Abstract
Enantioenriched methylenecyclopentanes are synthesized by stereospecific, nickel-catalyzed Heck cyclizations of secondary benzylic ethers. The reaction proceeds in high yield and enantiospecificity for benzylic ethers of both π-extended and simple arenes. Ethers with pendant 1,2-disubstituted olefins form trisubstituted olefins with control of both absolute configuration and alkene geometry. Diastereoselective synthesis of a polycyclic furan is demonstrated.
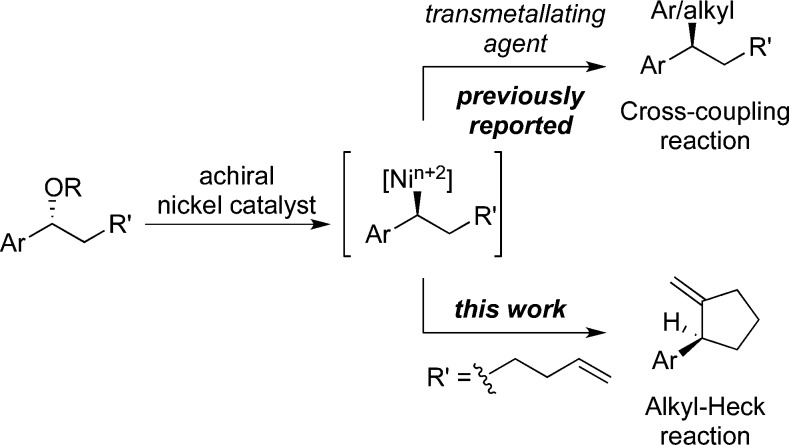
The Mizoroki–Heck reaction is part of the foundation of modern organometallic chemistry and is a key disconnection in the synthetic chemist’s repertoire.1 Creative advances continue to expand our synthetic capabilities and refine our understanding of transition-metal-catalyzed reactions.2 Traditional Heck reactions employ an aryl or vinyl halide or pseudohalide. Development of “alkyl-Heck reactions,” where the electrophilic partner is an alkyl halide or pseudohalide, is undergoing revitalization,3−7 in part due to synergy with recent advances in alkyl cross-coupling reactions.8 Exciting results employing primary alkyl halides have been reported, where catalyst control suppresses undesired side reactions and provides regioselectivity and asymmetric induction in the migratory insertion step.6,3d,3e Heck-like reactions of secondary alkyl iodides that proceed through radical intermediates have also been reported and provide substituted tetrahydrofurans and cyclopentanones with high diastereoselectivity.7 Important challenges remain. For example, in reactions of secondary alkyl electrophiles, control of the absolute configuration at the site of oxidative addition has not been reported.
We hypothesized that secondary ethers functionalized with a pendant alkene should undergo nickel-catalyzed Heck cyclization and that the reactions would be highly stereospecific (Scheme 1). This work builds on our development of related stereospecific nickel-catalyzed cross-coupling reactions of benzylic ethers.9−11 We propose that oxidative addition occurs with inversion, providing a single enantiomer of the key secondary alkylnickel intermediate that can continue through the cross-coupling or Heck catalytic cycle.
Scheme 1. Stereospecific Nickel-Catalyzed Reactions of Benzylic Ethers and Esters.
We designed substrates to test for stereospecific Heck cyclization, informed by our prior development of stereospecific cross-coupling reactions of esters and ethers. Employing benzylic pivalate 1 with Cs2CO3 as the base, however, failed to furnish any of the desired methylenecyclopentane 2 (Table 1, entry 1).12 Next, dimethylzinc was examined as a terminal reductant for the reaction, and a small amount of the desired methylenecyclopentane 2 was observed (entry 2). Encouraged by this result, we replaced dimethylzinc with methylmagnesium iodide, a stronger terminal reducing agent. To avoid unwanted side reactions of the leaving group with a Grignard reagent, we replaced pivalate 1 with methyl ether 5. Under these conditions, consumption of starting material increased significantly (entries 3–6).13 When bidentate phosphine ligands were added to the reaction, the undesired product 4, resulting from simple Kumada coupling, was the major product (entries 5 and 6). Remarkably, Kumada product 4 was not observed when catalysts ligated by monodentate phosphines were used in the reaction (entries 3 and 4). The desired Heck cyclization to afford methylenecyclopentane 2 proceeded in good yield with PCy3 as the ligand (entry 3). Our optimized reaction conditions took advantage of the air-stable NiCl2(PCy3)2 catalyst and afforded the desired product in high yield (entry 7).
