Abstract
Tumor relapse after radiotherapy may be due to the upregulation of programmed cell death ligand 1 (PD-L1). We demonstrated that anti-PD-L1 antibody synergizes with radiation to control local and distal tumors. CD8+T cells mediated antitumor effects of the combination therapy by the reduction of myeloid-derived suppressor cells (MDSCs) via tumor-necrosis factor (TNF)-mediated signaling. Our study provides insight into immune- and radiation-based combinational therapies.
Keywords: antibody therapy, myeloid-derived suppressor cell, negative signal, PD-L1, radiation therapy, T-cell activation, TNF
Radiotherapy is broadly used clinically to treat primary and metastatic malignancies in approximately 50% of all cancer patients.1 Although the accurate delivery of the irradiation dose has been greatly improved, many patients suffer from local tumor recurrences following radiotherapy.1,2 Therefore, efforts to improve the efficacy of radiotherapy via combination treatment with other treatment regimens could provide clinical benefit. Recently, high-dose ionizing radiation has been shown to induce cancer cell death and tumor-specific adaptive immune responses.3,4 Conversely, inadequate antitumor responses or radiation-induced suppressive immunity may also account for a large part of treatment failures. However, the mechanisms of how radiation modulates immunosuppressive factors and biological activities remain to be fully elucidated.
Immunosuppressive factors are utilized by the host to constrain immune responses and prevent immune hyper-activation from harming normal tissues.5 Cancers take advantage of this native ability by exploiting various immune escape mechanisms to persist despite anticancer treatments.5 Immune-checkpoint pathways have been shown to be major mechanisms of immune resistance.6 Checkpoint blockade immunotherapies, such as antibodies targeting programmed cell death ligand 1 (PD-L1) and its receptor (PDCD1, better known as PD-1), have significantly increased the objective response rate to ~20–30% in the treatment of several types of cancers.7,8 Although anti-PD-1 and anti-PD-L1 antibodies have provided a new benchmark for antitumor activity in immunotherapy, their scope of application could be broadened with the combination of other conventional treatments, especially radiotherapy. Therefore, it is imperative to investigate whether radiotherapy synergizes with PD-L1/PD-1 axis inhibitors in clinical settings.
We hypothesized the following potential principles providing rationale for the combination of radiation and PD-L1/PD-1 axis inhibitors: 1) Irradiation increases tumor destruction and triggers immune infiltration into tumors, concomitantly with a radiation-induced local inflammatory responses stimulating an upregulation of PD-L1 expression to constrain local tissue damage, and 2) Irradiation-induced immunity and enhanced PD-L1 expression together provide a window of opportunity for robust pharmacological actions of PD-L1/PD-1 axis inhibitors, reducing radio-resistance and increasing the anti-PD-L1 immunotherapy response rate.
In order to test these theories, first, we determined the expression level of PD-L1 in different myeloid cell populations and tumor cells in response to radiation therapy. The upregulation of PD-L1 was observed in the irradiated tumors in mice,9 suggesting that alteration of PD-L1 levels in the tumor microenvironments may block the antitumor function of infiltrating effector T cells induced by irradiation. Next, we combined irradiation with anti-PD-L1 antibody therapy to treat 2 allograft tumor models, TUBO breast cancer and MC38 colon cancer. We found that radiation and anti-PD-L1 antibody synergized in both tumor models, whereas there was only a slight impact on tumor growth after radiation therapy alone. Furthermore, the combination treatment not only lead to prolonged antitumor immunity upon tumor re-challenge, but also induced an abscopal effect, thereby controlling secondary tumors distant from the irradiated primary tumor in both tumor models. These results demonstrate that the combination of irradiation and anti-PD-L1 antibody can potentially control both local and distal tumors.9
The antitumor effect of irradiation has been attributed largely to the adaptive immune response.3,4 Considering that PD-1 has been considered to be a key biomarker of T cell exhaustion,6 adaptive immune responses are deemed critical mediators of the combination therapy. The expression profile of PD-1 on T cells indicates that irradiation-induced T cells are replenished and newly infiltrating T cells are subsequently exhausted immediately during reconstitution of the tumor microenvironment. We next investigated whether adaptive immunity is required for the efficacy of the combination therapy and whether the therapy restores the functionality of cytotoxic T cells (CTLs). The depletion experiments indicated that CD8+ T cells, but not CD4+ T cells, were required for the efficacy of the combined radiation and checkpoint blockade therapy. Combination therapy could also induce a robust tumor antigen-specific T cell response, confirming the pivotal contribution of CD8+ T cell effector functions. Thus, these results not only reveal that CD8+ T cells are essential for the synergy of irradiation and anti-PD-L1 antibody therapy, but also that the effector functions of replenished CTLs in the tumor microenvironment following irradiation are restored by PD-L1 blockade.9
