Abstract
The presence of obstructive sleep apnea (OSA) in patients with cancer appears to be accompanied by poorer outcomes. However, the mechanisms underlying such association are unknown. We hypothesize that the constitutive characteristics of OSA, namely, intermittent hypoxia and sleep fragmentation, promote changes in the tumor microenvironment that ultimately lead to a disadvantageous immunosurveillance, thereby accelerating tumor proliferation and enhancing its invasiveness.
Keywords: sleep fragmentation, macrophage polarity, sleep apnea, angiogenesis, intermittent hypoxia, invasion
Obstructive Sleep Apnea
Obstructive sleep apnea syndrome (OSA) is a highly prevalent disorder affecting approximately 4 to 10% of adults that has been implicated in a large spectrum of end-organ morbidities. OSA is characterized by repetitive obstructions of the upper airway during sleep that result in intermittent hypoxia (IH), increased inspiratory efforts, and ultimately sleep fragmentation (SF). A large body of research has extensively examined some of the mechanisms underlying the cardiovascular, cognitive, and metabolic co-morbidities associated with OSA in both patient cohorts and murine models. In this setting, activation and propagation of inflammatory and oxidative stress pathways have emerged as leading mechanistic processes.1
OSA, Sleep, and Cancer
OSA could interact with cancer and promote adverse effects on tumorigenesis and disease outcomes. This convergence of OSA and cancer likely is primarily a consequence of the episodic hypoxia that characterizes this frequently occurring sleep disorder.2 Such assumptions are supported by recent seminal epidemiological studies linking enhanced cancer aggressiveness and mortality link to the severity and frequency of IH.3,4 Early in vitro and in vivo models of OSA further suggested that IH during the sleep cycle promotes increased melanoma tumor growth and metastastic potential, thereby lending biological plausibility to cohort-based studies, and tilting the emphasis of research efforts toward hypoxia-based models, with little, if any, attention being paid to the other major constitutive element of OSA, namely SF.5
Most of the work on correlations between sleep and cancer has focused on the impact of cancer therapies on altered sleep quality and attendant quality of life measures.6 Notwithstanding, sleep duration and overall sleep characteristics may affect overall cancer outcomes. The presence of either short or prolonged sleep duration appeared to incur either a higher incidence of cancer or were associated with adverse outcomes.7 However, assessment of sleep duration was restricted to a nominal single question “how many hours do you sleep every night?,” such that estimates were highly subjective, and failed to assess sleep disruption by underlying sleep disorders (e.g., OSA), or environmental sleep disruptors (e.g., noise pollution). To explore the potential implications of SF on tumor proliferation, we exposed mice to a SF paradigm that is non-stressful and preserves sleep duration.8 In this setting, 2 well-established syngeneic tumor models revealed that proliferative rates and tumor size and invasiveness were markedly increased in SF-exposed mice.9
Cancer and Tumor-Associated Macrophages (TAMs)
The immune system in general, and macrophages in particular, participate in multiple cancer-related processes. Among macrophages (Mϕ), 3 major sub-types have been enunciated with distinct origins. Classically-activated Mϕ are derived from stimulation with LPS or interferon-γ (IFNγ) and designated as M1. Alternatively-activated wound-healing Mϕ arise in response to IL-4 or IL-13 stimulation and termed M2a. Finally, Mϕ phenotype derived from IL-10 or transforming growth factor- β (TGFβ) stimulation are designated as M2b. Alteration in the polarity of Mϕ is clearly a major determinant of tumor modulatory properties. The tumor microenvironment delivers various signals that shape Mϕ phenotypes preferentially into those promoting tumor growth instead of those that attack tumor cells. Simplistically stated, pro-inflammatory cytokines produced by M1 perform antitumor functions, whereas on the other hand, tissue regenerative functions of M2 (e.g., angiogenesis, stromal and cytoskeleton proliferative signaling, etc.) help to promote tumor growth. Tumor-associated macrophages (TAMs) have therefore been extensively examined and M2-like Mϕ appear to support tumor progression and survival in most cancers by releasing a vast array of growth factors, cytokines, inflammatory mediators, and proteolytic enzymes that underlie key components of tumor growth and invasion.