Table 1. Optimization of Reaction Conditions.

| entry | R | ligand | base | yield 2 (%)a | yield 3 (%)a | yield 4 (%)a |
|---|---|---|---|---|---|---|
| 1 | Piv | PCy3 | Cs2CO3 | <2 | 37 | <2 |
| 2 | Piv | PCy3 | ZnMe2 | 7 | 39 | <2 |
| 3 | Me | PCy3 | MeMgI | 64 | 17 | <2 |
| 4 | Me | PPh3 | MeMgI | 18 | 76 | <2 |
| 5 | Me | DPEphos | MeMgI | 4 | 23 | 47 |
| 6 | Me | dppf | MeMgI | <2 | 27 | 66 |
| 7 | Me | NiCl2(PCy3)2b | MeMgI | 82 | 11 | <2 |
Yield determined by 1H NMR based on comparison with PhSiMe3 as internal standard.
NiCl2(PCy3)2 was used in place of Ni(cod)2 and added ligand.
Having established conditions for the cyclization of secondary ether 5, we synthesized enantioenriched (R)-5 with the goal of determining the stereospecificity of the reaction. Ether (R)-5 was prepared in high enantiomeric excess by CBS reduction of the corresponding ketone.14 Cyclization of (R)-5 resulted in good yield and excellent enantiospecificity to afford the methylenecyclopentane 2 with inversion at the benzylic stereocenter (Table 2, entry 1).15 The reaction is scalable with no observable deterioration in yield or enantiospecificity when performed on a 1.0 mmol scale of ether 5 (Table 2, entry 2).
Table 2. Scope of Intramolecular Heck Cyclization.


Determined by chiral SFC.
Isolated yield after column chromatography.
Enantiospecificity (es) = (eeproduct/eesubstrate) × 100.
Reaction run on 1.0 mmol scale.
Enantiomeric excesses determined by chiral GC.
Enantiospecificity was determined based on the ee of the corresponding alcohol, rather than the ether.
Reaction performed at 60 °C.
Reaction performed with added MgI2 (1 equiv).
Yield determined by 1H NMR based on comparison with PhSiMe3 as internal standard.
We next examined the cyclizations of a range of enantioenriched benzylic ethers. Benzylic methyl ethers of π-extended arenes proceed in excellent yield to afford highly enantioenriched methylenecyclopentanes (entries 1 and 3). Simple heteroarenes such as thiophene 8 and furan 10 also perform well under the reaction conditions (entries 4 and 5). Taking advantage of the Thorpe–Ingold effect by substitution of the alkyl chain with geminal dimethyl substituents improves the yield of the cyclization in general (entry 6).16 Simple benzylic substrates such as 16 presented a challenge, where high enantiospecificity but modest conversion was typically observed (entry 8). In this case, geminal disubstitution failed to improve yield (entry 9), but modification of the ether provided a solution (entries 10–12). Our laboratory has previously developed the methoxyethyl ether as a traceless directing group that accelerates sluggish cross-coupling reactions.9b This strategy proved fruitful in the context of Heck reactions as well; methoxyethyl ethers 20, 21, and 23 afforded the desired methylenecyclopentanes at 60 °C (entries 6–8). Yields of 17, 19, 22, and 24 could typically be further improved by approximately 10% with the addition of MgI2 (1 equiv).17,18
To further test the limits of the transformation, an alkyne insertion/Kumada domino reaction was examined.19 Benzylic ether 25 bearing a tethered TMS-protected alkyne was subjected to the reaction conditions to afford tetrasubstituted olefin 26 in good yield and excellent enantiospecificity (Scheme 2). A 1.9:1 mixture of stereoisomers was obtained which could be separated by flash column chromatography with silver nitrate impregnated silica gel.20 We hypothesize that the migratory insertion step proceeds with syn selectivity.21 Therefore, the stereoisomeric mixture of products present in the cyclization of alkyne 25 is predicted to be the result of isomerization of the vinylnickel intermediate prior to reductive elimination.22
Scheme 2. Alkyne Insertion–Kumada Reaction.
