Abstract
Touch spray, a spray-based ambient in-situ ionization method, uses a small probe, e.g. a teasing needle to pick up sample and the application of voltage and solvent to cause field-induced droplet emission. Compounds extracted from the microsample are incorporated into the sprayed micro droplets. Performance tests include disease state of tissue, microorganism identification, and therapeutic drug quantitation. Chemical derivatization is performed simultaneously with ionization.
Introduction
Ambient ionization produces ions outside the mass spectrometer from samples in their native state.1, 2 Desorption electrospray ionization (DESI)3 was reported in 2004, and since then more than forty ambient ionization methods have been described.4–6 In some experiments such as DESI a single agent (charged droplet impact) is used to desorb the analyte and to effect its ionization. In others, two separate agents are used to perform the key operations of desorption and ionization: For example, desorption can be achieved using a laser, plasma or droplet rt and ionization can take the form of electrospray ionization, atmospheric pressure chemical ionization or laser ionization. One of the main characteristics of ambient ionization is the speed of analysis - it requires a few seconds for the entire process of sampling, ionization and recording of mass spectra. This feature is the result of eliminating or greatly relaxing sample pre-treatment, including avoiding separation techniques prior to mass spectrometry. Ambient ionization mass spectrometry displays wide applicability combined with high sensitivity and the high molecular specificity characteristic of mass spectrometry. Ambient methods based upon spray ionization include DESI,3 nanospray desorption electrospray ionization (nanoDESI),7 liquid microjunction surface-sampling probe (LMJ-SSP),8 probe electrospray ionization (PESI),9 and others. In each case solvent and high voltage are used to generate the strong electric field needed to produce charged secondary droplets which leave the substrate carrying dissolved analyte into the mass spectrometer. The emitted charged droplets undergo coulombic fission when sufficient surface charge is accumulated as a result of solvent evaporation, eventually yielding analyte ions by mechanisms that parallel those in electrospray ionization.
A family of methods exist which rely on spray based ionization from substrates:10 these methods include paper spray (PS),11 PESI,12 and leaf spray (LS).13 Substrate spray methods generate ions from sharp tips, naturally present or created, and require a minute amount of sample. Direct electrospray probe (DEP) ionization was an early example, analyzing prepared solutions via manual addition to a metallic/glass probe.14–16 PESI followed, allowing the analysis of liquid samples. PESI utilizes smooth metallic objects with sharp tips (e.g. acupuncture needles) to transfer aliquots of sample solution for MS analysis and has been applied by Hiroaka et al. to tissue analysis in cancer diagnostics,17 protein analysis, and agrochemical analysis.18 Metallic probes such as those used in DEP and PESI provide a non-porous surface primarily serving as a means of analyte transport and application of high voltage. Organic materials (paper, wood, and plant parts) have been used in substrate-based electrospray sources. Substrates based on organic materials are not only capable of generating ions but also provide a varying ability for selective absorption of components of a mixture. Mass spectra were recorded from wooden toothpicks by Yao and colleagues19 who detected pharmaceuticals in applied homogenous solution, and similarly by Chen et al. using bamboo nibs, similar to those used in calligraphy.20 Analysis of powders, sampled from locations otherwise difficult to access was achieved using moistened wooden toothpicks.21 PS is similarly capable of in situ sampling with the detection of analytes by wiping as in the detection of agrochemicals on the surface of an orange.22
Touch spray (TS) ionization falls into this family of substrate spray methods, using a probe (e.g. needle) to sample material and solvent and an electrical potential to desorb analyte and transfer it in ionic form into a mass spectrometer. TS allows in situ sampling of materials from which ions are generated once solvent (as needed) and a potential are applied. It is closely related to the metallic probe substrate techniques such as PESI and organic substrate techniques such as PS, and characterised by the ability to sample and absorb materials such as solid powers, trace amounts of solids on surfaces, solutions, and heterogeneous matrices (e.g. tissue) onto a probe. Absorbed material can be transported from the point of origin to the MS in two stages, first by physical transfer of a small sample and then by transfer of dissolved analytes in the sprayed droplets. TS, as with other spray-based ambient ionization methods, allows nearly simultaneous chemical derivatization and ionization, generating derivatives that can increase signal and enhance qualitative identifications. This publication describes the methodology of TS and explores applications, with emphasis on in situ analysis via illustrative examples.
