Abstract
Tumors are composed of heterogeneous cell populations recruited by cancer cells to promote growth and metastasis. Among cells comprising the tumor stroma, myeloid-derived cells play pleiotropic roles in supporting tumorigenesis at distinct stages of tumor development. The tumor-infiltrating myeloid cell contingent is composed of mast cells, neutrophils, dendritic cells, macrophages, and myeloid-derived suppressor cells. Such cells are capable of evading the hostile tumor environment typically prone to immune cell destruction and can even promote angiogenesis, chronic inflammation, and invasion. This paper briefly summarizes the different myeloid-derived subsets that promote tumor development and the strategies that have been used to counteract the protumorigenic activity of these cells. These strategies include myeloid cell depletion, reduction of recruitment, and inactivation or remodeling of cell phenotype. Combining drugs designed to target tumor myeloid cells with immunotherapies that effectively trigger antitumor adaptive immune responses holds great promise in the development of novel cancer treatments.
Keywords: myeloid-derived suppressor cells, tumor-associated macrophages, tumor-associated neutrophils, dendritic cells, immunotherapy
The Tumor Microenvironment
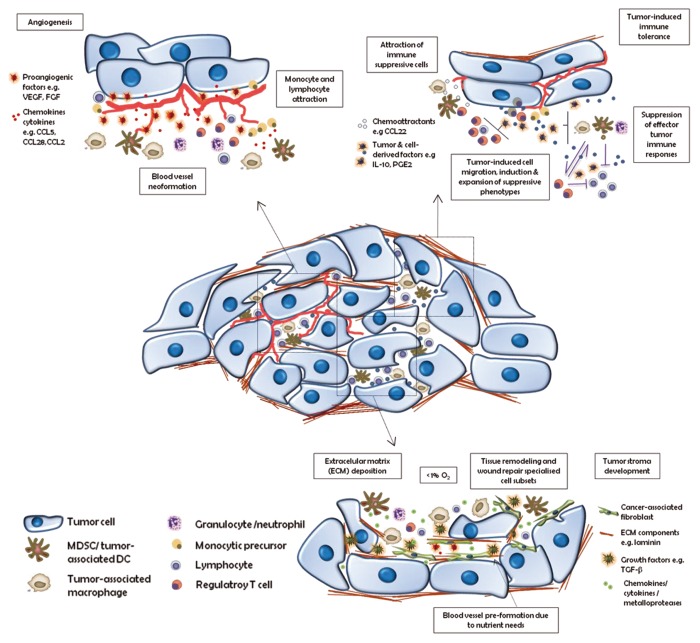
Tumors are more than simply masses of equivalent and proliferating cancer cells. Rather, they are heterogeneous by nature, being composed of multiple distinct cell types that participate in tangled interactions with one another (Fig. 1). Those cells which form the tumor-associated stroma are active contributors to tumor development. Over the last decade, accepted opinion has evolved from reductionism—perceiving a tumor as nothing more than a collection of relatively equivalent cancer cells—to the recognition of tumors as organs with interdependent cells whose complexity is somehow comparable to, or even exceeds that of, normal tissues. In fact, the tumor microenvironment serves as the key support system of a cancer, becoming the source of the 3-dimensional organization and architecture of the stroma, as well as providing all the protumorigenic factors that facilitate the growth, invasion, angiogenesis, and even metastatic ability of the neoplastic lesion. The tumor microenvironment contains malignant cells—those harboring genetic mutations—as well as other cell types that are activated and/or recruited such as fibroblasts, immune cells, and endothelial cells, many of which give rise to blood and lymphatic vessels. This heterogeneity of tumor cells is supported by tumor-derived factors that enhance the crosstalk between the cell populations and mediate tumor homeostasis.
Figure 1. Main cancer-promoting functions of tumor-infiltrating immune cells. Tumors are infiltrated by immune cells that support tumor growth by: 1) promoting angiogenesis; 2) driving immunosuppression; and 3) stimulating extracellular matrix remodeling. CCL, (C-C) motif chemokine; DC, dendritic cell; ECM, extracellular matrix; FGF, fibroblast growth factor; IL-10, interleukin-10; MDSC, myeloid-derived suppressor cell; PGE2, prostaglandin E2; TGFβ, transforming growth factor β; VEGF, vascular endothelial growth factor.
The first link between inflammation and cancer was proposed by Rudolph Virchow in the 19th century who noticed leukocytes infiltrating tumors. Later on, at the beginning of the 20th century, Paul Ehrlich predicted that the immune system has the capacity to suppress the growth of cancerous lesions. Currently, researchers are convinced that an inflammatory microenvironment is an essential component of tumor development. Thus, neoplasms can be recognized and eliminated by the action of the host immune system. Nevertheless, most tumors continue to grow and progress.
This paradox may be accounted for by inefficient functioning of the host immune system toward a developing tumor. The immune system detects pathogenic insults through innate immune cell populations that subsequently mount a specific adaptive immune response aimed at responding appropriately to the damage. In this way, tumors are placed under natural selective pressures that lead them to evolve several mechanisms to bypass the immune recognition machinery and elude immune system checkpoints. As is the case for immune cells, the tumor microenvironment creates a milieu that inhibits antitumor immune reactivity. Thus, tumors modulate host immunity to remain as “invisible” as possible and so continue their path to invasiveness and metastasis.
Invisibility in immunological terms is a complex issue. Tumors need to recruit immunosuppressive immune cells to control and overcome the host’s antitumor immune responses. As is the case with the systemic immune system, the tumor immune regulatory system is composed of both myeloid and lymphoid immune cells. Among a particular cell subset, there will be cells functionally specialized in specific duties, such as generating DNA damage through the release of toxic chemical molecules, recruiting suppressive cells by secreting chemokines and growth factors, or abrogating T cell proliferation. This hierarchic organization explains why different immunosuppressive cell subsets dominate in certain established tumors. Hence, a fuller and more detailed understanding of the interactions between the immunosuppressive cell subsets will open the gates to new therapeutic approaches.
Tumor-Infiltrating Myeloid Cells
Myeloid cells are an immune cell division that, along with natural killer (NK) cells, makes up the innate immune system. Innate immunity defends the organism against infection in a non-specific manner, responding to pathogens in a generic way. This arm of the immune system constitutes an evolutionarily older defense strategy and plays a pivotal role in both the onset and resolution of the tissue inflammatory process. However, when tissue homeostasis is chronically perturbed, the imbalance between innate and adaptive immunity can result in excessive tissue repair. This affects tissue architecture and produces several molecules such as free oxygen radicals which induce DNA damage in epithelial cells potentially leading to tumor development in some circumstances. Once neoplastic cells arise and persist, innate immune cells produce cytokines and chemokines—based on their physiological tissue remodeling machinery—helping epithelial cells and fibroblasts to create the tumor stroma. They also attract other immune cells to constitute an immunosuppressive milieu.
