly enough, this concept has been met for many years with great skepticism from mainstream protein scientists, until its author was awarded with a Nobel Prize.
Iron and Cancer
Accumulating evidence suggests that a correlation exists between increased blood concentration of unbound iron and the incidence of cancer in humans.13,14 Furthermore, iron level reduction may prevent cancer morbidity and mortality.15-17 It should be noted that it is only trivalent iron (Fe3+) and not divalent (Fe2+) which participate in the generation of hydroxyl radicals and subsequent formation of insoluble parafibrin from soluble plasma fibrinogen.8 However, when hemoglobin is released from the hemolyzed erythrocytes, the divalent ferrous ions are enzymatically converted to ferric ions. Thus, any pathologic condition in which erythrocyte membranes are damaged, e.g., in response to infections and/or exposure to environmental toxins, may contribute to the excessive body storage of trivalent iron. It should be noted that this form of iron accumulates with age due to the fact that there is no mechanism for its physiologic elimination, and may therefore underlie prior observations of associations between cancer incidence and aging.
Current Issues of Cancer Immunotherapies
It is of extreme importance to remember that the presence of fibrin-like deposits, identified as thrombosis, has been reported for almost a century.18-20 However, no explanations have been offered why such deposits are not being removed by the fibrinolytic enzymes frequently found to be dramatically activated in tumors, particularly in prostate cancer.6 Apparently, there must be some post-translational modifications of fibrinogen/fibrin structure that render the tumor thrombi resistant the proteolytic degradation. The same phenomenon may also be responsible for the disappointing results of chemotherapy for the most types of solid tumors. In the past decade, attempts have being made to develop alternative methods using adaptive and/or adoptive immunotherapies.21 It is of interest to note that while some the adoptive modalities work perfectly well in artificially contrived systems in vitro they lose their efficacies in the physiologically relevant environment in vivo. Obviously the difference is in the exposure of tumor and immune cells to blood and its components as emphasized by Placke et al.1 As mentioned before another important hemostatic factor is plasma fibrinogen that, in the presence of free iron, is converted to parafibrin. We propose that this insoluble polymer forms a protective barrier around tumor cells by means of the hydrophobic interaction, similar to those operating in the phenomena of erythrocyte and bacterial cells aggregations. The role of parafibrin in tumor immune evasion can be compared with that ascribed to the desmoplastic stroma in pancreatic cancer.21 It should be born in mind, however, that even the most active NK cells generated ex vivo cannot degrade this barrier because hydrophobic forces holding it together do not represent a substrate for the action of proteolytic enzymes.9
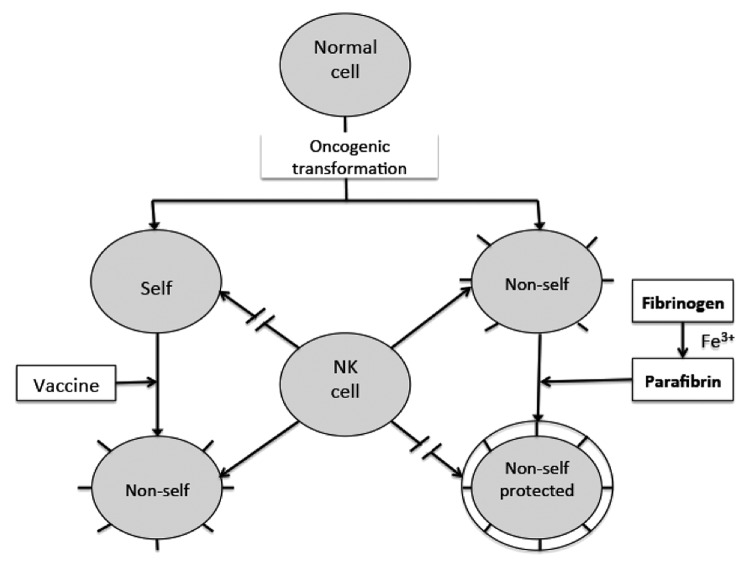
The dual role of parafibrin in the tumor evasion is graphically presented in Figure 1. First, because the antigenic properties of this polymer are almost identical to plasma fibrinogen and/or fibrin, parafibrin is seen by the innate immune system as “self.” Second, and more importantly, even if parafibrin attracts immune cells, it cannot be removed due to its remarkable resistance to degradative proteases normally liberated from activated NK cells.22
Figure 1. Conceptual scenarios of the interaction between natural killer cells and cancer cells. Natural killer (NK) cells attack those tumor cells that appear to as “non-self,” but spare the “self” identified cancer cells, including those coated with “self” parafibrin polypetide chains.
The Role of Polyphenols and Other Dietary Agents
In view of the known cancer protective effect of Mediterranean diet23,24 it is quite possible that certain polyphenolic substances present in this diet, such as EGCG,25 ferulic acid,26 and curcumin27 may exert their preventive and/or therapeutic effects by helping remove the protective barrier from the tumor membranes. The proposed mechanism of the action of polyphenols is based on their amphiphilic character that allows parafibrin to be displaced in a zipper-like manner (Fig. 2).