Results and discussion
Touch Spray Ionization
Touch spray ionization is aimed specifically at in situ analysis of complex samples. Analytes are transferred from a particular location in the sample using a roughed probe. Subsequently, spray ionization (see Supplemental Fig 1) transfers the ionized analyte to the mass spectrometer for analysis. The TS method incorporates a manual method of collecting a MS sample: sampling is remote to the mass spectrometer and after manual transfer onto a probe, MS is performed directly from the sampling device. It is not necessary to use a specific probe material or that it have a particular physical form: the transfer of a minute amount of material for MS analysis can be achieved by touching, scratching, dipping, swiping, or otherwise attaching sample material. The probe is then aligned with the mass spectrometer, high voltage is applied, solvent is added, and mass spectra are recorded. In these experiments the touch spray probe was aligned with the atmospheric inlet (0.5–20 mm away) and an appropriate voltage (3.0–5.0 kV) was applied to generate a stable electrospray signal without a corona discharge. Solvent was either applied manually via pipette (0.1–2 μL, which provided mass spectral signals lasting only a few seconds) or continuously via a syringe pump (yielding continuous signal until analyte exhaustion, typically > 1 min).
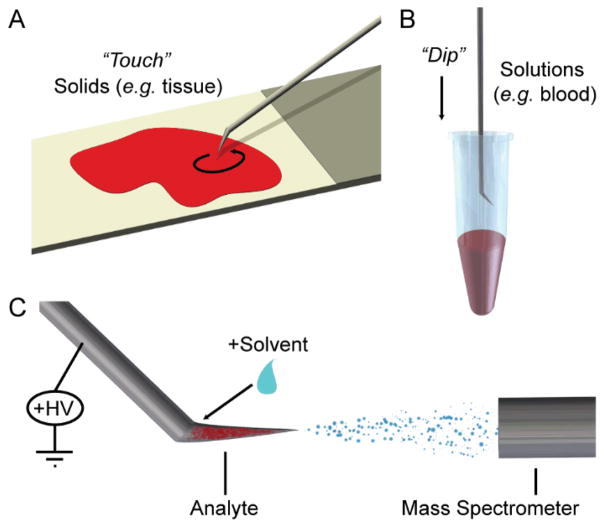
One suitable probe is a teasing needle (Fig. 1); these are metallic, possess a sharp tip, and are roughened. The metallic and roughened features appear to be beneficial when sampling and transferring material, such as biological tissue. The crevasses in the roughened surface hold material during sample transfer and analysis, facilitating analyte extraction. In addition, in the case of teasing needles, the angled feature was found to increase reliability, as it accommodated solvent application and was observed to promote solvent flow. Complete wetting of the probe’s surface improved extraction of analytes and emission of solvent microdroplets, a feature which was not observed in all probes evaluated.
Fig. 1.
Sampling of (A) solids and (B) liquids by touch spray ionization using an angled teasing needle. (C) Application of high voltage and solvent at mass spectrometer causes release of analyte-containing charged droplets.
Touch spray is user-guided, placing limits on the rate and reproducibility of sampling. It is therefore semi-quantitative with performance dependent on user experience. However, it allows ready assessment of the presence or absence of analytes in situ, information that is critical for applications in fields such as forensics and quality control while also providing the ability to monitor relative changes in ion profiles in applications such as cancer diagnostics.