Apart from sharing a common progenitor cell, there is a panoply of myeloid immune cells that acquire their differentiated phenotype locally. For instance, blood monocytes migrate through the circulatory system reaching virtually all tissues where, dependent upon their ultimate location, these immature myeloid cells will differentiate into dendritic cells, macrophages, or osteoclasts. Mast cell progenitors also undergo differentiation when they reach their target tissue, adopting mast cell properties typically near vascular structures.1 The ability of myeloid cells to adapt their specialized functions depending on the tissue context is well manipulated by the tumor microenvironment to polarize myeloid cells through a paracrine or autocrine release of different molecules to promote a pro-tumorigenic myeloid phenotype. This property results in a plethora of myeloid-mediated tumor escape mechanisms. These include the predominance of immature myeloid cell subsets at the tumor site, the reduced antigen presentation and associated attenuation of T cell activation due to the loss of cell-to-cell contact between myeloid cells and T cells, and the interference with myeloid cell migration to secondary lymphoid organs. Thus, taken together, these tumor-guided myeloid functional deficiencies culminate in impaired antitumor immune response.2-6
Myeloid cell populations play a pivotal role in tumor development. Tumor-associated myeloid cell subsets are comprised of myeloid-derived suppressor cells at different stages of differentiation as well as neutrophils, dendritic cells, or macrophages and mast cells. In this context, these latter cell populations, keen to resolve inflammation when a pathogenic threat is present, serve to render the host immune cell subsets tolerant to tumor growth as well as to strengthen tumor stroma development. As for tumor-induced tolerance, this is achieved by either direct—e.g., induction of T-cell anergy by cell-to-cell contact—or indirect mechanisms, including the release of diverse molecules, such as transforming growth factor β (TGFβ) or interleukin 10 (IL-10) that polarize effector T-cell differentiation toward a regulatory-like phenotype.
Mast Cells
Mast cells are a myeloid subset with canonical functions in regulating allergic events and in T-cell mediated immunity. However, mast cells also accumulate at the sites of tumor growth in response to chemokines, such as (C-C) chemokine ligands CCL5 and CCL2.18 Mast cells have also been noted to amass in human invasive melanoma. Mast cells are well known by their characteristic release of secretory granules upon activation. These granules may contain a variety of molecules, such as heparin, histamine, vascular endothelial growth factor (VEGF), interleukin 1, and serine proteases which, under certain conditions, can directly promote the angiogenic switch.7 In addition, the soluble mediators released by mast cells promote a proinflammatory tumor microenvironment prone to the generation of Il-17 producing T helper (Th17) cells.8,9
Tumor-Associated Neutrophils (TANs)
Neutrophils are short-lived white blood cells derived from bone marrow precursors. They are among the first cells to arrive at the sites of infection, releasing chemokines and proteases to recruit innate and adaptive immune effector cells. However, in several transplantable tumor models TANs have been observed to both stimulate tumor angiogenesis (via the production of various proangiogenic factors) and suppress antitumor immunity.10,11 Furthermore, it has been shown that TANs also adopt a protumor polarized phenotype driven primarily by TGFβ signaling in established solid tumors, a polarization comparable to that of tumor-associated macrophages.12 Interestingly, intratumoral TGFβ blockade has been shown to alter the phenotype of TANs toward tumor-inhibitory properties. Thus, restricting TGFβ signaling may be critical to the maintenance of proinflammatory neutrophils that promote antitumor CD8+ T cell recruitment and activation via the secretion of T cell-attracting chemokines and proinflammatory cytokines.12,13
Dendritic Cells (DCs)
Known as professional antigen presenting cells, DCs are strategically positioned for bridging innate and adaptive immunity. DCs are a highly heterogeneous population of cells with remarkable plasticity that share common features, such as cell morphology and functional characteristics. These cells can be subdivided into 2 fundamentally distinct developmental stages termed immature dendritic cells (iDCs) and mature dendritic cells (mDCs). Whereas iDCs are localized primarily in peripheral tissues and perform the specialized functions of antigen uptake and processing, mDCs reside in lymphoid organs, where they interact with antigen-specific T cells and initiate immune responses. The chemokine receptor repertoire differs between iDCs and mDCs, such that the signals they perceive directing migration and homing to new sites are correspondingly different.2 DCs along this developmental spectrum then directly sense pathogens or altered cell components (such as from transformed cells), or other danger signals, and conveys the antigen captured in peripheral tissues to the lymphoid organs in order to mount an immune response.
However, in the context of tumors DCs often remain dysfunctional due to tumor-induced abnormalities. For instance, functional DCs may be eliminated in a tumor context either by abrogating their differentiation and maturation status or by the induction of apoptosis. These events have been clearly evinced by decreased numbers of circulating myeloid DCs detected in cancer patients.14 Moreover, tumor-associated DCs often appear to be incapable of migrating from the tumor site and further, have lost key components of their antigen processing and presentation machinery. Indeed, the tumor microenvironment abrogates the release of chemokines that normally attract functional DCs, thus reducing their numbers at the tumor site.15,16 Abnormal levels of circulating plasmacytoid dendritic cells in cancer patients and the marked increase of immature DC subsets both in patient and mouse tumor lesions highlight the pivotal role of tumor cells and stroma in polarizing tumor-associated immune cell subsets.17
Thus, the production of tumor-derived factors which affect DCs at molecular and transcriptional levels might lead to abnormalities in DC maturation, suppress DC survival, and impair DC function at the tumor site. For example, tumor derived TGFβ and IL-10 significantly reduce MHC class II and costimulatory surface molecule expression, diminish IL-12 production, abrogate DC maturation, and expand functional regulatory T cells (Tregs).18 The serum level of VEGF, another immunomodulatory tumor-derived factor, has been shown to inversely correlate with DC numbers in patients with colorectal cancer. Furthermore, monocyte-derived DCs cultured with exogenous VEGF are prone to apoptosis and an attenuated maturation status.19 VEGF has also been shown to be involved in recruiting immature DCs from the bone marrow to the tumor microenvironment.20 Catalysis of arachidonic acid, a member of the inflammatory-associated eicosanoid signaling cascade, by prostaglandin-endoperoxide synthase 2 generates prostaglandins such as prostaglandin E2, that impedes DC maturation and interleukin 12 production but considerably increases IL-10 levels in vivo.21 With regard to intrinsic transcription factors, activation of signal transducer and activation of transcription 3 (STAT3) by tumor-derived factors such as epidermal growth factor (EGF), VEGF, IL-10, and colony stimulating factor 2 (CSF2, also known as GMCSF) has been observed to block DC maturation and function.2,22
Altogether, DCs dysregulation in tumors is important for tumor immune subversion, but DCs are not mere passive spectators of this process. Certain DCs subsets play crucial roles in tumor escape. For instance, tumor-induced immature myeloid DCs have been reported to promote the proliferation of Tregs in a TGFβ-dependent manner in murine melanoma. Furthermore, the tumor microenvironment abrogates the native ability of DCs to present tumor antigens-thereby blocking their induction of tumor-specific cytotoxic T lymphocytes (CTLs)-and stimulates the upregulation of programmed cell death ligand 1 (PD-L1) on tumor DCs that further inhibits antitumor T cell-mediated immunity.23,24
