Abstract
The long-term efficacy of anti-angiogenesis drugs targeting vascular endothelial growth factor (VEGF) and VEGF receptors in the treatment of renal cell carcinoma (RCC) has been lacking. We have shown that the ELR+CXCL cytokines and their (C-X-C) chemokine receptors, namely CXCR1 and CXCR2, stimulate cancer cell proliferation, tumor inflammation, and angiogenesis. Hence, this essential molecular nexus regulating cancer growth represents a key therapeutic target.
Keywords: angiogenesis, inflammation, renal cell carcinoma, ELR+CXCL, CXCR1, CXCR2, CXCL7
The process of tumor neo-vascularization (angiogenesis) is considered a major target in the treatment of cancer, as are the growth factors that drive the proliferation of constituent vasculature endothelial cells. The general aim of this therapeutic approach is to asphyxiate tumors by depriving them of oxygen and nutrients obtained from the blood. Anti-angiogenesis therapies typically comprise inhibitors of these angiogenic growth factors or their cognate receptors and such agents have been approved for the treatment of colon, breast, lung, kidney, brain, and ovarian cancers. The neo-vascularization in tumors is principally driven by the vascular endothelial growth factor (VEGF). Thus, the first approved anti-angiogenic therapy approved for clinical applications targeted VEGF. Termed bevacizumab (BVZ, trade name Avastin®), this targeted therapy is a recombinant humanized monoclonal IgG1 antibody that binds to and inhibits all the biologically active forms of VEGF.
BVZ is no longer the sole anti-angiogenic agent on the market, and ATP mimetics inhibiting the activity of tyrosine kinase receptors (RTKs) that have been implicated in neo-angiogenesis processes are currently preferred to BVZ since patients undergoing treatment do not require hospitalization. In the case of metastatic renal cell carcinoma (RCC) first line treatment is sunitinib, a tyrosine kinase inhibitor (TKI) that inhibits the activity of members of the RTK family, such as the VEGF receptors 1 (FLT1), 2 (KDR), 3 (FLT4), as well as platelet-derived growth factor (PDGF) receptor, colony stimulating factor receptor 1 (CSFR1), and c-KIT.1 Despite an increase in the time to disease progression, disappointingly, no major effects on overall survival were reported among sunitinib-treated RCC patients.2 Although physicians observed a decrease in tumor size during the first months of treatment, the large majority of patients harbored remaining malignant cells that disseminated to distant sites from the primary tumor if not resected. In addition, metastases that initially decreased in size upon treatment with TKI, ultimately re-grew. Hence, the selective pressure initially exerted by anti-angiogenic therapeutic agents ultimately gave rise to tumor cells with specific acquired properties permitting treatment evasion.
Conceptually, these therapeutic agents should only target endothelial cells. However, tumor cells aberrantly express the receptors targeted by the drugs and often become “addicted” to signaling pathways mediated by such receptors. Hence, the above treatments are not restricted in nature to anti-angiogenesis actions only because they may inhibit both endothelial and cancer cell proliferation.
One of the major hypotheses to explain how tumors can evade such treatments aiming to block the de novo generation of new vasculature within the tumor is the possible presence of alternative angiogenic factors. At the time that we begun our study, a major publication by Sparmann and Bar-Sagi3 highlighted the role of VEGF and interleukin 8 (IL-8) in tumor development and angiogenesis. We then focused our attention on IL-8 (also named CXCL8), which belongs to a family of pro-angiogenic/pro-inflammatory cytokines named ELR+CXCL (so named for their N-terminal glutamate, leucine, and arginine tripeptide motif preceeding the C-X-C chemokine motif) comprising CXCL1, 2, 3, 5, 6, 7, and 8. Our hypothesis pointed to the presence of VEGF and member(s) of the ELR+CXCL family in tumors. The challenge was to demonstrate if ELR+CXCL cytokines were better prognostic markers of progression free/overall survival than VEGF.