Detection of Lipids from Biological Tissue
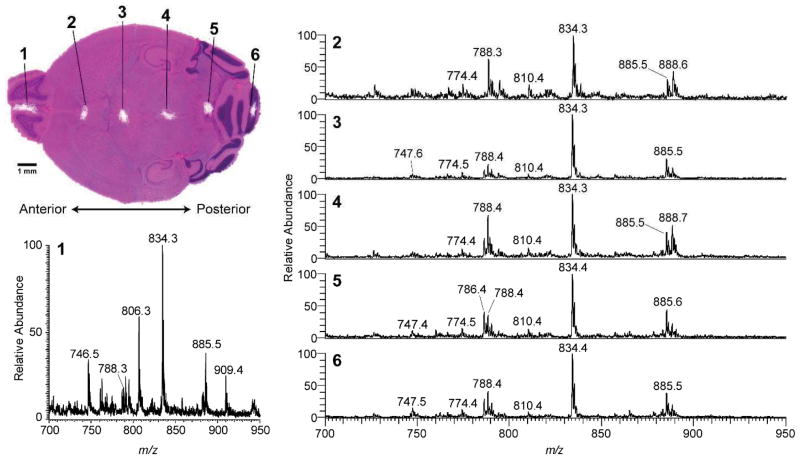
The lipid composition of mouse brain tissue has been extensively studied using ambient ionization mass spectrometry (e.g. DESI)23 and therefore constitutes a biological standard by which to qualitatively measure the performance of touch spray ionization. A minute amount of cellular and extracellular material can be transferred to a touch spray probe using light abrasive force on the biological material. For the sake of demonstration, mouse brain tissue sections were sampled in this manner removing material in a circle (diameter <1 mm). Touch spray mass spectra displayed in Fig 2 are similar to those acquired with DESI, Supplemental Fig 2, in the negative ion mode is dominated by signals due to fatty acids (m/z <300), fatty acid dimers (m/z 300–700), and glycerophospholipids (m/z 700–1000). The relative abundances of m/z 888.5 (main constituent from previous studies, sulfatide 24:1), 788.5 (phosphatidylserine 36:1), 834.5 (phosphatidylserine 40:6), and 885.5 (phosphatidylinositol 38:4) were used to qualitatively assess neural composition (white and grey matter) as shown in Fig 2. The transverse plane of mouse brain possesses left-right hemisphere symmetry, visible in the various staining features (e.g. cerebellum and corpus callosum). Touch spray was performed at six positions (Fig. 2) annotated upon the H&E stain. The touch spray mass spectra from the mouse olfactory bulb (Fig 2, point 1) displaced unique ions detected at m/z 806.5 (PS 38:6) and 909.5 (PI 40:6) corresponding to parallel findings using DESI.23 Similarly, the mass spectra recorded from point 2 indicated a high percentage of white matter, corresponding to the corpus callosum. The spectra recorded at point 5 show a high percentage of gray matter (periaqueductal gray and/or cerebellum). At other points a mixture of gray and white matter was observed with point 3 corresponding to the thalamus, point 4 to periaqueductal gray with white matter potentially from the posterior commissure, and point 6 primarily white matter from the granular layer of the cerebellum with some gray matter.
Fig. 2.
(Upper left) H&E stain of a transverse mouse brain section after repeat sampling, annotated, by TS-MS (Lower left) Sampling point 1, corresponding to the olfactory bulb which contained unique lipids detected at m/z 806.3 and 909.4 (Right) TS-MS from annotated sampling points displaying various levels of white and gray matter
The reproducibility of touch spray was assessed using coronal mouse brain sections. These sections are comprised of either grey or white matter each possessing different glycerophospholipids, reflected in the spectra, and whose distribution is symmetrical between right and left hemispheres. Touch spray was performed at six equally spaced points across one coronal section (Supplemental Fig 3). Mass spectra were reproducible in terms of the prominent glycerophospholipids ions seen and also in terms of their approximate relative intensities. They were similar between points of related neural composition; for example, A and F indicated grey matter which produces patterns including a base peak at m/z 834.5 and major peaks at m/z 788.5 and 885.5 whereas B and E displayed an ion m/z 888.5 with a higher ratio of m/z 888.5 to 834.5 than m/z 788.5 or 885.5 to 834.5 indicating the presence of white matter.
Ex vivo Detection of Malignant Prostate Cancer
The ability to discriminate prostate malignancy from non-malignant states is vital for improving the care of patients, and could be furthered by advances in molecular-based diagnostics. DESI-MS can be used to distinguish cancer from normal tissue in human brain tumors and to classify tumor subtypes, grades, and tumor cell concentrations.24 TS is being applied to prostate cancer to assess malignant and non-malignant states. Prostate cancer tissue from radical prostatectomy specimens was evaluated with TS using DESI imaging to validate the TS data. Samples were collected using a disposable biopsy gun and sectioned to allow evaluation by histopathology in serial sections (<50 μm).