Tumor-Associated Macrophages (TAMs)
Macrophages are present in most solid tumors, representing up to 50% of the cell mass.25 Blood monocytes are recruited to the tumor stroma where they differentiate to macrophages.26 The soluble factors that promote the accumulation of macrophages and are produced by cancer and stromal cells of the tumor include both chemokines such as CCL2, CCL5, CCL7, CXCL8, and CXCL12, as well as cytokines such as VEGF, platelet-derived growth factor (PDGF), and CSF-1.27,28 Once present in the tumor stroma, macrophages promote all phases of tumorigenesis, such as tumor growth, invasion, and metastasis, as well as stimulating tumor-promoting processes such as angiogenesis and immune suppression. Clinical data reveal that macrophage infiltration strongly correlates with poor prognosis in a variety of human cancers.29 TAMs stimulate tumor growth through the release of soluble factors. Thus, invasion and metastasis are promoted by the release of proteins such as tumor necrosis factor (TNF), TGFβ, IL-1, or matrix metallopeptidase 9 (MMP9).29,30 The process of angiogenesis required to sustain tumor growth is enhanced by the release of growth factors such as VEGF, PDGF, and TGFβ.31 Finally, the suppression of antitumor immune responses is mediated by the pattern of cytokines released which is characterized by the production of high levels of IL-10 and low levels of IL-12.32 Inducible nitric oxide synthase 2 (NOS2) and arginase-1 also contribute to the TAM-mediated immune suppression. Nitric oxide (NO) production by the NOS2 pathway leads to T cell cytotoxicity and apoptosis, whereas arginase-1 converts arginine into putrescine and L-ornithine, metabolites used by cancer cells to proliferate and inhibit T cell proliferation. Both enzymes produce reactive oxygen species (ROS), suppressing tumor infiltration by lymphocytes.33
Altogether, macrophages are the best example of how tumors manipulate their microenvironment during the process of malignant disease progression. The macrophages infiltrating tumors resemble “alternatively activated” (M2) macrophages but their tumor-promoting activity is reinforced by the expression of some M1-associated molecules typically associated with classically activated macrophages.30 This unique, tumor-associated subset of macrophages with amazing polarization properties is present at every stage of tumor development, continuously adapting to the tumor requirements for progression.
Myeloid-Derived Suppressor Cells
Myeloid-derived suppressor cells (MDSCs) comprise myeloid progenitors and immature myeloid cells at distinct stages along a spectrum of differentiation. In a physiological context, these cells would give rise to terminally differentiated DCs, macrophages, monocytes, or granulocytes. However, in response to a pathologic condition such as cancer, these cells become blocked in their maturation and tend to accumulate and expand precociously. Indeed, MDSCs are present in almost all cancer patients.34 However, myeloid cells which morphologically resemble MDSCs cell subsets have been found in healthy individuals, but do not have the same suppressive characteristics. Thus, it appears that MDSCs specifically act under certain pathological conditions where they accumulate in peripheral lymphoid organs and attenuate immunity.35
MDSCs do not express canonical DC, monocyte or macrophage surface markers but their morphology resembles that of granulocytes (Gr-MDSCs) or monocytes (Mo-MDSCs). MDSC subsets can be identified by the surface expression of CD11b and Gr-1. The monoclonal antibody RB6–8C5, that recognizes Gr-1, binds to 2 members of the Ly6 family of leukocyte-expressed markers: Ly6C, a monocyte/macrophage marker, and Ly6G, a classical neutrophil marker. High Gr-1 expression that coincides with Ly6GhighLy6Clow populations is associated with granulocytes, termed Gr-MDSCs. However, this marker is lowly expressed on monocytic cells, such as monocyte and macrophage precursors, which can be alternatively defined by Ly6ChighLy6Glow expression and are known as Mo-MDSCs.36 In the presence of the appropriate stimuli, Mo-MDSCs may differentiate to become DCs or macrophages in a hypoxia-dependent process both in vitro and in vivo.37 However, in cancer Mo-MDSCs preferentially differentiate toward Gr-MDSCs in a process governed by epigenetic silencing of the retinoblastoma (Rb) gene controlled by histone deacetylase 2 (HDAC2).38 MDSC nomenclature in PBMCs from cancer patients is still a matter of debate. However, markers for the classification of Mo-MDSCs as CD14+CD11b+HLA-DRlow/− cells and Gr-MDSCs as LIN−HLA-DR−CD33+CD11b+ cells have been extensively reported throughout the literature.39
The expansion and accumulation of MDSCs is governed by numerous tumor-derived factors. Most of these factors are principal components of the inflammatory response, such as CSF2, IL-6, IL-1, or VEGF. These cell-to-cell signaling molecules also function to regulation the expansion of other myeloid cell subsets, such as DCs, mast cells, TANs, or TAMs. Recently, cancer-stimulated microRNAs have been shown to be factors inducing MDSC accumulation and myeloid cell dysfunction.40-42 In the case of MDSCs, tumor-related signaling molecules trigger the phosphorylation and activation of STAT3, a key transcription factor regulating myeloid progenitor cell function and proliferation, and thus, MDSC expansion.43,44 STAT3 activation in myeloid progenitor cells lead to the production of S-100 calcium binding protein family members S100A8 and S100A9 proteins, which abrogate myeloid cell maturation and promote MDSC accumulation in the tumor bed as well as their migration to metastatic sites.45,46
The activation of MDSCs mainly depends on factors derived from both activated T cells and tumor stromal cells, that regulate immunomodulatory molecules such as arginase-1, NOS2, ROS, and TGFβ through the action of STAT-family transcription factors STAT1 and STAT6 and nuclear factor-κ B (NF-κB) signaling pathways. Thus, downstream extracellular signaling factors, such as IL-4, IL-13, interferon-γ (IFNγ), and TGFβ mediate, the immunosuppressive activity of MDSCs.44 Furthermore, the STAT6 transcription factor has been shown to be involved in MDSCs turnover. In support, Suzanne Ostrand-Rosenberg’s group has shown that MDSCs express the cell surface death receptor Fas and can activate CD8+ T cells expressing Fas ligand, such that MDSCs initiated T cell activation subsequently induces MDSC apoptosis.47
In the same way as TAMs, MDSC–based T cell immune suppression at the tumor site is antigen non-specific, and therefore MDSCs also share the mechanisms by which they abrogate T cell proliferation and functionality, such as arginine metabolism by NOS2 and arginase-1 or the production of ROS. However, Gr-MDSCs are prone to produce high levels of ROS, low levels of NO and thus high levels of arginase-1-mediated arginine catabolism, whereas Mo-MDSCs, via the NOS2 system, induce low levels of ROS and high levels of NO.36 Moreover, MDSCs efficiently induce immunosuppressive Tregs and promote their expansion in vivo through the release of IL-10 and TGFβ.48
Modulation of Tumor-Infiltrating Myeloid Cells by Immunotherapy
Tumor-associated myeloid cells constitute one of the many barriers that antitumor immunotherapy must overcome to successfully eradicate established tumors. However, myeloid cells also play a part in effector immune responses. These cells are not only involved in innate immune responses but are also important effector cells in adaptive immune responses. Indeed, the relevance of myeloid cells in enhancing antitumor immunity has been underestimated due to the critical role of other effector cells such as T lymphocytes or B cells in adaptive immune responses. Therefore, in order to achieve potent antitumor effects and defeat cancer, all the “troops” must be recruited to the battlefield, including antitumor myeloid cells.