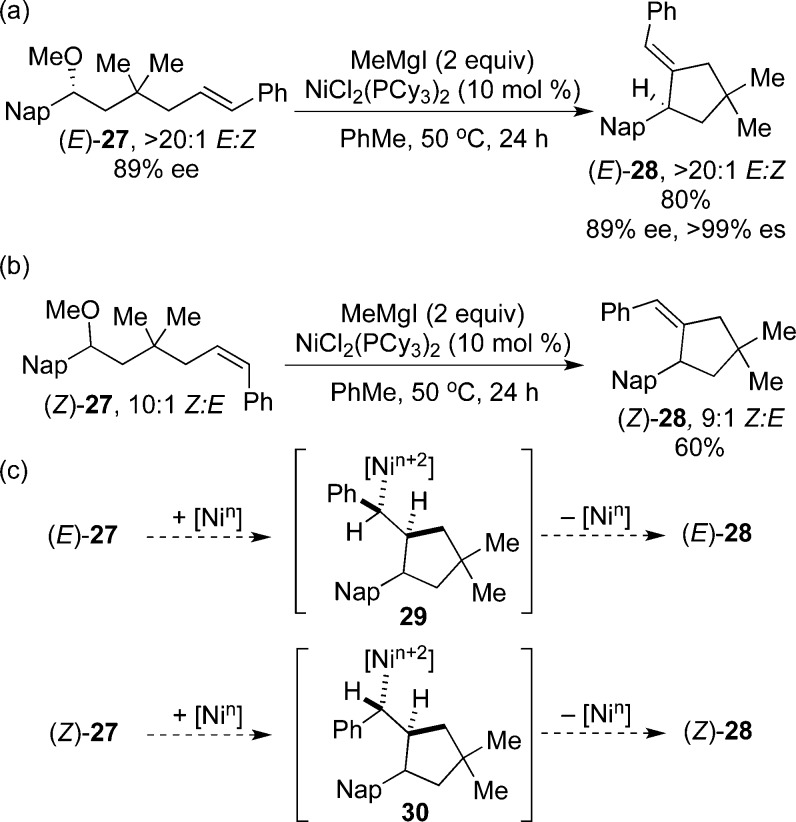
Trisubstituted olefins are valuable synthetic targets found in natural products, intermediates in biosynthetic pathways of steroids, and important building blocks for further functionalization by asymmetric catalysis.23,24 Heck cyclization of 1,2-disubstituted olefins affords a strategy for the synthesis of trisubstituted olefins as single stereoisomers, based on the stereochemical requirements of migratory insertion and β-hydride elimination.21,25 Indeed, when (E)-27 is subjected to the reaction conditions, the trisubstituted olefin (E)-28 is formed in high yield and with high enantiospecificity at the benzylic stereocenter. The product is formed as a single olefin isomer in >20:1 dr (Scheme 3a). Next, (Z)-27 was cyclized in good yield to form (Z)-28 (Scheme 3b). Therefore, either isomer of the product can be accessed simply by selecting the appropriate isomer of starting material.
Scheme 3. Stereoselective Synthesis of Trisubstituted Olefins.
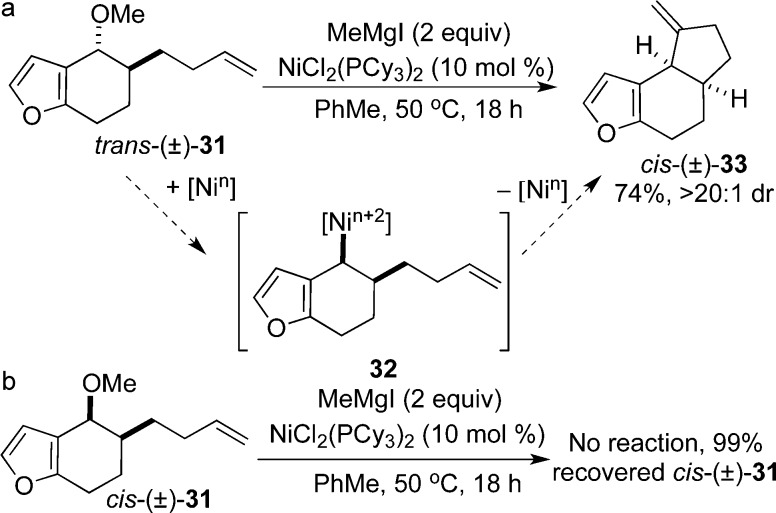
The classic Heck reaction has had a transformative impact on natural product synthesis because it can provide rapid assembly of complex polycyclic architectures.1d To challenge our alkyl-Heck reaction, we synthesized trans-31 from the corresponding dihydrobenzofuranone by α-alkylation and reduction.26 Cyclization of trans-31 provided cis-33 as the major product in >20:1 diastereoselectivity (Scheme 4a). The ring fusion was assigned as cis based on NOE correlations and a comparison of the J-coupling constants to calculated values for 33 and to literature values for related tricyclic terpenoids.26 Tricyclic cis-33 maps onto the core of furan terpenoid natural products such as pseudoferic acid C, and lactones such as nepalensolides A–C and brothenolide.27−29
Scheme 4. Diastereoselective Tricyclic Ring Formation with Inversion at the Benzylic Position.