To this end, we obtained RCC tumor samples and their normal counterparts to test for the presence of VEGF and ELR+CXCL cytokines and determine if there was any correlation between the levels of these molecules and patient outcome. Despite observations that the amount of VEGF was higher in the tumor extracts4 than in normal tissues, the VEGF levels among cancer patients did not correlate well with patient outcome. Among all the ELR+CXCL tested, CXCL7 was a prognostic factor for survival independent of the Fuhrman grade as well as the presence of metastasis at diagnosis, two relevant clinical markers. CXCL1 was also statistically indicative of survival in an univariate analysis. CXCL8 tended to be informative of survival without reaching statistical significance.5
To support our clinical observations, we obtained xenografts of RCC model cells (786-O, RCC10, Caki-2) for further study in nude mice. In these experiments, we reported that the VEGF monoclonal antibody therapy BVZ unexpectedly accelerated tumor development by normalizing the blood vessel network and by selecting more aggressive tumor cells with an increased proliferative index.4 Surprisingly, no prior preclinical experiments in mice had been performed with RCC models, despite the fact that BVZ has been tested in 14 independent models of cancers.6 Hence, our results were among the first to counter the general dogma that anti-VEGF therapy should be efficient in all models of tumors.
Our animal model mimics the progression phase in patients a temporal window in which anti-VEGF therapy is probably no more efficient than the initial window of treatment just after surgical resection. In similar experiments, we showed that commercially available CXCL7 antibodies actually arrested tumor development5. On the other hand, tumors obtained with cells overexpressing CXCL7 grew more rapidly5. However, extracts from experimental tumors contained high levels of human CXCL7 whereas the CXCL7 levels produced by the cultured cancer cell lines from which our xenograft models were derived were negligible. This discrepancy could be explained by the fact that interleukin 1 β (IL-1β) produced by the inflammatory cells present in the tumors grown in vivo stimulated CXCL7 production. This phenomenon was recapitulated in vitro by stimulating 786-O cells with exogenously applied IL-1β.
CXCL7 and other ELR+CXCL cytokines could also generate an autocrine proliferation loop since RCC cells from both our cell lines as well as primary carcinoma cells (obtained from freshly resected tumors) expressed the receptors for ELR+CXCL, namely (C-X-C) chemokine receptor type 1 (CXCR1) and type 2 (CXCR2). These G-protein coupled receptors are physiologically expressed on endothelial cells and neutrophils/macrophages, an expression pattern that likely accounts for their role in both angiogenesis and inflammatory processes. A pharmacological inhibitor of these receptors, SB225002, has been found to decrease RCC and endothelial cell proliferation in vitro and also induced their apoptosis. This inhibitor has a strong anti-tumor potential in vivo by reducing the tumor cell proliferative index and tumor vascularization.7
ELR+CXCL chemokine family and their receptors, CXCR1 and CXCR2, represent a signaling axis that is potentially an independent pathway at the cross-roads of tumor and endothelial cell proliferation, as well as the maintenance of tumor inflammation (Fig. 1). Moreover, ELR+CXCL and CXCR1/CXCR2 may maintain tumor cell stemness8,9 and control tumor cell senescence.10 This new avenue deserves consideration among current treatments targeting the VEGF/VEGF receptor pathway, plus the receptors implicated in vessel integrity (e.g., PDGFR and c-KIT). Our results highlight a new therapeutic niche for targeting ELR+CXCL cytokines, particularly CXCL7, in the treatment of kidney cancer.