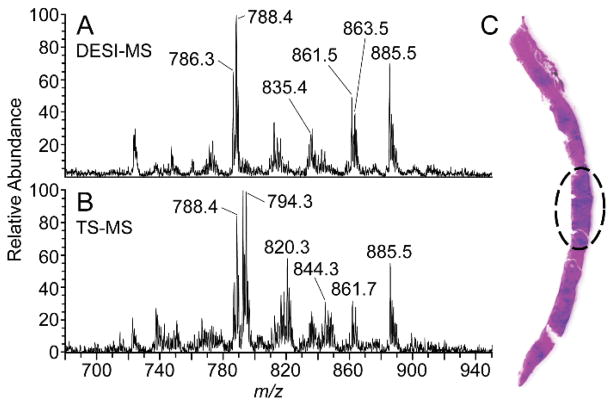
DESI and TS spectra acquired from a region of malignant prostate cancer, determined by histopathology, are presented in Fig 3, A and B, respectively. The H&E stained biopsy section shown in Fig 3C is a sample analyzed by DESI-MS with the cancerous region outlined. TS-MS was performed on the adjacent section. The phospholipid region (m/z 720–900) was observed to possess many similarities between the two methods such as the ratio and abundance of ions at m/z 786.5 (PS 36:2), 788.5 (PS 36:1), 861.5 (PI 36:4), 863.5 (PI 36:3), and 885.5 (PI 38:4). The major differences seen between the spectra were the chlorinated phosphotidylcholine adducts present in the TS spectrum at m/z 792.5 (PC 34:2)/794.5 (PC 34:1), 818.5 (PC 36:3)/820.5 (PC 36:2), and 844.5 (PC 38:4)/846.5 (PC 38:3). The chlorinated adducts in the TS spectrum indicate a lower salt tolerance in TS than in DESI.25
Fig. 3.
(A) DESI-MS and (B) TS-MS of region outlined in (C) corresponding to prostate malignancy as determined by histopathological evaluation
In vitro Detection of Bacteria
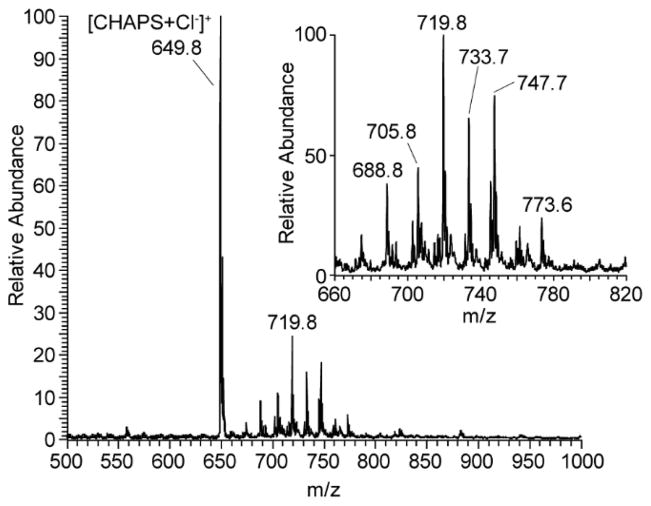
Interest in in vitro detection and identification of bacteria by mass spectrometry has increased significantly.26 The ability to directly detect biomolecules such as proteins and lipids by matrix assisted laser desorption ionization (MALDI)27 and electrospray ionization (ESI), respectively, has reduced diagnosis time while improving accuracy due to high molecular specificity. Touch spray was investigated for its applicability to in vitro detection. A minute amount of material, as little as 1% of a single bacterial colony, was required for mass spectral analysis. The data presented in Fig 4 were acquired in the negative ionization mode with automatic gain control (AGC) in a burst of signal lasting just a few seconds. Repeated sampling of the E. coli culture yielded ionized lipids of identical m/z values and relative spectral intensity, while absolute intensity varied with sampling. The spectra contained phospholipids in a profile from approximately m/z 660–780, including odd-carbon number fatty acid phospholipids (e.g. m/z 719.5, phosphatidylglycerol 32:1). It was determined empirically that the addition of CHAPS, a surfactant, to the methanol spray solvent increased and stabilized the TS spectra. Addition of CHAPS at 0.01% was determined to have the most beneficial effect without undue interference from the CHAPS chloride adduct (m/z 649.8). The observed surfactant effect on improving spectra in ambient ionization due to reduction in surface tension is well known.28
Fig. 4.
Negative ion mode TS-MS displaying E. coli phospholipids in a sample consisting of 1% of a single colony, showing phospholipids and the chloride adduct of CHAPS, a surfactant added to the spray solution.