Accumulating evidence has shown that myeloid-derived suppressor cells (MDSCs) contribute to the attenuation of immune responses during cancer progression, including after treatment.10 This raises the possibility that the combinatorial therapeutic regimen used in our tumor models might also alter the distribution of MDSCs, unmasking the intensity of replenished CTLs functions. Our study revealed that concurrent therapy significantly and markedly reduced tumor-associated MDSCs in comparison to single therapy. It has been well documented that MDSCs regulate T-cell activation and proliferation by various routes, such as the induction of regulatory T cells, the release of oxidizing molecules, the amino acid deprivation of T cells, and interference of T-cell migration and viability.10 However, the increase of CTLs functions coincident with the decrease of MDSCs proportion prompted us to reconsider their potential interaction in this context. We hypothesized that CD8+ T cells induced by irradiation and anti-PD-L1 antibody are responsible for the elimination of tumor-infiltrating MDSCs. In our in vivo model, depletion of CD8+ T cells following combination therapies recovered the population of MDSCs within tumors. Immunofluorescence-based co-localization assay revealed that the residual Gr1+ cells and CD8+ T cells were located significantly closer spatially in tumors that received combination treatment, compared with untreated controls. An in vitro co-culture assay showed that activated CD8+ T cells induced an increase in MDSC apoptosis in a tumor necrosis factor (TNF)-dependent manner. Furthermore, blockade of TNF in vivo abrogated the efficacy of combination treatment. Collectively, these results indicate that combination of irradiation and anti-PD-L1 antibody therapies achieved effective tumor control by enhancing CTLs effector functions, which, in turn, negatively regulates the accumulation of MDSCs through TNF signaling.9
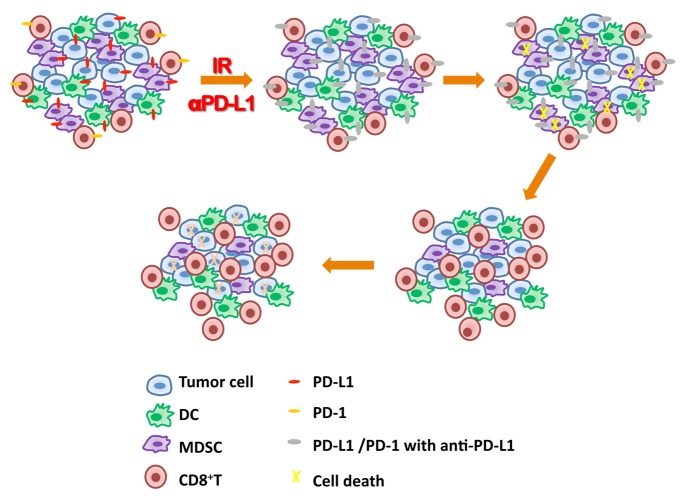
This study is timely and clinically relevant as it not only reveals that PD-L1 blockade synergizes with radiation therapy, but also indicates that the combination reduces radio-resistance and increases host response to antibody treatment (Fig. 1). We also demonstrate that CD8+ T cells are essential in the treatment response, partly by inducing MDSC cell death (Fig. 1). This study broadens the scope of current endeavors to manipulate the suppressive tumor environment and provides new insights into the design of combination cancer therapies for clinical applications.
Figure 1. Impact of the combination of irradiation and anti-PD-L1 antibody on the tumor microenvironment. Irradiation (IR) can induce programmed cell death ligand 1 (PD-L1) upregulation in the tumor microenvironment. Blockade of PD-L1/PD-1 signaling via anti-PDL1 antibody therapy (αPD-L1) restores the function of CD8+T cells following irradiation. Subsequently, the activated CD8+T cells infiltrate tumors and constrain the accumulation of myeloid-derived suppressor cells (MDSCs) through the cytotoxic action of tumor necrosis factor (TNF). Lacking MDSC support, tumor cells are effective extinguished by activated CD8+ T cells responding to the combination treatment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Deng L, Liang H, Burnette B, weicheslbaum r, Fu YX. Radiation and anti-PD-L1 treatment induces T cell-mediated depletion of myeloid-derived suppressor cells and tumor regression. OncoImmunology 2014; 10.4161/onci.28499
References
- 1.Begg AC, Stewart FA, Vens C. Strategies to improve radiotherapy with targeted drugs. Nat Rev Cancer. 2011;11:239–53. doi: 10.1038/nrc3007. [DOI] [PubMed] [Google Scholar]
- 2.Liauw SL, Connell PP, Weichselbaum RR. New paradigms and future challenges in radiation oncology: an update of biological targets and technology. Sci Transl Med. 2013;5:173sr2. doi: 10.1126/scitranslmed.3005148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang H, Deng L, Chmura S, Burnette B, Liadis N, Darga T, Beckett MA, Lingen MW, Witt M, Weichselbaum RR, et al. Radiation-induced equilibrium is a balance between tumor cell proliferation and T cell-mediated killing. J Immunol. 2013;190:5874–81. doi: 10.4049/jimmunol.1202612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366:2517–9. doi: 10.1056/NEJMe1205943. [DOI] [PubMed] [Google Scholar]
- 6.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, Wolchok JD, Hersey P, Joseph RW, Weber JS, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]