This substrate class also provides mechanistic insight into the stereochemical and mechanistic aspects of the Heck cyclization. Cyclization of trans-31 to afford cis-33 is consistent with inversion at the benzylic stereogenic center. We attribute this outcome to inversion during the oxidative addition event to generate a cis substituted benzylnickel intermediate (32). Subsequent steps in the catalytic cycle, migratory insertion and β-hydride elimination, should not affect the benzylic stereogenic center. When the diastereomer, cis-31, was subjected to cyclization conditions, starting material was recovered in quantitative yield (Scheme 4b). It is worthwhile to note that cis-31 does not appear to undergo side reactions such as elimination or Kumada coupling.30 This observation is consistent with coordination of the olefin to the catalyst prior to oxidative addition.
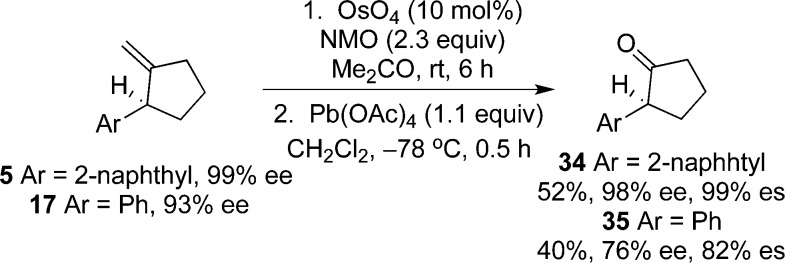
Methylenecyclopentanes are valuable synthetic intermediates; the exocyclic olefin provides a synthetic handle for further elaboration to more complex products. For example, methylenecyclopentanes 5 and 17 are readily converted to the corresponding enantioenriched α-aryl cyclopentanones by a two-step procedure. Dihydroxylation of the olefin with OsO4 followed by mild oxidative cleavage of the resultant diol with Pb(OAc)4 affords α-aryl cyclopentanones 34 and 35 in good to excellent levels of enantiopurity (Scheme 5).
Scheme 5. Synthesis of Enantioenriched α-Aryl Cyclopentanones.
In summary, selective formation of methylenecyclopentanes containing tertiary stereocenters has been achieved by stereospecific, nickel-catalyzed intramolecular Heck cyclization of secondary ethers. The reaction proceeds in high yield and enantiospecificity and has been applied to the formation of synthetically challenging trisubstituted olefins. An alkyne insertion–Kumada domino reaction to prepare tetrasubstituted olefins is also demonstrated. Efforts to expand the scope of the transformation and elucidate mechanistic details are underway.
Acknowledgments
This work was supported by NIH NIGMS (R01GM100212), the University of California (Chancellor’s Fellowship to M.R.H.), the Ford Family Foundation (predoctoral fellowship to M.R.H.), and the National Science Foundation (Graduate Research Fellowship Grant Number DGE-1321846 to M.R.H.). Dr. Joseph Ziller is acknowledged for X-ray crystallographic analysis. Dr. Ivelina Yonova is thanked for helpful discussions.