Forensics: Bulk and Trace Detection of Illicit Drugs
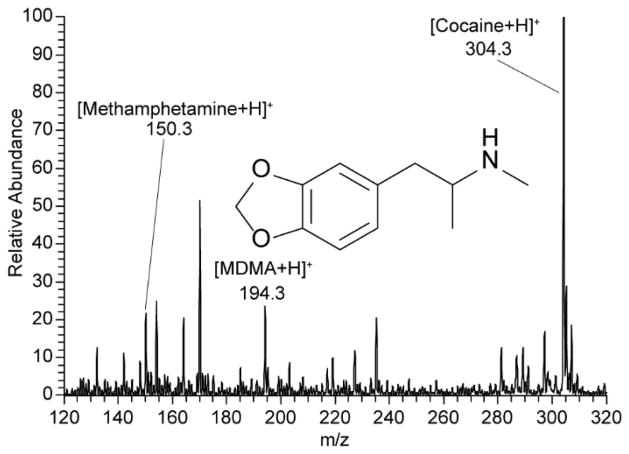
Forensics, in particular illicit drug analysis, often requires the ability to detect compounds in situ from matrices including powders, drug residues on surfaces including clothing, and illicit drugs in solution. Mass spectrometry is certainly capable of analyzing all of these types of samples when using various types of sample preparation. Touch spray offers a method by which to analyze these sample types without preparative steps. Drug residues on clothing typically require extraction in solvents whereas with TS the material can be lightly rubbed absorbing minute amount of drugs while removing negligible amounts of the cloth. This ability was demonstrated by the detection of cocaine from a dried blood spot containing 10 ng of the spiked drug on blue cloth, Supplemental Fig 4. A homogenous solution containing 400 ppb each of MDMA, cocaine, and methamphetamine was sampled by dipping the TS probe into the solution and dried at ambient temperature (<1 minute). The mass spectrum (Fig 5) was recorded by the addition of spray solvent and a high voltage; it displays the ions at m/z 150.3, 194.3, and 304.3 for each of the drugs in the solution, respectively.
Fig. 5.
Positive ion mode TS-MS of illicit drugs, methamphetamine, MDMA, and cocaine, at 400 ppb in homogenous solution sampled via a single dip of a touch spray probe
Therapeutic Drugs
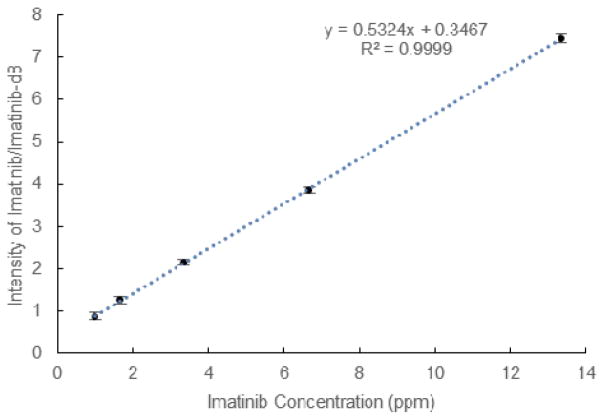
Therapeutic drug monitoring aids in maintaining drug concentrations within the beneficial range, maximizing therapeutic effect while minimizing the risk of harmful overdosing or wasteful undosing. Ambient ionization methods such as PS have used whole dried blood or whole blood mixed with a coagulant to quantitatively measure the concentrations of pharmaceuticals.29 Whole bovine blood spiked with imatinib, a therapeutic used for the treatment of chronic myelogenous leukemia, and with its deuterated isotopomer added as internal standard, were analyzed over a range of concentrations. Using the teasing probe, blood was sampled by dipping once directly into the blood to a fixed depth, waiting <1 min to dry after dipping, and analyzing directly afterwards. The quantitative performance was similar to that of PS with a linear response across the concentration range tested (Fig 6).30 It could be increased further using MS/MS. Furthermore, the percentage error (n=5) was <10% for imatinib concentrations greater than 1 ppm, a value within FDA guidelines (see Supplemental Table 1). It is envisioned that a modified TS probe may be useful as a semi-quantitative tool that could be used as a finger-prick device which could be directly used to measure the concentration of therapeutics in whole blood.
Fig. 6.
Full scan calibration curve of Imatinib. Data points represent the average value for repeat analysis (N=5) and error bars corresponding to the standard deviation. Linear regression performed by unweighted least squares analysis from 1 ppm to 13.3 ppm.