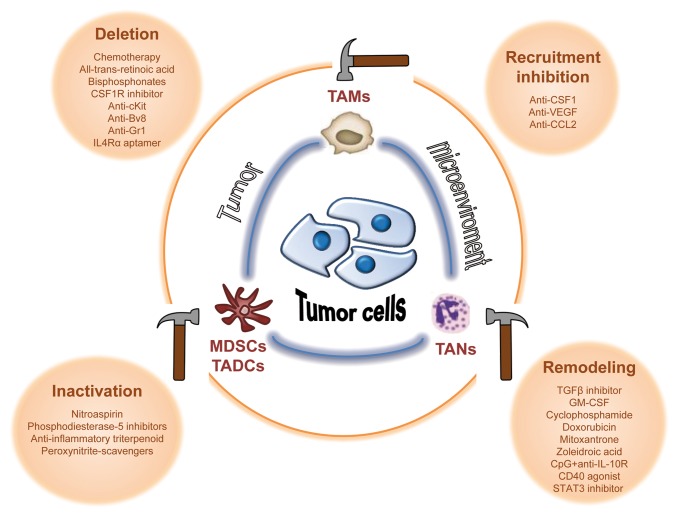
Conceptually, the immunosuppressive activity of tumor-associated myeloid cells can be abrogated by several distinct routes. These include methods to deplete myeloid suppressor cell levels via chemotherapy. Other approaches include agents to reduce myeloid recruitment to the tumor microenvironment, as well as means to attenuate their protumorigenic functions. This step paves the way for the generation of antitumor effector myeloid cells. These myeloid cells can originate by conversion of tumor-associated myeloid cells into antitumor myeloid cells or, alternatively, by recruitment of a new subset of myeloid cells into the tumor stroma (Fig. 2).
Figure 2. Strategies to modulate tumor-associated myeloid cells. The immunosuppressive activity of tumor-associated myeloid cells can be abrogated by immunotherapeutic agents aiming to: 1) deplete these cells; 2) reduce their recruitment to the tumor microenvironment; 3) inactivate their tumor-promoting functions; or 4) remodel tumor-infiltrating myeloid cells to convert suppressive myeloid subtypes to those with antitumor properties. Bv8, prokineticin 2; CCL22, C-C motif chemokine 22; c-Kit, cellular Kit proto-oncogene; CpG, CpG oligodeoxynucleotides; CSF1, colony stimulating factor 1; CSF1R, CSF1 receptor; GR-1, granulocyte-differentiation antigen-1; IL-4Rα, interleukin 4 α-chain receptor; STAT3, signal transducer and activator of transcription 3; TGFβ, transforming growth factor-β; VEGF, vascular endothelial growth factor.
Depletion of Myeloid Suppressor Cells
The most promising and feasible strategy to reduce the intratumoral numbers of myeloid suppressor cells is the use of low doses of approved chemotherapy drugs. Ugel et al.49 evaluated 12 widely used anticancer drugs for their ability to deplete myeloid suppressor cells and to restore immune responsiveness in tumor-bearing mice. They found that 7 of these drugs were able to effectively reduce tumor-infiltrating MDSCs: cyclophosphamide, 5-fluorouracil, fludarabine, gemcitabine, bortezomib, sorafenib, and sunitinib. This comparative study confirmed prior reports, since the majority of these anticancer drugs had been previously observed to reduce MDSCs tumor-infiltration in various tumor models.50-58 In addition to anticancer drugs, other small molecules have been found to block the expansion of MDSCs. Among these drugs are vitamin derivatives,59-61 amino-bisphosphonate,62,63 and antibodies or antagonists, such as an IL-4Rα RNA aptamer, that target molecules required for myeloid suppressive cell expansion and recruitment.64-68
With regard to TAMs, clodronate-loaded liposomes are the most widely used drug to deplete macrophages in animal models.69 In breast cancer patients, treatment with these liposomal bisphosphonates reportedly reduced the formation of new metastases.70 Another compound that can be used to deplete TAMs is trabectedin, a recently approved chemotherapy drug originally isolated from a marine tunicate Ecteinascidia.71 This drug was originally developed due to its potent tumoricidal ability, but recently trabectedin has also been shown to possess potent immunomodulatory activities. Trabectedin appears to selectively deplete monocytes and macrophages and further, blocks angiogenesis and monocyte tumor recruitment via reducing the expression of VEGF and CCL2 in tumor vessels.72 This intriguing mechanism of a new chemotherapy drug highlights the importance of studying the effects of conventional chemotherapeutic agents on immunity in an attempt to maximize their antitumor efficacy.
Blockade of Macrophage Recruitment to the Tumor Microenvironment
Another strategy to reduce TAM levels is to interfere with molecules that recruit macrophages to the tumor bed. Importantly, attenuated macrophage tumor-infiltration manifests as reduced tumor growth and metastatic spread. The most widely studied strategy to date is blockade of colony stimulating factor 1 (CSF1) signaling, an essential regulator of macrophage homeostasis. Blockade of the receptor for CSF1 was first described as reducing TAMs, and thereby increasing CD8+ T cells,28 but it can also effectively reduce the infiltration of MDSCs.66 CSF1 signaling can be abrogated using inhibitors of the protein tyrosine kinase activity of the CSF1 receptor or by exogenous application of molecules that block CSF1 ligand-receptor binding. Among the inhibitors of tyrosine kinase activity, Ki20227 has been shown to be relatively selective for the CSF1 receptor73 but most other tyrosine kinase inhibitors are broad spectrum and inhibit other tyrosine kinases. For instance, PLX3397, which shows potent antitumor activity when combined with chemotherapy, can block CSF1 and cKIT receptor tyrosine kinases.74 Other strategies that have been implemented to specifically prevent the binding of CSF1 to its cognate receptor include the use of small interfering RNAs against CSF175 and the use of blocking antibodies against CSF1 or its receptor.73,74 Investigations of therapeutic applications of blockade of CSF1 as a cancer treatment has reached clinical trials. A Phase I/II clinical trial of an anti-CSF1 antibody has been recently performed (NCT00757757) and several clinical trials with inhibitors of protein tyrosine kinase activity are ongoing (NCT01499043, NCT01349049 and NCT01349036).
