Abstract
Objective To examine whether there are socioeconomic gradients in the incidence, prevalence, treatment, and follow up of patients with heart failure in primary care.
Design Population based study.
Setting 53 general practices (307 741 patients) participating in the Scottish continuous morbidity recording project between 1April 1999 and 31 March 2000.
Participants 2186 adults with heart failure.
Main outcome measures Comorbid diagnoses, frequency of visits to general practitioner, and prescribed drugs.
Results 2186 patients with heart failure were seen (prevalence 7.1 per 1000 population, incidence 2.0 per 1000 population). The age and sex standardised incidence of heart failure increased with greater socioeconomic deprivation, from 1.8 per 1000 population in the most affluent stratum to 2.6 per 1000 population in the most deprived stratum (odds ratio 1.44, P = 0.0003). On average, patients were seen 2.4 times yearly, but follow up rates were less frequent with increasing socioeconomic deprivation (from 2.6 yearly in the most affluent subgroup to 2.0 yearly in the most deprived subgroup, P = 0.00009). Overall, 812 (80.6%) patients were prescribed diuretics, 396 (39.3%) angiotensin converting enzyme inhibitors, 216 (21.4%) β blockers, 208 (20.7%) digoxin, and 86 (8.5%) spironolactone. The wide discrepancies in prescribing between different general practices disappeared after adjustment for patient age and sex. Prescribing patterns did not vary by deprivation categories on univariate or multivariate analyses.
Conclusions Compared with affluent patients, socioeconomically deprived patients were 44% more likely to develop heart failure but 23% less likely to see their general practitioner on an ongoing basis. Prescribed treatment did not differ across socioeconomic gradients.
Introduction
The adverse impact of socioeconomic deprivation on health, and particularly cardiovascular health, is well recognised.1 Although this increased risk is multifactorial, key modifiable factors need to be identified to properly direct efforts to reduce these gradients. For example, the higher mortality for acute coronary syndromes in socioeconomically deprived people seem to be due largely to four factors: a higher prevalence of atherosclerotic risk factors, earlier onset of symptomatic coronary atheroma, reduced access to specialist care, and suboptimal application of proved efficacious therapies.2-4
Socioeconomic deprivation is associated with higher rates of admission to hospital and case fatality in heart failure, but the mechanisms are unclear—indeed, this excess risk seems to depend on age, sex, comorbidities, severity of disease, and adherence to treatment.5-7 It may be intriguing to speculate about socioeconomic gradients in access to general practitioners and outpatient pharmacotherapy being the key causative factors, but there is a paucity of high quality research on heart failure in primary care.8 This question is important as heart failure accounts for almost a quarter of all admissions to hospital for cardiovascular events, has a high mortality (median survival around 18 months), and places a great burden on all healthcare systems (estimated direct costs of £905m ($1650m; €1350m) in the United Kingdom in 2000, 2% of total NHS expenditure).7,9,10
We used data from the Scottish continuous morbidity recording project to examine whether there are socioeconomic gradients in the incidence, prevalence, and follow up of patients with heart failure. We also examined the influence of socioeconomic deprivation on the prescribing patterns of general practitioners.
Methods
In Scotland the continuous morbidity recording project is coordinated by the Information and Statistics Division of the Common Services Agency, NHS, and involves prospective data collection from face to face contacts between doctors and patients from selected general practices. At the time of our study, these 53 practices had a total registered practice population of 307 741 patients (around 6% of the total population in Scotland) and were representative of the Scottish population for age, sex, socioeconomic status, and mix of rural and urban locations.11 As such this scheme allows accurate estimates of the national prevalence, incidence, and consultation rates for heart failure in primary care. Comprehensive information is collected about the index condition (including whether it is a first occurrence, recurrent, or persistent), up to nine concomitant medical problems, and prescriptions issued or renewed.12 Data are entered on to the computer system of the General Practice Administration System for Scotland. The Information and Statistics Division of the Common Services Agency, NHS, conducts internal quality assurance of the project, involving a rolling programme of visits to practices to compare the morbidity data with practice held records: in 1999-2000 the completeness of capture of contacts was 91% and the accuracy of Read coding was 91% (L Graham, personal communication, 29 November 2003).
Using data from 1 April 1999 to 31 March 2000, we examined the prevalence and contact rates (number of consultations for that diagnosis in that year) for heart failure. We also estimated the incidence of heart failure from the number of patients with a diagnosis of heart failure entered by their general practitioner with the modifier of “first.” The table shows the crude rates and the age and sex standardised rates.
Table 1.
Rates per 1000 population for incidence, prevalence, and contacts of patients with heart failure in primary care, stratified by socioeconomic status
|
Prevalence
|
Incidence
|
Contacts
|
Annual contacts per patient
|
Estimated mean survival (years)*
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Deprivation category | Sample size | Crude | Adjusted | Crude | Adjusted | Crude | Adjusted | ||
| 1 (most affluent) | 70961 | 6.3 | 6.4 | 1.8 | 1.8 | 16.8 | 17.1 | 2.6 | 3.5 |
| 2 | 66633 | 7.5 | 7.4 | 1.7 | 1.6 | 20.0 | 19.6 | 2.7 | 4.4 |
| 3 | 93258 | 7.3 | 7.5 | 1.9 | 1.9 | 17.5 | 19.6 | 2.4 | 3.8 |
| 4 | 34627 | 7.3 | 7.5 | 2.6 | 2.7 | 16.6 | 17.9 | 2.3 | 2.8 |
| 5 (most deprived) | 28633 | 6.7 | 7.2 | 2.4 | 2.6 | 13.4 | 14.3 | 2.0 | 2.8 |
| Odds ratio between categories 5 and 1 | 1.06 | 1.13 | 1.33 | 1.44 | 0.80 | 0.84 | 0.77 | 0.80 | |
| P for trend | 0.27 | 0.06 | 0.002 | 0.0003 | <0.001 | 0.07 | <0.001 | <0.001 | |
Adjusted rates are age and sex standardised to distribution found in entire continuous morbidity recording practice population.