Residual Agrochemical Detection in Foods
Agrochemicals are applied to foods in an attempt to prolong crop quality while attempting to limit potentially adverse health effects. Oranges and other citrus fruits are commonly treated (systemically or sprayed post-harvest) with fungicides such as thiabendazole, leaving a trace amount of material on the surface of the orange peel. Agrochemical levels are monitored and regulated in the United States, typically by chromatographic separation prior to MS analysis. This procedure is not readily accomplishing in situ, limiting thorough screening of foodstuffs; however, fungicides have previously been reported to be monitored using paper spray ionization in situ.31 A non-organic orange purchased from a national grocer was subjected to analysis by TS, in which the probe was used to sample ~4 cm2 area of the peel. The spectrum, Supplemental Fig 5, includes ions due to protonated thiabendazole (m/z 202) and imazalil (m/z 297). The acquired spectrum matches previously reported fungicides detected from non-organic oranges using additional ambient ionization methods (i.e. low temperature plasma).22
Elaborations
Reactive Touch Spray Ionization
TS-MS experiments exploiting simultaneous chemical derivatization and ionization (i.e. reactive ambient ionization) were explored. Derivatized versions of analytes often give greater signals in MS analysis of complex mixtures. The use of appropriate reagents allows reduction of complex spectra via analyte signal enhancement or characteristic m/z value shifts. Reactive touch spray was explored with known types of ambient reactions including non-covalent adduct formation, (e.g. silver adduction of olefins32) and covalent bond formation (e.g. betaine aldehyde formation alcohols33). Cholesteroyl lineolate and adrenosterone were sampled from homogenous solution in a dipping fashion by TS. The unsaturated aliphatic functionality of cholesteroyl lineolate was reacted with silver nitrate (4 ppm, acetonitrile) forming non-covalent adducts detected at m/z 755.4 and 757.4 (Supplemental Fig 6) corresponding to [107Ag+cholesteroyl lineolate] and [109Ag+cholesteroyl lineolate]. Covalent bond forming reactions targeting the ketone functional group in adrenosterone, a cholesterol-related hormone, were accomplished using Girard’s Reagent P and hydroxylamine (Supplemental Fig 7). The detection of cholesterol from bovine blood was accomplished using betaine aldehyde which reacts with a poorly ionizable hydroxyl functionality creating a positively-charged quaternary amine derivative (Supplemental Fig 8).
Continuous Touch Spray Ionization
Most applications of touch spray only require brief recording of the MS signal to obtain the desired information, as outlined above. However, if the application dictates a longer signal duration, touch spray can be coupled to a solvent delivery system (solvent pump) to provide solvent at controlled flow rate to produce stable ion currents. The solvent is added continuously at the same location where discrete additions of solvent produce spectra, yielding a signal that lasts until analyte exhaustion. An illustrative figure is presented in Supplemental Fig 9 including parameters determined used with the bent teasing probe.
Conclusions
Touch spray (TS) ionization is a spray-based ambient ionization method capable of in situ sampling of complex mixtures. The rapid, reproducible, and specific chemical information obtainable from biological tissue includes mouse brain and human prostate cancer. Differentiation of specific anatomical regions and disease states based on lipids detected in the negative ionization mode appears to be possible. Application in the detection of bacteria is promising, the detected lipids allowing for differentiation of bacteria. TS-MS of solutions, including blood, could allow for numerous applications in forensics (e.g. illicit drugs) and medicine (e.g. therapeutic drug monitoring). The user-guided nature of TS allows for the unique sampling abilities while hindering absolute quantitation. Further, as is the case for many ambient ionization techniques, simultaneous ioinization and chemical derivatization is enhances the signal for specific analytes or groups of analytes.
Further development of TS-MS including the sampling methodology, probe design, and probe material could lead to improved quantitative performance and reproducibility. The ultimate application of the TS technology would be in combination with miniaturized mass spectrometer systems to allow for in situ sampling, ionization, and mass analysis.
Experimental
Touch Spray Probe
The TS probe used is a commercially available teasing needle purchased from Fisher Scientific (Pittsburgh, PA, USA) while multiple variants also were tested.
Materials
The spray solvent used for MS acquisition was methanol, purchased from Mallinckrodt Baker Inc. (Phillipsburg, NJ), and applied manually via an adjustable pipette (Eppendorf Research-2.5 μL). Additional spray additives including 3-[(3-Cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) was purchased from Sigma-Aldrich (St. Louis, MO). Therapeutic drugs were acquired from Cerilliant (Round Rock, Texas, USA). Drugs were stored at −20° C until diluted in bovine blood to appropriate concentrations. Illicit drugs were acquired from Cerilliant (Round Rock, Texas, USA). Drugs were stored at −20° C until diluted to appropriate concentrations. Non-organic orange was purchased from national grocer for agrochemical MS experiments.