Another cytokine that promotes macrophage infiltration into tumors is VEGF. Tumor-associated macrophages express the VEGF receptor variant VEGFR2 (also known as kinase insert domain receptor, KDR) and VEGF-blocking antibodies reduce the number of TAMs.76 Chemokines are another potential target that may be manipulated to interfere with macrophage recruitment. For instance, antibodies blocking CCL2 have been successfully used to reduce TAM migration77,78 and CXCR2 antagonists display antitumor activities and reduce metastatic dissemination by impeding Gr-1+ cell accumulation.46,79
Functional Inactivation of Protumorigenic Myeloid Suppressor Cells
The protumorigenic activity of myeloid cells can be attenuated not only by reducing the number of these subsets in the tumor but also by blocking the effector molecules that mediate the deleterious effects of myeloid cells. A common effector molecule used by several myeloid cell subsets are ROS. Acute release of ROS is among antitumor immune mediators, however chronic exposure to ROS actually promotes tumor progression by dampening effector immune cells and promoting DNA damage and chromosomal instability in tumor cells. An anti-inflammatory triterpenoid with the ability to reduce ROS release has been shown to dampen the activity of MDSCs.80 An alternative strategy to block the suppressor activity of these cells is to inhibit the catabolic enzymes overexpressed by MDSCs, namely arginase 1 and NOS2. These enzymes have been blocked by nitroaspirin and by inhibitors of phosphodiesterase-5 (such as sildenafil). Inhibition of both of these molecules has been shown to reduce the activity of MDSCs and enhance antitumor immunity.81-83 Finally, production of ROS by tumors can nitrate chemokines84 and promote the hyporesponsiveness of T cells to stimulation through the tyrosine phosphorylation of several proteins, including the CD3ζ chain of the T cell receptor complex.85 These immunosuppressive effects can be blocked by peroxynitrite-scavenging drugs.84
Remodeling of Tumor-infiltrating Myeloid Cells
Several treatments have been shown to remodel the intratumoral myeloid cell compartment, switching from a suppressive myeloid cell milieu toward antitumor myeloid cell types. Among chemotherapy drugs, cyclophosphamide has a special ability to induce tumor myeloid cell remodeling. Ibe et al. reported that cyclophosphamide induces a fast switch in intratumoral macrophages from tumor-suppressive M2 to antitumor M1 subtypes.86 The antitumor activity of this drug is enhanced when the modulation of tumor-infiltrating myeloid cells is combined with immunostimulatory treatment. For instance, in combination with an antitumor vaccine and CpG, cyclophosphamide depleted MDSCs and promoted the appearance of antitumor neutrophils, thereby leading to the eradication of large tumors.87 Moreover IL-12 combined with cyclophosphamide modified the intratumoral myeloid cell compartment by a combination of proinflammatory monocytes and neutrophils that acquired direct tumor killing capabilities and promoted antitumor CD8+ T-cell infiltration.88,89 Salem et al. reported an expansion of immature myeloid cells in the recovery phase after cyclophosphamide treatment, thereby boosting the antitumor effect of a vaccine composed of gp100 melanoma peptide and the toll-like receptor 3 (TLR3) ligand, poly(I:C).90 Interestingly, other chemotherapy components can trigger intratumoral myeloid cell remodeling. Recently, Ma et al. showed that recruitment of a DC-like CD11c+CD11b+Ly6Chi myeloid cell population was critical for the immunostimulatory effect of chemotherapies such as doxorubicin or mitoxantrone that trigger an immunogenic cell death.91
Other therapeutic regimens can trigger this functional remodeling. For instance, the switch between N2 to N1-subtypes of intratumoral neutrophils can be achieved by blocking TGFβ.12,92 In addition, some reports have linked the antitumor activity of CSF2 to its ability to activate the killing activity of neutrophils.93,94 In regards to macrophages, the switch from M2 to M1 can be achieved by the use of zoleidronic acid95 or by the combination of CpG with an antibody to block the IL-10 receptor96 In both cases, a potent antitumor innate immune response is exerted by remodeling the type of macrophages present within the tumor. Zoleidronic acid is an anti-resorptive agent that has been observed to exhibit antitumor effects both in vitro and in vivo. The proposed mechanism of action is the modulation of the mevalonate pathway that affects protein prenylation in both tumor cells and macrophages, thereby decreasing the viability of these cells. In addition, zoleidronic acid promotes the translocation of NF-κB to the nucleus, inducing the production of inflammatory cytokines.95,97 Disrupting another critical regulator of myeloid cell fate, STAT3 has been shown to be crucial for tumor-induced myeloid cell remodeling toward tumor-resolving inflammation.98,99 The STAT3 inhibitor JSI-124 induces antitumor immunity by maturing MDSCs toward DCs in vivo100 and there is an ongoing clinical trial investigating the use of an antisense STAT3 inhibitor in cancer patients. Finally, CD40 agonists alone or in combination with IL-2 have been shown to switch immunosuppressive TAMs into potent antitumor macrophages in preclinical settings and, more importantly, have been found to induce objective clinical responses in patients.101-103
Conclusions
Myeloid cells are essential components of the tumor stroma that support all phases of tumor development. These cells promote tumor growth and metastasis by facilitating tumor transformation and angiogenesis, as well as by suppressing antitumor effector immune responses. Therefore, tumor-associated myeloid cells are excellent drug targets to therapeutically interfere with tumor development. Several strategies have been tested to deplete myeloid cells infiltrating tumors, including the inhibition of their recruitment, agents to abrogate their immunosuppressive functions or to remodel their tumor composition. These treatments usually achieve modest results in terms of tumor rejection but can have synergistic effects when combined with other cancer treatments such as chemotherapy, radiotherapy, monoclonal antibodies or other immunotherapies designed to boost the adaptive immune system. The clinical development of drug combinations is cumbersome and expensive, especially when single agents do not display potent antitumor effects. However, cancer is a complex disease with many players involved in the tumorigenic process. In order to achieve tumor rejections, several pathways will likely need to be simultaneously targeted.