Supporting Information Available
Experimental procedures and characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- a Seechurn C. C. C. J.; Kitching M. O.; Colacot T. J.; Snieckus V. Angew. Chem., Int. Ed. 2012, 51, 5062–5085. [DOI] [PubMed] [Google Scholar]; b Heck R. F. Org. React. 1982, 27, 345–390. [Google Scholar]; c The Mizoroki–Heck Reaction; Oestreich M., Ed.; Wiley: Chichester, 2009. [Google Scholar]; d Review of asymmetric Heck reactions in synthesis: Dounay A. B.; Overman L. E. Chem. Rev. 2003, 103, 2945–2964. [DOI] [PubMed] [Google Scholar]; d Review of industrial applications of the Heck reaction: Torborg C.; Beller M. Adv. Synth. Catal. 2009, 351, 3027–3043. [Google Scholar]
- For a recent discussion, see:Oestreich M. Angew. Chem., Int. Ed. 2014, 53, 2282–2285. [DOI] [PubMed] [Google Scholar]
- For examples of primary benzylic electrophiles:; a Heck R. F.; Nolley J. P. J. Org. Chem. 1972, 37, 2320–2322. [Google Scholar]; b Wu G.-Z.; Lamaty F.; Negishi E.-i. J. Org. Chem. 1989, 54, 2507–2508. [Google Scholar]; c Matsubara R.; Gutierrez A. C.; Jamison T. J. J. Am. Chem. Soc. 2011, 133, 19020–19023. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Standley E. A.; Jamison T. F. J. Am. Chem. Soc. 2013, 135, 1585–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Yang Z.; Zhou J. J. Am. Chem. Soc. 2012, 134, 11833–11835. [DOI] [PubMed] [Google Scholar]
- For a recent example of Heck-type reactions of α-halocarbonyls:Liu C.; Tang S.; Liu D.; Yuan J.; Zheng L.; Meng L.; Lei A. Angew. Chem., Int. Ed. 2012, 51, 3638–3641. [DOI] [PubMed] [Google Scholar]
- Cobalt-catalyzed alkyl-Heck-type reactions:; a Ikeda Y.; Nakamura T.; Yorimitsu H.; Oshima K. J. Am. Chem. Soc. 2002, 124, 6514–6515. [DOI] [PubMed] [Google Scholar]; b Affo W.; Ohmiya H.; Fujioka T.; Ikeda Y.; Nakamura T.; Yorimitsu H.; Oshima K.; Imamura Y.; Mizuta T.; Miyoshi K. J. Am. Chem. Soc. 2006, 128, 8068–8077. [DOI] [PubMed] [Google Scholar]
- Firmansjah L.; Fu G. C. J. Am. Chem. Soc. 2007, 129, 11340–11341. [DOI] [PubMed] [Google Scholar]
- a Bloome K. S.; Alexanian E. J. J. Am. Chem. Soc. 2010, 132, 12823–12825. [DOI] [PubMed] [Google Scholar]; b Bloome K. S.; McMahen R. L.; Alexanian E. J. J. Am. Chem. Soc. 2011, 133, 20146–20148. [DOI] [PubMed] [Google Scholar]
- For reviews, see:; a Swift E. C.; Jarvo E. R. Tetrahedron 2013, 69, 5799–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Jana R.; Pathak T. P.; Sigman M. S. Chem. Rev. 2011, 111, 1417–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Taylor B. L. H.; Swift E. C.; Waetzig J. D.; Jarvo E. R. J. Am. Chem. Soc. 2011, 133, 389–391. [DOI] [PubMed] [Google Scholar]; b Greene M. A.; Yonova I. M.; Williams F. J.; Jarvo E. R. Org. Lett. 2012, 14, 4293–4296. [DOI] [PubMed] [Google Scholar]; c Taylor B. L. H.; Harris M. R.; Jarvo E. R. Angew. Chem., Int. Ed. 2012, 51, 7790–7793. [DOI] [PubMed] [Google Scholar]; d Harris M. R.; Hanna L. E.; Greene M. A.; Moore C. E.; Jarvo E. R. J. Am. Chem. Soc. 2013, 135, 3303–3306. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Wisniewska H. M.; Swift E. C.; Jarvo E. R. J. Am. Chem. Soc. 2013, 135, 9083–9090. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Yonova I. M.; Johnson A. G.; Osborne C. A.; Moore C. E.; Morrissette N. S.; Jarvo E. R. Angew. Chem., Int. Ed. 2014, 53, 2422–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For related transformations, see:Zhou Q.; Srinivas H. D.; Dasgupta S.; Watson M. P. J. Am. Chem. Soc. 2013, 135, 3307–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For a lead reference of the complementary stereoconvergent strategy, see:Do H.-Q.; Chandrashekar E. R. R.; Fu G. C. J. Am. Chem. Soc. 2013, 135, 16288–16291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Additional bases, ligands, and TESOTf as an additive were examined, but the only product observed was 3. For acceleration of nickel-catalyzed Heck reactions using TESOTf, see:Matsubara R.; Jamison T. F. J. Am. Chem. Soc. 2010, 132, 6880–6881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substitution of the Grignard reagent with other bases in Heck cyclization of ether 5 resulted in quantitative recovery of 5.