Mouse Brain Tissue
Mouse brain tissue was acquired from JAX® Mice and Services (Bar Harbor, Maine, USA). A mouse brains was sectioned at 15 μm and thaw mounted onto glass microscope slides. The slides were stored in closed containers at −20 °C; prior to analysis they were allowed to come to room temperature and dried in an electronic desiccator for approximately 20 minutes.
Prostate Tissue
Radical prostatectomy specimens were obtained from consented patients via Indiana School of Medicine (Indianapolis, IN). Biopsies were obtained using a disposable biopsy gun in accordance with an IRB approved protocol. Biopsies were frozen, cryosectioned at 15 μm and thaw mounted to glass microscope slides. Sections were stored at −80° C prior to analysis. Histopathology was performed upon H&E staining prostate biopsies by an expert pathology.
Bacteria Culturing
An E. coli stain was supplied by bioMérieux, Inc. (Hazelwood, MO) and cultured from a frozen sample stored at −80 °C in TSAB. Bacteria were cultured on TSA with 5% sheep blood (Remel, Lenexa, KS) and incubated in a VWR incubator (Chicago, IL) at 37±1°C for approximately 24 h, sub-cultured for an additional 24 h, and then used in TS-MS experiments.
Instrumentation
A LTQ linear ion trap (Thermo Fisher Scientific) was used in experiments unless otherwise specified and carried out with the auto gain control (AGC) activated.
Supplementary Material
Acknowledgments
Funding for this work was provided by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health (NIH) under award number R21EB015722. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors acknowledge contributions by Purdue coworkers Ryan Espy and the assistance of Ahmed Hamid. As well as contributions by Indiana School of Medicine collaborators Liang Cheng, Timothy Masterson, and Michael Koch.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/b000000x/
Notes and references
- 1.Monge ME, Harris GA, Dwivedi P, Fernández FM. Chemical Reviews. 2013;113:2269–2308. doi: 10.1021/cr300309q. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Gamez G, Zenobi R. Journal of the American Society for Mass Spectrometry. 2009;20:1947–1963. doi: 10.1016/j.jasms.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 3.Takats Z, Wiseman JM, Cooks RG. Journal of Mass Spectrometry. 2005;40:1261–1275. doi: 10.1002/jms.922. [DOI] [PubMed] [Google Scholar]
- 4.Huang MZ, Yuan CH, Cheng SC, Cho YT, Shiea J. Annual Review of Analytical Chemistry. 2010;3:43–65. doi: 10.1146/annurev.anchem.111808.073702. [DOI] [PubMed] [Google Scholar]
- 5.Badu-Tawiah AK, Eberlin LS, Ouyang Z, Cooks RG. Annual Review of Physical Chemistry. 2013 doi: 10.1146/annurev-physchem-040412-110026. [DOI] [PubMed] [Google Scholar]
- 6.Nemes P, Vertes A. TrAC Trends in Analytical Chemistry. 2012;34:22–34. [Google Scholar]
- 7.Roach PJ, Laskin J, Laskin A. Analyst. 2010;135:2233–2236. doi: 10.1039/c0an00312c. [DOI] [PubMed] [Google Scholar]
- 8.Van Berkel GJ, Kertesz V, Koeplinger KA, Vavrek M, Kong ANT. Journal of Mass Spectrometry. 2008;43:500–508. doi: 10.1002/jms.1340. [DOI] [PubMed] [Google Scholar]
- 9.Hiraoka K, Nishidate K, Mori K, Asakawa D, Suzuki S. Rapid Communications in Mass Spectrometry. 2007;21:3139–3144. doi: 10.1002/rcm.3201. [DOI] [PubMed] [Google Scholar]
- 10.Venter AR, Douglass KA, Shelley JT, Hasman G, Honarvar E. Analytical Chemistry. 2013;86:233–249. doi: 10.1021/ac4038569. [DOI] [PubMed] [Google Scholar]
- 11.Wang H, Liu J, Cooks RG, Ouyang Z. Angewandte Chemie. 2010;122:889–892. doi: 10.1002/anie.200906314. [DOI] [PubMed] [Google Scholar]