The most feasible and inexpensive strategy to modulate tumor-associated myeloid cells is the use of low-doses of approved chemotherapy drugs. However, the doses and the timing of such drugs must be carefully examined in clinical trials in order to maximize their efficacy on myeloid cells and achieve the desired anticancer immunostimulatory effects. Positive clinical results obtained by modulating myeloid cells with such compounds will pave the way for the development of new drugs targeting these cells to boost the successful application of immunotherapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by “Fondo de Investigaciones Sanitarias,” “Fundación Mutua Madrileña” and by the agreement between “Fundación para la Investigación Médica Aplicada” and the “Unión Temporal de Empresas proyecto Centro de Investigación Médica Aplicada.” J.M.E. was supported by a fellowship of “Fondo de Investigaciones Sanitarias.” P.B. was supported by a Miguel Servet contract from “Fondo de Investigaciones Sanitarias.”
Glossary
Abbreviations:
- CSF1
colony stimulating factor 1
- DCs
dendritic cells
- Gr-MDSCs
granulocytic myeloid-derived suppressor cells
- iDCs
immature dendritic cells
- NOS2
inducible nitric oxide synthase 2
- IL-10
interleukin 10
- mDCs
mature dendritic cells
- Mo-MDSCs
monocytic myeloid-derived suppressor cells
- MDSCs
myeloid-derived suppressor cells
- NO
nitric oxide
- ROS
reactive oxygen species
- TGF-β
transforming growth factor beta
- TAMs
tumor-associated macrophages
- TANs
tumor-associated neutrophils
- VEGF
vascular endothelial growth factor
Citation: Medina-Echeverz J, Aranda F, Berraondo P. Myeloid-derived cells are key targets of tumor immunotherapy. OncoImmunology 2014; 3:e28398; 10.4161/onci.28398
References
- 1.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 1999;13:1382–97. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fricke I, Gabrilovich DI. Dendritic cells and tumor microenvironment: a dangerous liaison. Immunol Invest. 2006;35:459–83. doi: 10.1080/08820130600803429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nyberg P, Salo T, Kalluri R. Tumor microenvironment and angiogenesis. Front Biosci. 2008;13:6537–53. doi: 10.2741/3173. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–70. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 5.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–50. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 6.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 7.Theoharides TC, Conti P. Mast cells: the Jekyll and Hyde of tumor growth. Trends Immunol. 2004;25:235–41. doi: 10.1016/j.it.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Piconese S, Gri G, Tripodo C, Musio S, Gorzanelli A, Frossi B, Pedotti R, Pucillo CE, Colombo MP. Mast cells counteract regulatory T-cell suppression through interleukin-6 and OX40/OX40L axis toward Th17-cell differentiation. Blood. 2009;114:2639–48. doi: 10.1182/blood-2009-05-220004. [DOI] [PubMed] [Google Scholar]
- 9.Tripodo C, Gri G, Piccaluga PP, Frossi B, Guarnotta C, Piconese S, Franco G, Vetri V, Pucillo CE, Florena AM, et al. Mast cells and Th17 cells contribute to the lymphoma-associated pro-inflammatory microenvironment of angioimmunoblastic T-cell lymphoma. Am J Pathol. 2010;177:792–802. doi: 10.2353/ajpath.2010.091286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A. 2006;103:12493–8. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–60. [PubMed] [Google Scholar]
- 12.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065X.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhao F, Falk C, Osen W, Kato M, Schadendorf D, Umansky V. Activation of p38 mitogen-activated protein kinase drives dendritic cells to become tolerogenic in ret transgenic mice spontaneously developing melanoma. Clin Cancer Res. 2009;15:4382–90. doi: 10.1158/1078-0432.CCR-09-0399. [DOI] [PubMed] [Google Scholar]
- 15.Feijoó E, Alfaro C, Mazzolini G, Serra P, Peñuelas I, Arina A, Huarte E, Tirapu I, Palencia B, Murillo O, et al. Dendritic cells delivered inside human carcinomas are sequestered by interleukin-8. Int J Cancer. 2005;116:275–81. doi: 10.1002/ijc.21046. [DOI] [PubMed] [Google Scholar]
- 16.Yurkovetsky ZR, Yurkovetsky GN. Trafficking of dendritic cells in the tumor environment. Dendritic Cells in Cancer: Springer, 2009:271-89. [Google Scholar]
- 17.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–67. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-beta-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–29. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alfaro C, Suarez N, Gonzalez A, Solano S, Erro L, Dubrot J, Palazon A, Hervas-Stubbs S, Gurpide A, Lopez-Picazo JM, et al. Influence of bevacizumab, sunitinib and sorafenib as single agents or in combination on the inhibitory effects of VEGF on human dendritic cell differentiation from monocytes. Br J Cancer. 2009;100:1111–9. doi: 10.1038/sj.bjc.6604965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kandalaft LE, Motz GT, Busch J, Coukos G. Angiogenesis and the tumor vasculature as antitumor immune modulators: the role of vascular endothelial growth factor and endothelin. Cancer Immunology and Immunotherapy: Springer, 2011:129-48. [DOI] [PubMed] [Google Scholar]
- 21.Harizi H, Juzan M, Pitard V, Moreau JF, Gualde N. Cyclooxygenase-2-issued prostaglandin e(2) enhances the production of endogenous IL-10, which down-regulates dendritic cell functions. J Immunol. 2002;168:2255–63. doi: 10.4049/jimmunol.168.5.2255. [DOI] [PubMed] [Google Scholar]
- 22.Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, Corzo A, Cho HI, Celis E, Lennox B, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–6. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sa C. Immunobiology of Dendritic Cells in Cancer. In: Hurwitz Ga, ed. Tumor-Induced Immune Suppression: Springer Verlag, 2008. [Google Scholar]
- 24.Lipscomb. Dendritic Cell Maturation Versus Polarization in Tumor Escape. In: Shurin MR, ed. Dendritic Cells in Cancer: Springer Verlag, 2009. [Google Scholar]
- 25.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–73. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 26.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 27.Bottazzi B, Polentarutti N, Acero R, Balsari A, Boraschi D, Ghezzi P, Salmona M, Mantovani A. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983;220:210–2. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- 28.Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–40. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 30.Van Ginderachter JA, Movahedi K, Hassanzadeh Ghassabeh G, Meerschaut S, Beschin A, Raes G, De Baetselier P. Classical and alternative activation of mononuclear phagocytes: picking the best of both worlds for tumor promotion. Immunobiology. 2006;211:487–501. doi: 10.1016/j.imbio.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/S1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 32.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 33.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 34.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40:2969–75. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tirelli U, Spina M, Sandri S, Serraino D, Gobitti C, Fasan M, Sinicco A, Garavelli P, Ridolfo AL, Vaccher E, The Italian Cooperative Group on AIDS and Tumors Lung carcinoma in 36 patients with human immunodeficiency virus infection. Cancer. 