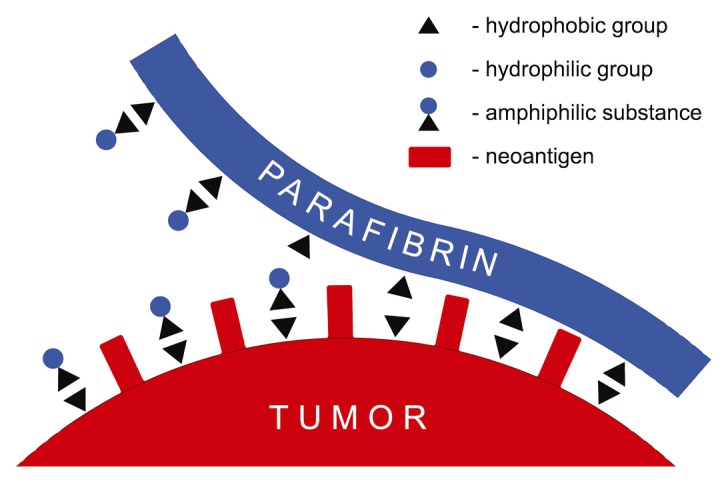
Figure 2. Displacement of parafibrin protective coat from the cancer cell membrane by an amphiphilic substance. The hydrophobic groups of the amphiphilate form strong complexes with those of cancer cell membrane, thus diplacing the polypeptide backbone of parafibrin thereby increasing its water solubility due to hydrophilic group enrichment.
This mechanism is similar to that operating in the removal of fat by detergents, in which the hydrophobic groups of a detergent react with the non-polar regions of the fat with the exposure of hydrophilic sites to the aqueous milieu. In addition to these organic substances suggested here to displace parafibrin from the tumor cell surfaces, there are two minerals that may also play an important role in this phenomenon. Thus, magnesium ions contained in green leaves and vegetables may exert their anticancer properties28 by inhibiting the intrinsic blood coagulation and in this way limiting the formation of parafibrin as indicated in the pathogenesis of Alzheimer disease11 The other essential, albeit not generally recognized mineral, is selenium that in a specific chemical form as sodium selenite blocks the protein thiol groups and subsequently inhibit protein unfolding and scrambled refolding.29 Finally, it should be emphasized that the present concept of parafibrin formation and its role in tumor evasion would not be possible without the pioneering work on the protein structure-function relationship elucidated by the great American protein chemist, Chris Anfinsen.30
Conclusions
Our perspective is that a singular electron transfer event from the redox iron ion into the hydroxyl group of water can initiate a chain of events that can explain the enigmatic phenomenon of immune evasion. This novel concept offers an additional scenario accounting for improper tumor immunoediting, as well as fostering a defective cellular immune system. However, a philosophical question arises whether such a simple idea can find its place in this day-and-age of the enormous complexity and technical sophistication of the current cancer research.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- EGCG
epigallo-3-catechin gallate
- FBG
fibrinogen
- FM
fibrin monomer
- HO
hydroxyl radical
- NK
natural killer
- tPA
tissue plasminogen activator
References
- 1.Placke T, Kopp H-G, Salih HR. The wolf in sheep’s clothing: Platelet-derived “pseudo self” impairs cancer cell “missing self” recognition by NK cells. Oncoimmunology. 2012;1:557–9. doi: 10.4161/onci.19367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niewiarowski S, Budzynski AZ, Lipinski B. Significance of the intact polypeptide chains of human fibrinogen in ADP-induced platelet aggregation. Blood. 1977;49:635–44. [PubMed] [Google Scholar]
- 3.van Oss CJ. Surface properties of fibrinogen and fibrin. J Protein Chem. 1990;9:487–91. doi: 10.1007/BF01024625. [DOI] [PubMed] [Google Scholar]
- 4.Lipiński B, Worowski K, Myśliwiec M, Farbiszewski R. Erythrocyte sedimentation and soluble fibrin monomer complexes. Thromb Diath Haemorrh. 1969;21:196–202. [PubMed] [Google Scholar]
- 5.Lipinski B, Hawiger J, Jeljaszewicz J. Staphylococcal clumping with soluble fibrin monomer. J Exp Med. 1967;120:160–8. doi: 10.1084/jem.126.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipinski B. Prostate cancer vaccines, fibrin and selenium: A conceptual review. Open Prostate Cancer J. 2010;3:69–73. doi: 10.2174/1876822901003010069. [DOI] [Google Scholar]
- 7.Lipinski B, Egyud LG. Thiol-induced crosslinking of human blood proteins: Implications for tumor immunity. Bioorg Med Chem Lett. 1992;2:919–24. doi: 10.1016/S0960-894X(00)80588-0. [DOI] [Google Scholar]
- 8.Lipinski B, Pretorius E. Novel pathway of iron‑induced blood coagulation: implications for diabetes mellitus and its complications. Pol Arch Med Wewn. 2012;122:115–22. [PubMed] [Google Scholar]