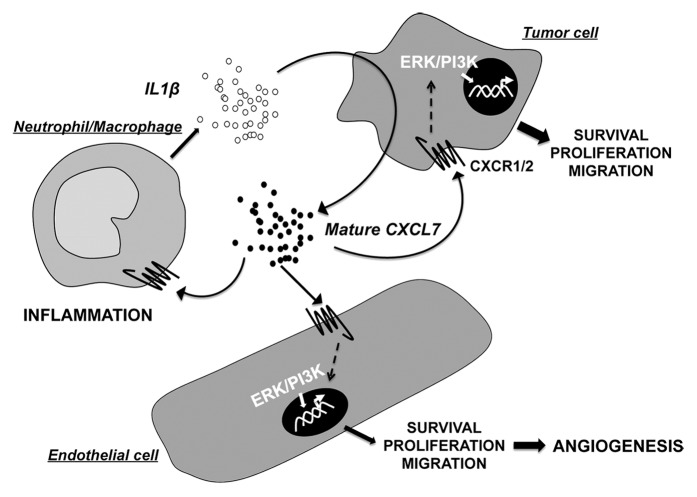
Figure 1. The role of the ELR+CXCL chemokine family member CXCL7 in autocrine and paracrine activation loops between cells in the tumor microenvironment. Cross-talk exists between malignant cells and normal cells of the microenvironment through the intermediary of the ELR+CXCL chemokine family, particularly CXCL7 and their (C-X-C) receptors, CXCR1 and CXCR2. CXCL7, potentially up-regulated by IL-1β secreted by inflammatory cells, activates signaling cascades (e.g., ERK/PI3K) stimulates cancer and endothelial cell proliferation and participates in the attraction of neutrophils and macrophages. These mechanisms are dependent upon the expression of CXCR1 and CXCR2, physiologically expressed by normal cells of the tumor microenvironment but aberrantly expressed by neoplastic cells. Hence, the CXCL7 and CXCR1/CXCR2 signaling axis is crucial for tumor growth and angiogenesis, as well as for the maintenance of the tumor inflammatory context.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr Brahimi-Horn for editorial assistance. Financial support: Contract VEGFIL from the National Institute of Cancer (INCA), the French Association for Cancer Research (ARC), the Foundation de France, the “Conseil Général des Alpes Maritimes,” Roche France and The “Association pour la Recherche sur les Tumeurs du Rein (ARTuR).”
Citation: Giuliano S, Guyot M, Grépin R, Pagès G. The ELR+CXCL chemokines and their receptors CXCR1/CXCR2: A signaling axis and new target for the treatment of renal cell carcinoma. OncoImmunology 2014; 3:e28399; 10.4161/onci.28399
References
- 1.Eisen T, Sternberg CN, Robert C, Mulders P, Pyle L, Zbinden S, Izzedine H, Escudier B. Targeted therapies for renal cell carcinoma: review of adverse event management strategies. J Natl Cancer Inst. 2012;104:93–113. doi: 10.1093/jnci/djr511. [DOI] [PubMed] [Google Scholar]
- 2.Escudier B. Sunitinib for the management of advanced renal cell carcinoma. Expert Rev Anticancer Ther. 2010;10:305–17. doi: 10.1586/era.10.26. [DOI] [PubMed] [Google Scholar]
- 3.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell. 2004;6:447–58. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 4.Grepin R, Guyot M, Jacquin M, Durivault J, Chamorey E, Sudaka A, Serdjebi C, Lacarelle B, Scoazec JY, Negrier S, et al. Acceleration of clear cell renal cell carcinoma growth in mice following bevacizumab/Avastin treatment: the role of CXCL cytokines. Oncogene. 2012;31:1683–94. doi: 10.1038/onc.2011.360. [DOI] [PubMed] [Google Scholar]
- 5.Grépin R, Guyot M, Giuliano S, Boncompagni M, Ambrosetti D, Chamorey E, Scoazec JY, Negrier S, Simonnet H, Pagès G. The CXCL7/CXCR1/2 axis is a key driver in the growth of clear cell renal cell carcinoma. Cancer Res. 2014;74:873–83. doi: 10.1158/0008-5472.CAN-13-1267. [DOI] [PubMed] [Google Scholar]
- 6.Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65:671–80. [PubMed] [Google Scholar]
- 7.Levashova ZB, Sharma N, Timofeeva OA, Dome JS, Perantoni AO. ELR+-CXC chemokines and their receptors in early metanephric development. J Am Soc Nephrol. 2007;;18:2359–70. doi: 10.1681/ASN.2006040380. [DOI] [PubMed] [Google Scholar]
- 8.Ginestier C, Liu S, Diebel ME, Korkaya H, Luo M, Brown M, Wicinski J, Cabaud O, Charafe-Jauffret E, Birnbaum D, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485–97. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer CG, et al. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614–24. doi: 10.1158/0008-5472.CAN-10-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acosta JC, Gil J. A role for CXCR2 in senescence, but what about in cancer? Cancer Res. 2009;69:2167–70. doi: 10.1158/0008-5472.CAN-08-3772. [DOI] [PubMed] [Google Scholar]