Abstract
We recently demonstrated that simultaneous targeting of CD137 co-stimulatory and programmed cell death 1 (PD-1) co-inhibitory molecules synergistically induced an anticancer immune response in the ID8 syngeneic orthotopic mouse ovarian carcinoma model. We further showed that the therapeutic efficacy was enhanced by treatment with cisplatin. These findings provide a rationale for evaluating dual targeting of CD137/PD-1 co-signaling molecules in ovarian cancer patients.
Keywords: CD137, PD-1, co-signaling molecules, ovarian cancer, immunotherapy, monoclonal antibody
Ovarian cancer is the most deadly gynecologic malignancy. Surgical debulking in combination with platinum-based adjuvant chemotherapy remains the current standard-of-care treatment to which approximately 80% of patients will respond favorably. However, more than 60% of patients who initially achieve remission eventually relapse and less than 40% will survive beyond 5 y. Thus, novel treatments for ovarian cancer are urgently needed.
Ovarian cancer expresses antigens that can be recognized by the patients’ immune system and should, conceptually, be amenable to immunotherapy.1-3 Although clinical trials of various immunotherapeutic modalities have not yet yielded significant benefit,1 the failures to date can be understood in light of recent advances in our comprehension of the complexities of immune regulation, potentially leading to the development of more efficacious immunotherapies.
In order to generate an effective anticancer immune response, a cancer-immunity cycle must be executed, starting with the release of cancer antigens. These cancer targeting epitopes must then be presented by antigen-presenting cells (APCs) to prime and activate cognate T cells that then home to tumors culminating in the recognition and killing of cancer cells by effector T cells.4 Tumors develop multiple strategies to evade nearly every step of this cycle. These include deregulating APCs, the establishment of a physical barrier to prevent the homing of effector lymphocytes into the neoplastic lesion, and the inhibition of effector lymphocyte function via immunosuppressive cells and molecules such as indoleamine 2,3-dioxygenase 1 (IDO1) and transforming growth factor-β (TGFβ).5 To overcome this problem one needs to simultaneously activate the antitumor immune response coincident with inhibition of the immunosuppressive mechanisms which are strongest within the tumor microenvironment and in the tumor-draining lymph nodes.2,3
Co-stimulatory and co-inhibitory molecules play a crucial role in regulating tumor immunity, and the immune response can be modified by applying agonistic or antagonistic monoclonal antibodies (mAbs) to modulate the activity of such immunoregulatory molecules.6 mAbs blocking the co-inhibitory molecules cytotoxic T-lymphocyte associated protein 4 (CTLA-4) and/or programmed cell death 1 (PDCD1, better known as PD-1) have already shown encouraging activity in clinical trials against multiple solid tumors.6
We recently evaluated the antitumor efficacy of agonistic and antagonistic mAbs against multiple co-signaling molecules (both individually and in combinations) utilizing the well-established ID8 syngeneic orthotopic mouse model of ovarian cancer. In this model, small intraperitoneal tumor nests appear about 10 d post-implantation and solid tumors, as well as malignant ascites, develop rapidly thereafter leading to death approximately 30 d after tumor transplantation. Intraperitoneal injections of single mAb antagonists against co-inhibitory receptors (PD-1, CTLA-4, TIM-3, and LAG-3) or agonistic mAb targeting co-stimulatory molecules (CD137, OX40, GITR, and CD40) in mice that had been transplanted 10 d earlier with ID8 cells were found to be ineffective.2,3 However, treatment with a combination of anti-CD137 and anti-PD-1 mAbs significantly prolonged survival, and addition of a mAb to CTLA-4 further enhanced the antitumor efficacy.2,3 A mechanistic investigation demonstrated that the mAb combination greatly increased the number of tumor-associated CD8+ effector T cells in the peritoneal cavity and decreased the number of immunosuppressive regulatory T (Treg) cells and myeloid-derived suppressor cells (MDSCs) so that there was a prominent increase in the T effector to T suppressive cell ratios.2,3 Furthermore, the combined treatment rescued the function of exhausted T cells with a PD-1+TIM3+ or PD-1+TIM3– phenotype.3
Importantly, pretreatment of the ID8-bearing mice with cisplatin, a drug routinely used to treat patients with advanced ovarian carcinoma, synergized with the dual mAb combination and presaged an enduring, complete, and antigen-specific tumor regression in 80% of the mice.3 Likewise, Coukos et al. showed a synergistic antitumor effect by PD-1/PD-L1 pathway blockade combined with CD137 activation in the ID8 model and with 75% long-term survival when the mAb therapy was combined with a vaccine composed of irradiated cytokine-secreting whole tumor cells.7 Furthermore, combined anti-CD137/PD-1 antibody treatment synergized with radiotherapy in mice harboring established AT-3 triple-negative breast cancer.8 In other cancers, combined PD-1 blockade and CD137 activation has also shown significant immunotherapeutic effects in mice bearing B16 or SW-1 melanomas or TC-1 lung carcinoma.2,3 Also found to exhibit anticancer efficacy was the combination of the 2 mAbs with cisplatin in the TC-1 model.3
It remains elusive why established ID8 tumors are resistant to single PD-1 blockade or CD137 activation. It may be relevant that they are poorly infiltrated by T cells, as are about 50% of human ovarian cancers.5,7 It is possible that any tumor-infiltrating lymphoid cells are functionally exhausted and that a single blockade of the PD-1 pathway is not sufficient to mount an effective antitumor response unless there is activation via CD137 (Fig. 1). Combination with an appropriate chemotherapeutic drug, cancer vaccination or radiotherapy is likely to further increase the pool of tumor-reactive T cells that have escaped immunosuppression.3,8 Interestingly, combined CTLA-4 blockade and CD137 triggering was not effective against ID8 tumors, indicating that PD-1-mediated negative signaling plays a more important role than CTLA-4 in the evasion of these ovarian cancer cells from immunological control.