OSA and Polarity of TAMs
As mentioned above, application of IH during sleep promoted accelerated melanoma tumor growth and metastatic potential. In a recent set of experiments, we not only expanded these observations to the TC-1 tumor model but further sought to discern whether IH directly promotes tumor cell proliferation. Furthermore, we also examined whether TAMs are necessary and potentially sufficient to account for the increased tumor size and invasion observed in mice exposed to IH during sleep.10 Our experiments support the notion that rather than the direct effects of IH on cancer cells, IH-induced alterations in TAM polarity indirectly effect the tumor as a whole, thereby underlying the adverse cancer outcomes reported in patients afflicted with OSA. Indeed, IH-exposures both in vitro and in vivo shifted the polarity of TAMs toward a M2 phenotype that in turn accelerated tumor cell proliferation and increased tumor cell invasiveness.10
Coincidentally, similar findings emerged in the aforementioned SF experiments. First, TAMs bearing M2 macrophage markers were significantly more numerous and preferentially located in the TC-1 tumor periphery among SF-exposed mice. Second, unbiased quantitative proteomic characterization of TAM membrane proteins in normally sleeping mice and in SF-exposed mice confirmed major shifts in Mϕ polarity. Finally, based on the significant increases in toll-like receptor 4 (TLR4) expression in SF-exposed TAMs, experiments in tlr4−/− mice or in mice lacking 1 of the 2 major signaling pathways of TLR4, namely the myeloid differentiation factor MYD88 or the toll-like receptor adaptor molecule TRIF, revealed that the SF-induced differences in tumor growth were completely abrogated only in tlr4−/− mice.9
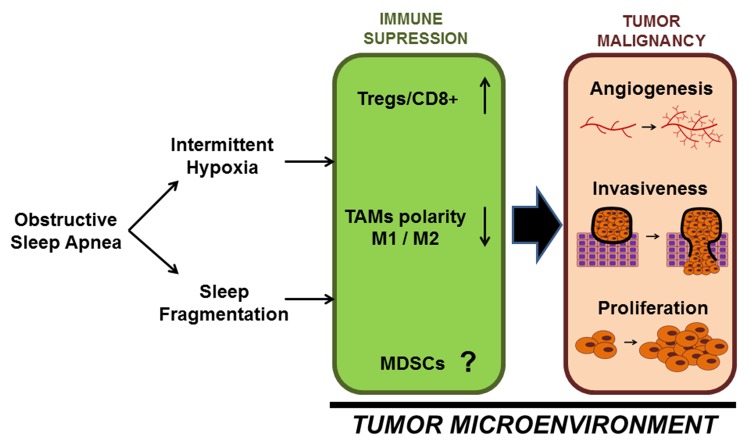
Taken together, it appears that the constitutive perturbations that characterize OSA, i.e., IH and SF, promote the occurrence of immunological alterations in the host response to the tumor that ultimately result in markedly adverse tumor properties. A tentative hypothetical schema of this conceptual framework is presented in Figure 1 and is primarily based on such preliminary findings. However, the restricted sets of experiments described heretofore in no way enable a comprehensive understanding of the complex landscape of the mechanisms and nature of the immunological alterations that link sleep perturbations, cyclical hypoxia, and cancer biology. Notwithstanding, these initial findings provide biological plausibility to the concept that sleep and sleep disorders intervene in fundamentally critical oncoimmunological processes.
Figure 1. Schematic diagram illustrating potential interactions between OSA and immunological processes in the tumor microenvironment. Obstructive sleep apnea (OSA) causes both intermittent hypoxia and sleep fragmentation that shift the balance of tumor-associated macrophage (TAM) polarity from M1 to M2 and increase the ratio of regulatory T (Tregs) to CD8+ cytotoxic T cells in the tumor microenvironment thereby promoting immune suppression and enhancing malignant disease progression.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
D.G. is supported by National Institutes of Health grants HL-65270, HL-086662, and HL-107160. I.A. is supported by Beatriu de Pinós fellowship from Generalitat de Catalunya (2010 BP_A 00238).
Citation: Almendros I, Hakim F, Gozal D. Sleep Apnea Awakes Cancer: A Unifying Immunological Hypothesis. OncoImmunology 2014; 3:e28326; 10.4161/onci.28326
References
- 1.Wang Y, Zhang SX, Gozal D. Reactive oxygen species and the brain in sleep apnea. Respir Physiol Neurobiol. 2010;174:307–16. doi: 10.1016/j.resp.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abrams B. Cancer and sleep apnea--the hypoxia connection. Med Hypotheses. 2007;68:232. doi: 10.1016/j.mehy.2006.06.037. [DOI] [PubMed] [Google Scholar]
- 3.Martínez-García MA, Martorell-Calatayud A, Nagore E, Valero I, Selma MJ, Chiner E, Landete P, Montserrat JM, Carreras C, Pérez-Gil A, et al. Association between sleep disordered-breathing and cutaneous melanoma aggressiveness. Eur Respir J. 2014 doi: 10.1183/09031936.00115413. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 4.Nieto FJ, Peppard PE, Young T, Finn L, Hla KM, Farré R. Sleep-disordered breathing and cancer mortality: results from the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2012;186:190–4. doi: 10.1164/rccm.201201-0130OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Almendros I, Montserrat JM, Torres M, Dalmases M, Cabañas ML, Campos-Rodríguez F, Navajas D, Farré R. Intermittent hypoxia increases melanoma metastasis to the lung in a mouse model of sleep apnea. Respir Physiol Neurobiol. 2013;186:303–7. doi: 10.1016/j.resp.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Haus EL, Smolensky MH. Shift work and cancer risk: potential mechanistic roles of circadian disruption, light at night, and sleep deprivation. Sleep Med Rev. 2013;17:273–84. doi: 10.1016/j.smrv.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59:131–6. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 8.Ramesh V, Nair D, Zhang SX, Hakim F, Kaushal N, Kayali F, Wang Y, Li RC, Carreras A, Gozal D. Disrupted sleep without sleep curtailment induces sleepiness and cognitive dysfunction via the tumor necrosis factor-α pathway. J Neuroinflammation. 2012;9:91. doi: 10.1186/1742-2094-9-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakim F, Wang Y, Zhang SX, Zheng J, Yolcu ES, Carreras A, Khalyfa A, Shirwan H, Almendros I, Gozal D. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res. 2014;74:1329–37. doi: 10.1158/0008-5472.CAN-13-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almendros I, Wang Y, Becker L, Lennon FE, Zheng J, Coats BR, Schoenfelt KS, Carreras A, Hakim F, Zhang SX, et al. Intermittent Hypoxia-induced Changes in Tumor-associated Macrophages and Tumor Malignancy in a Mouse Model of Sleep Apnea. Am J Respir Crit Care Med. 2014;189:593–601. doi: 10.1164/rccm.201310-1830OC. [DOI] [PMC free article] [PubMed] [Google Scholar]