Abstract
Tyrosine kinase inhibitor therapy has dramatically changed the outcome of chronic myeloid leukemia (CML) patients. However, the treatment is not considered to be curative and may present deleterious side effects, such that additional therapy options are warranted. Here, we discuss the beneficial immunomodulatory effects of interferon α (IFNα) therapy and the immunological changes related to optimal treatment responses.
Keywords: chronic myeloid leukemia, interferon-alpha, immunomodulation, curative treatment, T-cells, NK-cells, immunosurveillance
Chronic myeloid leukemia (CML) belongs to the group of myeloproliferative disorders and is characterized by an expansion of unmature myeloid cells in the bone marrow and peripheral blood. Its annual incidence is 1–2 cases per 100 000 individuals. CML is a model disease in cancer as its molecular oncogenesis is well characterized as induced by a 9;22 translocation, leading to the formation of BCR-ABL1 fusion gene and resultant fusion protein with a constitutive tyrosine kinase activity. This knowledge has enabled the discovery of targeted therapy with tyrosine kinase inhibitors (TKIs).1 Before the invention of TKI therapy, CML patients died within 5–6 y after diagnosis, whereas today most patients live a normal life span with chronic disease. Despite the success story of TKIs, the majority of patients relapse if they discontinue the treatment, and therefore TKIs are not considered curative. The life-long duration of TKI therapy has also raised the safety concerns as some severe side effects (such as vascular events, pleural effusions, and pulmonal hypertension) have been correlated with the TKI treatment. Furthermore, the annual drug costs are significant (around 40 000 eur/patient), and as a consequence of the improved therapy, the prevalence of CML is constantly increasing, thus causing tremendous economic burden in the future. Therefore, the discovery of curative treatment options is of substantial interest.
Before the TKI era, the first drug that was able to re-establish normal hematopoiesis in a minority of CML patients was interferon α (IFNα).2 Interestingly, it was also noted that some patients who achieved cytogenetic response to IFNα were able to discontinue the treatment without disease relapse even though they still had residual leukemia cells left.3 As CML is considered to be an immunogenic cancer, one hypothesis is that the IFNα treatment is able to activate the immune system, which could keep the leukemia cells under control. If this beneficial mechanism is understood, it could probably be used in the development of new, curative treatment strategies for CML. Unfortunately though, it is difficult to get suitable study material as TKIs are nowadays the treatment of choice in CML, and therefore, IFN-α monotherapy treated patients are extremely rare. However, we were able to collect a small cohort of patients who were either still using IFNα treatment with good therapy response (IFN-ON) or who had been able to stop the IFNα monotherapy without disease relapse (IFN-OFF). When studying the immune cell profile in these patients, we observed cytotoxic features, such as increased amounts of CD8+ T cells and in particular, the natural killer (NK) cell counts were significantly elevated in IFN-OFF patients. Moreover, IFN-α treated patients also presented a unique pattern of clonal γδ T cells, which was not observed in TKI treated CML patients4,5
Inspired by these results, we next aimed to characterize T and NK cells in more detail by phenotypic and functional assays. To our surprise, the results from NK cell cytotoxicity assays suggested that the NK cells did not posses an enhanced cytotoxic capacity when measured by conventional degranulation (CD107), cytotoxicity (against K562 cell line), and cytokine secretion (IFNα /TNFα) assays, even though their number was increased.6 However, more prominent phenotypic and functional changes were observed in the T-cell compartment. Compared with healthy controls, IFN-OFF patients had an increased proportion of CD4+ effector memory and CD8+ central memory T cells. Further, upon stimulation, the IFNγ/TNFα cytokine secretion by CD4+ T cells was significantly enhanced in IFN-OFF patients, and CD4+ effector and central memory cells were the main cytokine producers. No similar increase of effector memory cells was observed in the IFN-ON group.
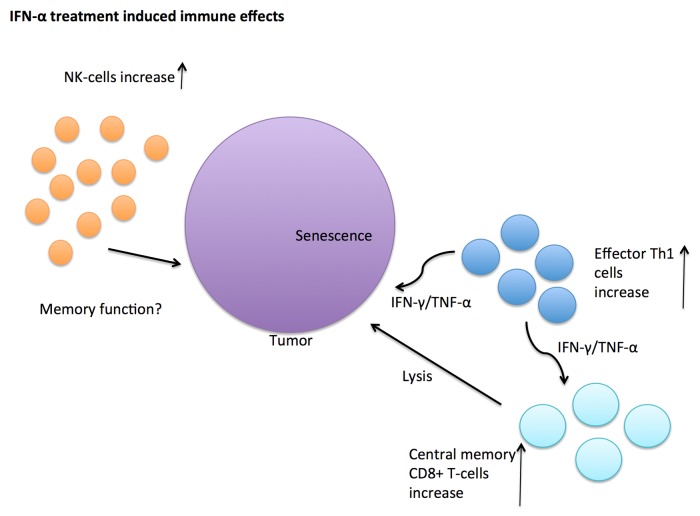
But how would these findings explain the remission status of IFN-α patients who still have residual leukemia cells left? As shown in Figure 1, it has previously been shown that T helper type 1 (Th1) CD4+ T cells have direct antitumor activity driving cancer cells to senescence.7 In addition, they are also able to stimulate the generation of cytotoxic CD8+ T cells.8 The specificity of the CD8+ central memory T cells in IFN-OFF patients is not known, but it is tempting to speculate that they may target residual leukemia cells. In addition to increased CD8+ central memory cells, IFN-OFF patients also had a lower proportion of recently activated CD8+ peripheral effector memory RA (TEMRA) cells. This is in accordance with the stable clinical status of the patients as they had been without any treatment for several years. The role of NK cells still remained open, but they may have memory function as it has recently been shown that NK cells also take part in the adaptive immunity.9 Taken together, the phenotypic and functional changes observed were not considered to be direct effects of IFN-α but rather long-term effects induced by the treatment.6
Figure 1. Antitumor immune effects induced by IFN-α treatment. Increased amount of natural killer (NK) cells, CD4+ effector memory T helper type 1 (Th1), and CD8+ central memory T cells have been found in interferon α (IFNα) treated CML patients who have been able to discontinue the therapy. These IFNα-induced immune cells secrete immunomodulatory cytokines such as interferon γ (IFNγ) or tumor necrosis factor α (TNFα) and may either directly, or indirectly, attack the tumor cells by driving them to senescence or by cytolysis.