- 12.Chen LC, Yoshimura K, Yu Z, Iwata R, Ito H, Suzuki H, Mori K, Ariyada O, Takeda S, Kubota T, Hiraoka K. Journal of Mass Spectrometry. 2009;44:1469–1477. doi: 10.1002/jms.1632. [DOI] [PubMed] [Google Scholar]
- 13.Liu J, Wang H, Cooks RG, Ouyang Z. Analytical Chemistry. 2011;83:7608–7613. doi: 10.1021/ac2020273. [DOI] [PubMed] [Google Scholar]
- 14.Kuo CP, Shiea J. Analytical Chemistry. 1999;71:4413–4417. doi: 10.1021/ac990049r. [DOI] [PubMed] [Google Scholar]
- 15.Kuo CP, Yuan CH, Shiea J. Journal of the American Society for Mass Spectrometry. 2000;11:464–467. doi: 10.1016/S1044-0305(00)00111-2. [DOI] [PubMed] [Google Scholar]
- 16.Jeng J, Shiea J. Rapid Communications in Mass Spectrometry. 2003;17:1709–1713. doi: 10.1002/rcm.1109. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimura K, Chen LC, Mandal MK, Nakazawa T, Yu Z, Uchiyama T, Hori H, Tanabe K, Kubota T, Fujii H. Journal of The American Society for Mass Spectrometry. 2012;23:1741–1749. doi: 10.1007/s13361-012-0447-2. [DOI] [PubMed] [Google Scholar]
- 18.Mandal MK, Ozawa T, Saha S, Rahman MM, Iwasa M, Shida Y, Nonami H, Hiraoka K. Journal of agricultural and food chemistry. 2013;61:7889–7895. doi: 10.1021/jf4014718. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Deng J, Yao ZP. Journal of the American Society for Mass Spectrometry. 2014;25:37–47. doi: 10.1007/s13361-013-0748-0. [DOI] [PubMed] [Google Scholar]
- 20.Chen HK, Lin CH, Liu JT, Lin CH. International Journal of Mass Spectrometry. 2013;356:37–40. [Google Scholar]
- 21.Hu B, So PK, Chen H, Yao ZP. Analytical Chemistry. 2011;83:8201–8207. doi: 10.1021/ac2017713. [DOI] [PubMed] [Google Scholar]
- 22.Soparawalla S, Tadjimukhamedov FK, Wiley JS, Ouyang Z, Cooks RG. Analyst. 2011;136:4392–4396. doi: 10.1039/c1an15493a. [DOI] [PubMed] [Google Scholar]
- 23.Eberlin LS, Ifa DR, Wu C, Cooks RG. Angewandte Chemie International Edition. 2010;49:873–876. doi: 10.1002/anie.200906283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eberlin LS, Norton I, Orringer D, Dunn IF, Liu X, Ide JL, Jarmusch AK, Ligon KL, Jolesz FA, Golby AJ, Santagata S, Agar NYR, Cooks RG. Proceedings of the National Academy of Sciences. 2013 doi: 10.1073/pnas.1215687110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson AU, Talaty N, Cooks RG, Van Berkel GJ. Journal of the American Society for Mass Spectrometry. 2007;18:2218–2225. doi: 10.1016/j.jasms.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Havlicek V, Lemr K, Schug KA. Analytical Chemistry. 2012;85:790–797. doi: 10.1021/ac3031866. [DOI] [PubMed] [Google Scholar]
- 27.Dubois D, Grare M, Prere M-F, Segonds C, Marty N, Oswald E. Journal of Clinical Microbiology. 2012 doi: 10.1128/JCM.00343-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badu-Tawiah A, Cooks RG. Journal of the American Society for Mass Spectrometry. 2010;21:1423–1431. doi: 10.1016/j.jasms.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Espy RD, Manicke NE, Ouyang Z, Cooks RG. The Analyst. 2012;137:2344–2349. doi: 10.1039/c2an35082c. [DOI] [PubMed] [Google Scholar]
- 30.Wang H, Liu J, Cooks RG, Ouyang Z. Angewandte Chemie. 2010;49:877–880. doi: 10.1002/anie.200906314. [DOI] [PubMed] [Google Scholar]
- 31.Wiley JS, García-Reyes JF, Harper JD, Charipar NA, Ouyang Z, Cooks RG. Analyst. 2010;135:971–979. doi: 10.1039/b919493b. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez-Serrano AF, Pirro V, Ferreira CR, Oliveri P, Eberlin LS, Heinzmann J, Lucas-Hahn A, Niemann H, Cooks RG. PloS one. 2013;8:e74981. doi: 10.1371/journal.pone.0074981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu C, Ifa DR, Manicke NE, Cooks RG. Analytical Chemistry. 2009;81:7618–7624. doi: 10.1021/ac901003u. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.