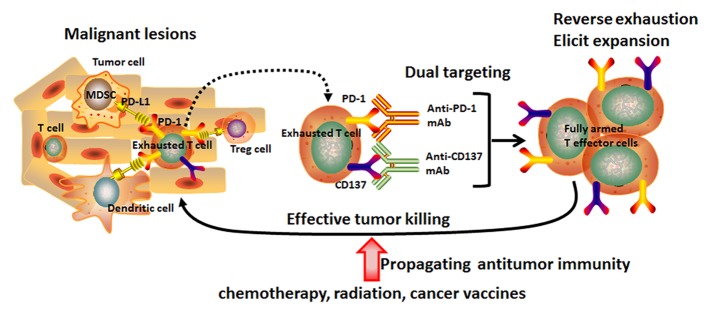
Figure 1. Induction of an anticancer immune response by dual targeting of CD137 co-stimulatory and PD-1 co-inhibitory molecules in combination with conventional cancer therapy. Immunosuppressive cells, including myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs) and tolerogenic dendritic cells (DCs) are present in the tumor microenvironment. Immunosuppressive cells and their associated cell-cell signaling molecules induce functional exhaustion of tumor-recognizing T cells via programmed cell death-1 (PD-1/PD-L1) and other inhibitory pathways. Dual targeting of CD137/PD-1 abolishes the local suppression and leads to functional rescue and expansion of effector T cells that subsequently kill the cancer cells. Combination with radiotherapy, certain chemotherapeutic drugs or cancer vaccines may also expand the population of tumor-reactive T cells relieved from suppression and cause tumor regression.
We conclude that dual targeting of CD137/PD-1 co-signaling molecules can induce a synergistic antitumor effect which further improves by combining the mAbs with other therapies (cisplatin, tumor vaccine, radiation) as demonstrated in multiple tumor models. These findings, as well as results from many ongoing clinical trials, reinforce the concept of targeting co-stimulatory and co-inhibitory molecules by immunomodulatory mAbs for cancer therapy.6 A variety of co-inhibitory and co-stimulatory receptors have been identified, and their significance as therapeutic targets, individually or in combinations, merits careful prospective preclinical characterization and clinical testing of the best candidates. “Translation” of our findings in the ID8 model to clinical trials in ovarian cancer patients should be seriously considered. Although we did not observe any toxicity in CD137-treated tumor-bearing mice, caution must be exercised since CD137 agonistic antibodies have been previously observed to cause liver toxicity in clinical trials.9 Restricting the biodistribution of the mAbs to the peritoneal cavity, such as by entrapment in nanoparticles, may provide one targeted approach to elicit effective antitumor immunity while minimizing systemic toxicity.10
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Wei H, Zhao L, Hellstrom I, Erik Hellstrom K, Guo Y. Dual targeting of CD137 co-stimulatory and PD-1 co-inhibitory molecules for ovarian cancer immunotherapy. OncoImmunology 2014; 3:e28248; 10.4161/onci.28248
References
- 1.Mantia-Smaldone GM, Corr B, Chu CS. Immunotherapy in ovarian cancer. Hum Vaccin Immunother. 2012;8:1179–91. doi: 10.4161/hv.20738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dai M, Wei H, Yip YY, Feng Q, He K, Popov V, Hellstrom I, Hellstrom KE. Long-lasting complete regression of established mouse tumors by counteracting Th2 inflammation. J Immunother. 2013;36:248–57. doi: 10.1097/CJI.0b013e3182943549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei H, Zhao L, Li W, Fan K, Qian W, Hou S, Wang H, Dai M, Hellstrom I, Hellstrom KE, et al. Combinatorial PD-1 blockade and CD137 activation has therapeutic efficacy in murine cancer models and synergizes with cisplatin. PLoS One. 2013;8:e84927. doi: 10.1371/journal.pone.0084927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 5.Motz GT, Coukos G. Deciphering and reversing tumor immune suppression. Immunity. 2013;39:61–73. doi: 10.1016/j.immuni.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melero I, Grimaldi AM, Perez-Gracia JL, Ascierto PA. Clinical development of immunostimulatory monoclonal antibodies and opportunities for combination. Clin Cancer Res. 2013;19:997–1008. doi: 10.1158/1078-0432.CCR-12-2214. [DOI] [PubMed] [Google Scholar]
- 7.Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73:6900–12. doi: 10.1158/0008-5472.CAN-13-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verbrugge I, Hagekyriakou J, Sharp LL, Galli M, West A, McLaughlin NM, Duret H, Yagita H, Johnstone RW, Smyth MJ, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 2012;72:3163–74. doi: 10.1158/0008-5472.CAN-12-0210. [DOI] [PubMed] [Google Scholar]
- 9.Ascierto PA, Simeone E, Sznol M, Fu YX, Melero I. Clinical experiences with anti-CD137 and anti-PD1 therapeutic antibodies. Semin Oncol. 2010;37:508–16. doi: 10.1053/j.seminoncol.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Lei C, Liu P, Chen B, Mao Y, Engelmann H, Shin Y, Jaffar J, Hellstrom I, Liu J, Hellstrom KE. Local release of highly loaded antibodies from functionalized nanoporous support for cancer immunotherapy. J Am Chem Soc. 2010;132:6906–7. doi: 10.1021/ja102414t. [DOI] [PMC free article] [PubMed] [Google Scholar]