- Corey E. J.; Bakshi R. K.; Shibata S. J. Am. Chem. Soc. 1987, 109, 5551–5553. [Google Scholar]
- The absolute configuration for starting material and product was determined for Table 2, entries 1 and 8, based on a comparison of optical rotations to literature values or X-ray crystallography. For full details, see the Supporting Information.
- Beesley R. M.; Ingold C. K.; Thorpe J. F. J. Chem. Soc. Trans. 1915, 107, 1080–1106. [Google Scholar]
- Greene M. A.Diastereoselective Synthesis of Seven Membered Ring trans-Alkenes and Development of Stereospecific Nickel-Catalyzed Cross-Coupling Reactions. Ph.D. Thesis, The University of California, Irvine, May 2013. [Google Scholar]
- Alternative tether lengths, for example, to generate the corresponding 3-, 4-, or 6-membered methylenecycloalkanes, do not provide desired products. For a summary of substrate classes that resist Heck cyclization, see the Supporting Information.
- For a recent example of an intramolecular palladium-catalyzed alkyne insertion/Suzuki reaction of alkyl iodides and aryl boronic esters, see:Monks B. A.; Cook S. P. J. Am. Chem. Soc. 2012, 134, 15297–15300. [DOI] [PubMed] [Google Scholar]
- Williams C. M.; Mander L. N. Tetrahedron 2001, 57, 425–447. [Google Scholar]
- Heck R. F. J. Am. Chem. Soc. 1969, 91, 6707–6714. [Google Scholar]
- For a discussion of isomerization pathways of vinyl metal complexes, see:; a Frohnapfel D. S.; Templeton J. L. Coord. Chem. Rev. 2000, 206–207, 199–235. [Google Scholar]; For selected examples, see:; b Tanke R. S.; Crabtree R. H. J. Am. Chem. Soc. 1990, 112, 7984–7989. [Google Scholar]; c Chung L. W.; Wu Y.-D.; Trost B. M.; Ball Z. T. J. Am. Chem. Soc. 2003, 125, 11578–11582. [DOI] [PubMed] [Google Scholar]
- Faulkner J. D. Synthesis 1971, 4, 175–189. [Google Scholar]
- Ojima I.Catalytic Asymmetric Synthesis, 2nd ed.; Wiley and Sons, Inc.: New York, 2005. [Google Scholar]
- Iimura S.; Overman L. E.; Paulini R.; Zakarian A. J. Am. Chem. Soc. 2006, 128, 13095–13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For details, see the Supporting Information.
- Wu X.-D.; He J.; Dong L.-B.; Pan Z.-H.; Xu G.; Gong X.; Song L.-D.; Leng Y.; Li Y.; Peng L.-Y.; Zhao Q.-S. Tetrahedron Lett. 2012, 53, 800–803. [Google Scholar]
- Asakawa Y.; Lin X.; Kondo K.; Fukuyama Y. Phytochemistry 1991, 30, 4019–4024. [Google Scholar]
- Takeda R.; Ohta Y.; Hirose Y. Bull. Chem. Soc. Jpn. 1983, 56, 1120–1124. [Google Scholar]
- The NiCl2(PCy3)2 catalyst is capable of catalyzing reactions of naphthylic
methyl ethers that lack a terminal olefin (e.g., 36).
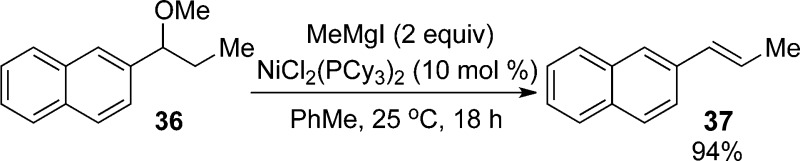
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.