- 9.Dyson HJ, Wright PE, Scheraga HA. The role of hydrophobic interactions in initiation and propagation of protein folding. Proc Natl Acad Sci U S A. 2006;103:13057–61. doi: 10.1073/pnas.0605504103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipinski B, Pretorius E. Iron-induced fibrin in cardiovascular disease. Curr Neurovasc Res. 2013;10:269–74. doi: 10.2174/15672026113109990016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipinski B, Pretorius E. The role of iron-Induced fibrin in the pathogenesis of Alzheimer's disease and the protective role of magnesium. Front Hum Neurosci. 2013;7:735–9. doi: 10.3389/fnhum.2013.00735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–83. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae YJ, Yeon JY, Sung CJ, Kim HS, Sung MK. Dietary intake and serum levels of iron in relation to oxidative stress in breast cancer patients. J Clin Biochem Nutr. 2009;45:355–60. doi: 10.3164/jcbn.09-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrucci LM, Cross AJ, Graubard BI, Brinton LA, McCarty CA, Ziegler RG, Ma X, Mayne ST, Sinha R. Intake of meat and iron and the risk of breast cancer in the prostate, lung, colorectal and ovarian cancer screening trial. Br J Cancer. 2009;101:178–84. doi: 10.1038/sj.bjc.6605118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedford MR, Ford SJ, Horniblow RD, Iqbal TH, Tselepis C. Iron chelation in the treatment of cancer: a new role for deferasirox? J Clin Pharmacol. 2013;53:885–91. doi: 10.1002/jcph.113. [DOI] [PubMed] [Google Scholar]
- 16.Zacharski LR, Chow BK, Howes PS, Shamayeva G, Baron JA, Dalman RL, Malenka DJ, Ozaki CK, Lavori PW. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: results from a randomized trial. J Natl Cancer Inst. 2008;100:996–1002. doi: 10.1093/jnci/djn209. [DOI] [PubMed] [Google Scholar]
- 17.Heath JL, Weiss JM, Lavau CP, Wechsler DS. Iron deprivation in cancer--potential therapeutic implications. Nutrients. 2013;5:2836–59. doi: 10.3390/nu5082836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trousseau A. Lectures on clinical medicine delivered at the Hotel-Dieu, Paris. Paris: The New Sydenham Society, 1872:5:282-332. [Google Scholar]
- 19.Schäfer M, Werner S. Cancer as an overhealing wound: an old hypothesis revisited. Nat Rev Mol Cell Biol. 2008;9:628–38. doi: 10.1038/nrm2455. [DOI] [PubMed] [Google Scholar]
- 20.Costantini V, Zacharski LR, Memoli VA, Kisiel W, Kudryk BJ, Rousseau SM. Fibrinogen deposition without thrombin generation in primary human breast cancer tissue. Cancer Res. 1991;51:349–53. [PubMed] [Google Scholar]
- 21.Watt J, Kocher HM. The desmoplastic stroma of pancreatic cancer is a barrier to immune cell infiltration. Oncoimmunology. 2013;2:e26788. doi: 10.4161/onci.26788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosetti C, Turati F, Dal Pont A, Ferraroni M, Polesel J, Negri E, Serraino D, Talamini R, La Vecchia C, Zeegers MP. The role of Mediterranean diet on the risk of pancreatic cancer. Br J Cancer. 2013;109:1360–6. doi: 10.1038/bjc.2013.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scoditti E, Calabriso N, Massaro M, Pellegrino M, Storelli C, Martines G, De Caterina R, Carluccio MA. Mediterranean diet polyphenols reduce inflammatory angiogenesis through MMP-9 and COX-2 inhibition in human vascular endothelial cells: a potentially protective mechanism in atherosclerotic vascular disease and cancer. Arch Biochem Biophys. 2012;527:81–9. doi: 10.1016/j.abb.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Schramm L. Going green: The role of the green tea component EGCG in chemoprevention. J Carcinog Mutagen. 2013;4:1000142. doi: 10.4172/2157-2518.1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srinivasan M, Sudheer AR, Menon VP. Ferulic Acid: therapeutic potential through its antioxidant property. J Clin Biochem Nutr. 2007;40:92–100. doi: 10.3164/jcbn.40.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heger M, van Golen RF, Broekgaarden M, Michel MC. The molecular basis for the pharmacokinetics and pharmacodynamics of curcumin and its metabolites in relation to cancer. Pharmacol Rev. 2014;66:222–307. doi: 10.1124/pr.110.004044. [DOI] [PubMed] [Google Scholar]
- 28.Anghileri LJ. Magnesium, calcium and cancer. Magnes Res. 2009;22:247–55. doi: 10.1684/mrh.2009.0173. [DOI] [PubMed] [Google Scholar]
- 29.Lipinski B. Rationale for the treatment of cancer with sodium selenite. Med Hypotheses. 2005;64:806–10. doi: 10.1016/j.mehy.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Moudrianakis EN. From protein coagulation and reversible denaturation to the protein folding problem: Chris Anfinsen defining the transition. FASEB J. 1996;10:179–83. doi: 10.1096/fasebj.10.1.8566540. [DOI] [PubMed] [Google Scholar]