2000;88:563–9. doi: 10.1002/(SICI)1097-0142(20000201)88:3<563::AID-CNCR11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 36.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, et al. HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–53. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, Villagra A, Antonia S, McCaffrey JC, Fishman M, et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol. 2013;14:211–20. doi: 10.1038/ni.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L, Zhang J, Diao W, Wang D, Wei Y, Zhang CY, Zen K. MicroRNA-155 and MicroRNA-21 promote the expansion of functional myeloid-derived suppressor cells. J Immunol. 2014;192:1034–43. doi: 10.4049/jimmunol.1301309. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Lai L, Chen Q, Song Y, Xu S, Ma F, Wang X, Wang J, Yu H, Cao X, et al. MicroRNA-494 is required for the accumulation and functions of tumor-expanded myeloid-derived suppressor cells via targeting of PTEN. J Immunol. 2012;188:5500–10. doi: 10.4049/jimmunol.1103505. [DOI] [PubMed] [Google Scholar]
- 42.Sonda N, Simonato F, Peranzoni E, Calì B, Bortoluzzi S, Bisognin A, Wang E, Marincola FM, Naldini L, Gentner B, et al. miR-142-3p prevents macrophage differentiation during cancer-induced myelopoiesis. Immunity. 2013;38:1236–49. doi: 10.1016/j.immuni.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 43.Lee H, Deng J, Kujawski M, Yang C, Liu Y, Herrmann A, Kortylewski M, Horne D, Somlo G, Forman S, et al. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med. 2010;16:1421–8. doi: 10.1038/nm.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bronte V. Myeloid-Derived Suppressor Cells in Cancer. In: Gabrilovich D, ed. Tumor-induced Immune Suppression: Springer Verlag, 2008. [Google Scholar]
- 45.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–49. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acharyya S, Oskarsson T, Vanharanta S, Malladi S, Kim J, Morris PG, Manova-Todorova K, Leversha M, Hogg N, Seshan VE, et al. A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell. 2012;150:165–78. doi: 10.1016/j.cell.2012.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinha P, Chornoguz O, Clements VK, Artemenko KA, Zubarev RA, Ostrand-Rosenberg S. Myeloid-derived suppressor cells express the death receptor Fas and apoptose in response to T cell-expressed FasL. Blood. 2011;117:5381–90. doi: 10.1182/blood-2010-11-321752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nagaraj S, Schrum AG, Cho HI, Celis E, Gabrilovich DI. Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol. 2010;184:3106–16. doi: 10.4049/jimmunol.0902661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ugel S, Peranzoni E, Desantis G, Chioda M, Walter S, Weinschenk T, Ochando JC, Cabrelle A, Mandruzzato S, Bronte V. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep. 2012;2:628–39. doi: 10.1016/j.celrep.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res. 2009;15:2148–57. doi: 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- 51.Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD, Rini B, Finke JH, Cohen PA. Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res. 2010;70:3526–36. doi: 10.1158/0008-5472.CAN-09-3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, Schwartz M, Divino CM, Pan PY, Chen SH. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–22. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Cruijsen H, van der Veldt AA, Vroling L, Oosterhoff D, Broxterman HJ, Scheper RJ, Giaccone G, Haanen JB, van den Eertwegh AJ, Boven E, et al. Sunitinib-induced myeloid lineage redistribution in renal cell cancer patients: CD1c+ dendritic cell frequency predicts progression-free survival. Clin Cancer Res. 2008;14:5884–92. doi: 10.1158/1078-0432.CCR-08-0656. [DOI] [PubMed] [Google Scholar]
- 54.Xin H, Zhang C, Herrmann A, Du Y, Figlin R, Yu H. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 2009;69:2506–13. doi: 10.1158/0008-5472.CAN-08-4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–83. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–21. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 57.Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Rébé C, Ghiringhelli F. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 58.Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lathers DM, Clark JI, Achille NJ, Young MR. Phase 1B study to improve immune responses in head and neck cancer patients using escalating doses of 25-hydroxyvitamin D3. Cancer Immunol Immunother. 2004;53:422–30. doi: 10.1007/s00262-003-0459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, Gabrilovich D. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63:4441–9. [PubMed] [Google Scholar]
- 62.Melani C, Sangaletti S, Barazzetta FM, Werb Z, Colombo MP. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007;67:11438–46. doi: 10.1158/0008-5472.CAN-07-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Hegmans JP, Aerts JG. Zoledronic acid impairs myeloid differentiation to tumour-associated macrophages in mesothelioma. Br J Cancer. 2010;103:629–41. doi: 10.1038/sj.bjc.6605814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, Chen SH. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–28. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, Blanchard D, Bais C, Peale FV, van Bruggen N, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–31. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 66.Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, Johnson M, Lusis AJ, Cohen DA, Iruela-Arispe ML, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of antiangiogenic therapy. Blood. 2010;115:1461–71. doi: 10.1182/blood-2009-08-237412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morales JK, Kmieciak M, Graham L, Feldmesser M, Bear HD, Manjili MH. Adoptive transfer of HER2/neu-specific T cells expanded with alternating gamma chain cytokines mediate tumor regression when combined with the depletion of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2009;58:941–53. doi: 10.1007/s00262-008-0609-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Roth F, De La Fuente AC, Vella JL, Zoso A, Inverardi L, Serafini P. Aptamer-mediated blockade of IL4Rα triggers apoptosis of MDSCs and limits tumor progression. Cancer Res. 2012;72:1373–83. doi: 10.1158/0008-5472.CAN-11-2772. [DOI] [PubMed] [Google Scholar]
- 69.Lepique AP, Daghastanli KR, Cuccovia IM, Villa LL. HPV16 tumor associated macrophages suppress antitumor T cell responses. Clin Cancer Res. 2009;15:4391–400. doi: 10.1158/1078-0432.CCR-09-0489. [DOI] [PubMed] [Google Scholar]
- 70.Diel IJ, Solomayer EF, Costa SD, Gollan C, Goerner R, Wallwiener D, Kaufmann M, Bastert G. Reduction in new metastases in breast cancer with adjuvant clodronate treatment. N Engl J Med. 1998;339:357–63. doi: 10.1056/NEJM199808063390601. [DOI] [PubMed] [Google Scholar]
