Abstract
Epstein–Barr virus (EBV) infection results in rapid loss of CD1d expression from the surface of infected B cells, thus enabling the virus to evade immune recognition by natural killer T (NKT) cells. Using pharmacologic means to boost CD1d expression, potent NKT cell effector functions can be elicited toward EBV-infected B cells, suggesting the promise of novel strategies to target EBV-associated diseases such as some B-cell malignancies.
Keywords: B cell lymphoma, Burkitt's lymphoma, CD1d, Epstein–Barr virus, NKT cells, X-linked lymphoproliferative disease, herpesvirus, immunodeficiency, infection, mononucleosis
Epstein–Barr virus (EBV) is a ubiquitous γ-herpesvirus that currently infects more than 90% of adults worldwide.1 Virus acquisition in childhood is typically sub-clinical, whereas primary infection during adolescence or early adulthood often manifests as infectious mononucleosis. EBV, isolated from cultures of Burkitt’s lymphoma cells, was the first identified human tumor virus and its association with an expanding list of human cancers led to the World Health Organization (WHO) classifying it as a “carcinogenic agent” in 1997. The virus has substantial transforming potential in vitro and has been widely used in laboratories to generate immortalized B cells known as lymphoblastoid cell lines (LCLs).
Primary acquisition of EBV is almost always through infection of pharyngeal epithelial cells, followed by viral entry into susceptible B cells. In immune competent hosts, EBV induces robust antigen-specific CD4+ and CD8+ T-cell responses that are important for controlling lytic viral replication. Subsequently, lifelong viral latency is established through the checks and balances of viral reactivation and host immune surveillance, resulting in an estimated frequency of 1 per 105 to 106 circulating B cells bearing EBV genomes.2 The observations that immunodeficient individuals, such as patients with AIDS or those undergoing transplantation or iatrogenic immunosuppression, are at greatly increased risk for the development of aggressive EBV-positive cancers, underscores the requirement for functional T cells to maintain tight control of viral replication and subsequent malignant disease. Despite decades of authoritative work describing innate and adaptive immune responses to the virus, there are still no effective EBV vaccines to prevent latent infection and the immune factors correlating with protection remain obscure.
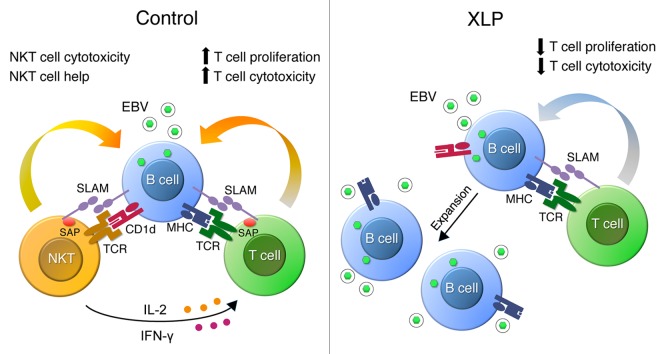
Studies of a rare primary immunodeficiency, X-linked lymphoproliferative disease (XLP), have yielded key insights into mechanisms of immune protection from EBV.3 Mutations in the SH2D1A gene encoding SLAM-associated protein (SAP) underlie XLP, a condition defined by exquisite and selective susceptibility to EBV such that these patients are apparently not at higher risk for infection with other pathogens.3 Boys with SH2D1A mutations are essentially asymptomatic until they are exposed to EBV, after which they develop fulminant, often fatal, infectious mononucleosis, massive expansions of EBV-infected B cells, and frequently, malignant B cell lymphomas. In 2005, along with 2 other groups, we reported that humans and mice with non-functional SH2D1A and Sh2d1a alleles completely lack natural killer T (NKT) cells.4 Based on these findings, we hypothesized that NKT cells may have a pivotal role in developing protective immunity to EBV (Fig. 1).
Figure 1. Proposed mechanism by which natural killer T cells act to restrict early Epstein–Barr virus replication. In healthy individuals, natural killer T (NKT) cells rapidly recognize and mediate direct cytotoxicity of newly-infected CD1d+ B cells, and provide “help” to stimulate NK cell (not shown) and antigen-specific T cell responses through the production of IFNγ and IL-2, thereby limiting EBV infection. By contrast, XLP patients, who lack NKT cells, have sub-optimal and slower adaptive responses to EBV infection. Delayed recognition of EBV-infected B cells may lead to an increased number of EBV-infected B cells and increased viral load. SLAM-SAP interactions may also be critical for mediating costimulation signals necessary for the optimal activation of T cells and NK cells. EBV, Epstein–Barr virus; IFNγ, interferon-γ; SAP, SLAM-associated protein; SLAM, signaling lymphocytic activation molecule; TCR, T-cell receptor, NK, natural killer; NKT, natural killer T; XLP, X-linked lymphoproliferative disease.