These beneficial immunomodulatory effects of IFN-α are of compelling interest in regards to combination therapies to treat leukemia specifically, as well as cancer treatment in general, are planned in the future. The combination of TKI (imatinib) with IFN-α has already been shown to induce superior therapeutic responses in comparison to TKI treatment alone.10 Furthermore, additional clinical studies combining IFN-α with more potent 2nd generation TKIs are already ongoing (http://clinicaltrial.gov NCT01657604, NCT01725204, NCT01872442). The advantage of IFN-α compared with more modern immunomodulatory drugs such as checkpoint inhibitors, could be a quite moderate and tolerable side-effect profile. However, it should be born in mind that only a proportion of patients appear to benefit from IFN-α therapy, and further studies are needed to understand whether there are some genetic predisposing factors or some unknown tumor-specific attributes, that could determine which patients will benefit from the immunomodulatory effects induced by IFN-α treatment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
- Abbrevations: CML
chronic myeloid leukemia
- TKI
tyrosine kinase inhibitor
- Ph
Philadelphia
- TNF-α
tumor necrosis factor alpha
- IFN-γ
interferon gamma
- IFN-α
interferon alpha
Citation: Ilander M, Kreutzman A, Mustjoki S. IFNα induces prolonged remissions modeling curative immunologic responses in chronic myeloid leukemia. OncoImmunology 2014; 3:e28781; 10.4161/onci.28781
References
- 1.Hehlmann R, Hochhaus A, Baccarani M, European LeukemiaNet Chronic myeloid leukaemia. Lancet. 2007;370:342–50. doi: 10.1016/S0140-6736(07)61165-9. [DOI] [PubMed] [Google Scholar]
- 2.Talpaz M, McCredie KB, Mavligit GM, Gutterman JU. Leukocyte interferon-induced myeloid cytoreduction in chronic myelogenous leukemia. Blood. 1983;62:689–92. [PubMed] [Google Scholar]
- 3.Mahon FX, Delbrel X, Cony-Makhoul P, Fabères C, Boiron JM, Barthe C, Bilhou-Nabéra C, Pigneux A, Marit G, Reiffers J. Follow-up of complete cytogenetic remission in patients with chronic myeloid leukemia after cessation of interferon alfa. J Clin Oncol. 2002;20:214–20. doi: 10.1200/JCO.20.1.214. [DOI] [PubMed] [Google Scholar]
- 4.Kreutzman A, Juvonen V, Kairisto V, Ekblom M, Stenke L, Seggewiss R, Porkka K, Mustjoki S. Mono/oligoclonal T and NK cells are common in chronic myeloid leukemia patients at diagnosis and expand during dasatinib therapy. Blood. 2010;116:772–82. doi: 10.1182/blood-2009-12-256800. [DOI] [PubMed] [Google Scholar]
- 5.Kreutzman A, Rohon P, Faber E, Indrak K, Juvonen V, Kairisto V, Voglová J, Sinisalo M, Flochová E, Vakkila J, et al. Chronic myeloid leukemia patients in prolonged remission following interferon-α monotherapy have distinct cytokine and oligoclonal lymphocyte profile. PLoS One. 2011;6:e23022. doi: 10.1371/journal.pone.0023022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ilander M, Kreutzman A, Rohon P, Melo T, Faber E, Porkka K, Vakkila J, Mustjoki S. Enlarged memory T-cell pool and enhanced Th1-type responses in chronic myeloid leukemia patients who have successfully discontinued IFN-α monotherapy. PLoS One. 2014;9:e87794. doi: 10.1371/journal.pone.0087794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braumüller H, Wieder T, Brenner E, Aßmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–5. doi: 10.1038/nature11824. [DOI] [PubMed] [Google Scholar]
- 8.Keene JA, Forman J. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J Exp Med. 1982;155:768–82. doi: 10.1084/jem.155.3.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–61. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simonsson B, Gedde-Dahl T, Markevärn B, Remes K, Stentoft J, Almqvist A, Björeman M, Flogegård M, Koskenvesa P, Lindblom A, et al. Nordic CML Study Group Combination of pegylated IFN-α2b with imatinib increases molecular response rates in patients with low- or intermediate-risk chronic myeloid leukemia. Blood. 2011;118:3228–35. doi: 10.1182/blood-2011-02-336685. [DOI] [PubMed] [Google Scholar]