- 71.García-Rocha M, García-Gravalos MD, Avila J. Characterisation of antimitotic products from marine organisms that disorganise the microtubule network: ecteinascidin 743, isohomohalichondrin-B and LL-15. Br J Cancer. 1996;73:875–83. doi: 10.1038/bjc.1996.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M, Pasqualini F, et al. Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell. 2013;23:249–62. doi: 10.1016/j.ccr.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 73.Kubota Y, Takubo K, Shimizu T, Ohno H, Kishi K, Shibuya M, Saya H, Suda T. M-CSF inhibition selectively targets pathological angiogenesis and lymphangiogenesis. J Exp Med. 2009;206:1089–102. doi: 10.1084/jem.20081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, Gallagher WM, Wadhwani N, Keil SD, Junaid SA, et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discov. 2011;1:54–67. doi: 10.1158/2159-8274.CD-10-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abraham D, Zins K, Sioud M, Lucas T, Schäfer R, Stanley ER, Aharinejad S. Stromal cell-derived CSF-1 blockade prolongs xenograft survival of CSF-1-negative neuroblastoma. Int J Cancer. 2010;126:1339–52. doi: 10.1002/ijc.24859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dineen SP, Lynn KD, Holloway SE, Miller AF, Sullivan JP, Shames DS, Beck AW, Barnett CC, Fleming JB, Brekken RA. Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res. 2008;68:4340–6. doi: 10.1158/0008-5472.CAN-07-6705. [DOI] [PubMed] [Google Scholar]
- 77.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang J, Lu Y, Pienta KJ. Multiple roles of chemokine (C-C motif) ligand 2 in promoting prostate cancer growth. J Natl Cancer Inst. 2010;102:522–8. doi: 10.1093/jnci/djq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tazzyman S, Barry ST, Ashton S, Wood P, Blakey D, Lewis CE, Murdoch C. Inhibition of neutrophil infiltration into A549 lung tumors in vitro and in vivo using a CXCR2-specific antagonist is associated with reduced tumor growth. Int J Cancer. 2011;129:847–58. doi: 10.1002/ijc.25987. [DOI] [PubMed] [Google Scholar]
- 80.Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, Meyer C, Becerra CR, Fishman M, Antonia S, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16:1812–23. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Santo C, Serafini P, Marigo I, Dolcetti L, Bolla M, Del Soldato P, Melani C, Guiducci C, Colombo MP, Iezzi M, et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc Natl Acad Sci U S A. 2005;102:4185–90. doi: 10.1073/pnas.0409783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meyer C, Sevko A, Ramacher M, Bazhin AV, Falk CS, Osen W, Borrello I, Kato M, Schadendorf D, Baniyash M, et al. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci U S A. 2011;108:17111–6. doi: 10.1073/pnas.1108121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, De Palma A, Mauri P, Monegal A, Rescigno M, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med. 2011;208:1949–62. doi: 10.1084/jem.20101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kasic T, Colombo P, Soldani C, Wang CM, Miranda E, Roncalli M, Bronte V, Viola A. Modulation of human T-cell functions by reactive nitrogen species. Eur J Immunol. 2011;41:1843–9. doi: 10.1002/eji.201040868. [DOI] [PubMed] [Google Scholar]
- 86.Ibe S, Qin Z, Schüler T, Preiss S, Blankenstein T. Tumor rejection by disturbing tumor stroma cell interactions. J Exp Med. 2001;194:1549–59. doi: 10.1084/jem.194.11.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berraondo P, Nouzé C, Préville X, Ladant D, Leclerc C. Eradication of large tumors in mice by a tritherapy targeting the innate, adaptive, and regulatory components of the immune system. Cancer Res. 2007;67:8847–55. doi: 10.1158/0008-5472.CAN-07-0321. [DOI] [PubMed] [Google Scholar]
- 88.Medina-Echeverz J, Fioravanti J, Zabala M, Ardaiz N, Prieto J, Berraondo P. Successful colon cancer eradication after chemoimmunotherapy is associated with profound phenotypic change of intratumoral myeloid cells. J Immunol. 2011;186:807–15. doi: 10.4049/jimmunol.1001483. [DOI] [PubMed] [Google Scholar]
- 89.Tsung K, Dolan JP, Tsung YL, Norton JA. Macrophages as effector cells in interleukin 12-induced T cell-dependent tumor rejection. Cancer Res. 2002;62:5069–75. [PubMed] [Google Scholar]
- 90.Salem ML, Díaz-Montero CM, Al-Khami AA, El-Naggar SA, Naga O, Montero AJ, Khafagy A, Cole DJ. Recovery from cyclophosphamide-induced lymphopenia results in expansion of immature dendritic cells which can mediate enhanced prime-boost vaccination antitumor responses in vivo when stimulated with the TLR3 agonist poly(I:C) J Immunol. 2009;182:2030–40. doi: 10.4049/jimmunol.0801829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38:729–41. doi: 10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 92.Brandau S. The dichotomy of neutrophil granulocytes in cancer. Semin Cancer Biol. 2013;23:139–40. doi: 10.1016/j.semcancer.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 93.Stoppacciaro A, Melani C, Parenza M, Mastracchio A, Bassi C, Baroni C, Parmiani G, Colombo MP. Regression of an established tumor genetically modified to release granulocyte colony-stimulating factor requires granulocyte-T cell cooperation and T cell-produced interferon gamma. J Exp Med. 1993;178:151–61. doi: 10.1084/jem.178.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Soiffer R, Hodi FS, Haluska F, Jung K, Gillessen S, Singer S, Tanabe K, Duda R, Mentzer S, Jaklitsch M, et al. Vaccination with irradiated, autologous melanoma cells engineered to secrete granulocyte-macrophage colony-stimulating factor by adenoviral-mediated gene transfer augments antitumor immunity in patients with metastatic melanoma. J Clin Oncol. 2003;21:3343–50. doi: 10.1200/JCO.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 95.Coscia M, Quaglino E, Iezzi M, Curcio C, Pantaleoni F, Riganti C, Holen I, Mönkkönen H, Boccadoro M, Forni G, et al. Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J Cell Mol Med. 2010;14:2803–15. doi: 10.1111/j.1582-4934.2009.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–46. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 97.Muratsu D, Yoshiga D, Taketomi T, Onimura T, Seki Y, Matsumoto A, Nakamura S. Zoledronic acid enhances lipopolysaccharide-stimulated proinflammatory reactions through controlled expression of SOCS1 in macrophages. PLoS One. 2013;8:e67906. doi: 10.1371/journal.pone.0067906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 99.Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114–23. doi: 10.1016/j.ccr.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nefedova Y, Nagaraj S, Rosenbauer A, Muro-Cacho C, Sebti SM, Gabrilovich DI. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 2005;65:9525–35. doi: 10.1158/0008-5472.CAN-05-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Weiss JM, Ridnour LA, Back T, Hussain SP, He P, Maciag AE, Keefer LK, Murphy WJ, Harris CC, Wink DA, et al. Macrophage-dependent nitric oxide expression regulates tumor cell detachment and metastasis after IL-2/anti-CD40 immunotherapy. J Exp Med. 2010;207:2455–67. doi: 10.1084/jem.20100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weiss JM, Back TC, Scarzello AJ, Subleski JJ, Hall VL, Stauffer JK, Chen X, Micic D, Alderson K, Murphy WJ, et al. Successful immunotherapy with IL-2/anti-CD40 induces the chemokine-mediated mitigation of an immunosuppressive tumor microenvironment. Proc Natl Acad Sci U S A. 2009;106:19455–60. doi: 10.1073/pnas.0909474106. [DOI] [PMC free article] [PubMed] [Google Scholar]