NKT cells are a distinct and conserved lineage of T cells expressing a semi-invariant TCR that recognizes glycolipid antigens in association with the conserved MHC class I-related molecule, CD1d.5 The most powerful NKT cell antigen characterized to date is a non-mammalian derived lipid, α-galactosylceramide (αGalCer), and the use of this reagent has been invaluable for stimulating and assessing the cellular functions of these unique immune cells. NKT cells connect innate and adaptive immunity by receiving signals from infected cells or antigen-presenting dendritic cells (DC) that have perceived infection and subsequently delivering secondary signals that efficiently prime and expand virus-specific T and B cells. The activation of NKT cells is rapid and critical for promoting optimal natural killer (NK) cell, dendritic cell (DC), and T cell activation. NKT cells can also directly lyse cancer cells and have been implicated in anti-viral responses in animal models of infection.6
To determine whether NKT cells might regulate immune responses against EBV, we first showed that depletion of NKT cells from human peripheral blood mononuclear cells (PBMCs) prior to infection with EBV increased the number of virally-infected B cells and viral genomes detected per culture relative to total PBMCs.7 In transwell experiments, the NKT effect was shown to depend upon cell-cell contact. However, enigmatically, subsequent experiments revealed that NKT cells failed to directly recognize pre-existent EBV-transformed B cells, even when αGalCer was added to cultures. This lack of NKT recognition was due to near complete downregulation of CD1d on the lymphoblastoid cell surface. Notably, other herpesviruses, including Kaposi’s sarcoma herpesvirus (KSHV) and herpes simplex virus 1 (HSV-1), have been postulated to escape NKT cell surveillance by silencing surface CD1d levels.8,9 Together, the findings suggest that NKT cells may be crucial for recognition of nascent EBV-infected B cells during the brief temporal window prior to the loss of CD1d expression.
To determine whether induced CD1d expression could restore NKT cell recognition of EBV-infected B cells, LCLs were treated with AM580, a synthetic retinoic acid receptor-α (RARα) agonist previously shown to induce CD1d transcription and surface expression on human DC.10 We found that AM580 induces CD1d expression on the lymphoblastoid cell surface and, that AM580-treated LCLs are capable of initiating robust responses from NKT cells, triggering both interferon-γ (IFNγ) secretion and cytotoxicity. Importantly, the findings that AM580-treated LCLs had the capacity to activate NKT cells even in the absence of αGalCer, and that the effect could be specifically blocked with anti-CD1d antibody, suggest that lymphoblasts can express an endogenous NKT cell lipid antigen.
Thus, one strategy to improve immune control of EBV-infected cells may be to maintain, restore or augment CD1d expression on the surface of target cells. This tactic may potentially be combined with the simultaneous boosting of NKT cell responses through administration of αGalCer or other NKT cell agonists. Such approaches seeking to manipulate the action of NKT cells to more efficiently recognize viral infection may improve current immunotherapy of EBV-associated malignancies and foster the development of EBV vaccines.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- DC
dendritic cell
- EBV
Epstein–Barr virus
- αGalCer
α-galactosylceramide
- LCL
lymphoblastoid cell line
- NK cell
natural killer cell
- NKT cell
natural killer T cell
- RARα
retinoic acid receptor-α
- SAP
SLAM-associated protein
- XLP
X-linked lymphoproliferative disease
Citation: Priatel JJ, Chung BK, Tsai K, Tan R. Natural killer T cell strategies to combat Epstein–Barr virus infection. OncoImmunology 2014; 3:e28329; 10.4161/onci.28329
References
- 1.Saha A, Robertson ES. Epstein-Barr virus-associated B-cell lymphomas: pathogenesis and clinical outcomes. Clin Cancer Res. 2011;17:3056–63. doi: 10.1158/1078-0432.CCR-10-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hsu JL, Glaser SL. Epstein-barr virus-associated malignancies: epidemiologic patterns and etiologic implications. Crit Rev Oncol Hematol. 2000;34:27–53. doi: 10.1016/S1040-8428(00)00046-9. [DOI] [PubMed] [Google Scholar]
- 3.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 4.Borowski C, Bendelac A. Signaling for NKT cell development: the SAP-FynT connection. J Exp Med. 2005;201:833–6. doi: 10.1084/jem.20050339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 6.Juno JA, Keynan Y, Fowke KR. Invariant NKT cells: regulation and function during viral infection. PLoS Pathog. 2012;8:e1002838. doi: 10.1371/journal.ppat.1002838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung BK, Tsai K, Allan LL, Zheng DJ, Nie JC, Biggs CM, Hasan MR, Kozak FK, van den Elzen P, Priatel JJ, et al. Innate immune control of EBV-infected B cells by invariant natural killer T cells. Blood. 2013;122:2600–8. doi: 10.1182/blood-2013-01-480665. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez DJ, Gumperz JE, Ganem D. Regulation of CD1d expression and function by a herpesvirus infection. J Clin Invest. 2005;115:1369–78. doi: 10.1172/JCI200524041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan W, Dasgupta A, Cresswell P. Herpes simplex virus evades natural killer T cell recognition by suppressing CD1d recycling. Nat Immunol. 2006;7:835–42. doi: 10.1038/ni1364. [DOI] [PubMed] [Google Scholar]
- 10.Szatmari I, Pap A, Rühl R, Ma JX, Illarionov PA, Besra GS, Rajnavolgyi E, Dezso B, Nagy L. PPARgamma controls CD1d expression by turning on retinoic acid synthesis in developing human dendritic cells. J Exp Med. 2006;203:2351–62. doi: 10.1084/jem.20060141. [DOI] [PMC free article] [PubMed] [Google Scholar]