Crude prevalence divided by crude incidence.
Prescription data were obtained from a representative subset (22 practices, 1007 patients with heart failure), and we included only those drugs that had been prescribed at least twice during the 12 months of the study. We restricted our analyses to loop diuretics, angiotensin converting enzyme inhibitors, β blockers, spironolactone, and digoxin.
Using postcodes of residence, we assigned a Carstairs deprivation category, from 1 (most affluent) to 5 (most deprived), to 294 112 patients (95.6% of total cohort)).13
Using general χ2 tests and χ2 tests for trend as appropriate, we compared the prevalence and incidence of heart failure and contact rates and prescribing data between Carstairs deprivation categories. We also performed multivariate logistic regression to examine the independent effects of age, sex, deprivation category, and general practitioner on prescriptions for each drug for heart failure. Using the drug of interest as the dependent variable in a binary logistic regression model, we used a backward stepwise selection, P value of 0.20 to enter and P value of 0.05 to remove, with age, sex, deprivation category, and general practitioner as the independent variables.
Results
Of the 307 741 patients registered in the general practices participating in the Scottish continuous morbidity recording project, 2186 were seen at least once for heart failure between 1 April 1999 and 31 March 2000 (prevalence 7.1 per 1000 population). Of these patients, 609 (27.9%) had a first diagnosis of heart failure (incidence 2.0 per 1000 population). The 2186 patients were seen 5285 times over the year (contacts 17.2 per 1000 population), with a mean number of contacts per patient of 2.4 each year.
The prevalence of heart failure differed between deprivation categories (table), with a non-significant 13% trend towards higher age and sex standardised prevalence in the most deprived group. The incidence of heart failure significantly increased with increasing social deprivation: socioeconomically deprived patients were 44% more likely to develop heart failure than affluent patients. In contrast, the association between socioeconomic deprivation and contacts or consultations was in the opposite direction: patients in the most deprived groups had 23% fewer follow up visits each year with their general practitioner (table). Although the age and sex standardised contacts differed significantly between the five strata, the P value for trend was not significant (P = 0.07) as there was little appreciable difference between categories 1 to 4. However, the most deprived subgroup had significantly lower standardised contact rates compared with deprivation categories 1 (odds ratio 0.81, 95% confidence interval 0.72 to 0.90), 2 (0.72, 0.65 to 0.81), 3 (0.72, 0.65 to 0.81), and 4 (0.79, 0.70 to 0.90). Contact rates did not differ across age groups or by sex. Estimated mean survival rates were significantly lower in the most deprived group (table).
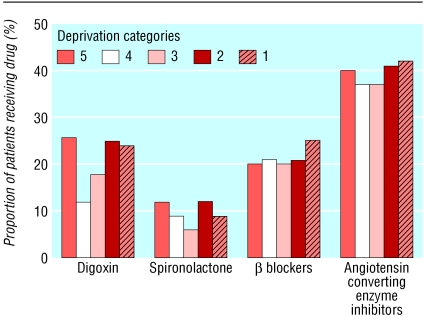
Prescribing data were available for all 1007 patients with heart failure (439 men and 568 women) in the 22 practices selected a priori. These patients had similar distributions for age, sex, and deprivation to the total sample of 2186 patients with heart failure (data not shown). Diuretics were prescribed for 812 (80.6%) of these 1007 patients, angiotensin converting enzyme inhibitors for 396 (39.3%), β blockers for 216 (21.4%), digoxin for 208 (20.7%), and spironolactone for 86 (8.5%). Both an angiotensin converting inhibitor and a β blocker were prescribed for 111 (11.0%) patients, and 13 (1.3%) were prescribed a combination of angiotensin converting enzyme inhibitor, β blocker, and spironolactone. Prescribing patterns did not vary by deprivation category on bivariate or multivariate analyses (figure). Wide discrepancies were found in prescribing between general practitioners; these disappeared after adjusting for differences in patient characteristics. For example, the variables associated with prescribing of angiotensin converting enzyme inhibitor on multivariate analysis were sex (odds ratio 1.42 for males compared with females) and age (0.60 for patients aged 75-84 years and 0.39 for patients older than 85 years compared with patients younger than 65 years). Deprivation category and general practitioner were not independently associated with prescriptions for angiotensin converting enzyme inhibitors.
Figure 1.
Prescribing patterns in patients with heart failure, stratified by socioeconomic status
Discussion
Socioeconomically deprived individuals are more likely to develop heart failure but less likely to see their general practitioner on an ongoing basis. Contrary to speculation, we did not find any relation between prescribing practices of general practitioners and socioeconomic status.
Although the finding of an increased incidence of heart failure in socioeconomically deprived individuals has not previously been reported, a study from the United States did show an inverse association between incidence of heart failure and educational attainment.14 However, our finding is not unexpected given data showing increased admissions to hospital for heart failure in deprived patients.5,7 Furthermore, as the risk factors for heart failure (similar to those for coronary artery disease) are more prevalent in socioeconomically deprived groups, it is plausible that the incidence of heart failure would be higher in these groups.15,16 Despite the noticeable gradient in incidence, there was only a trend towards differences in prevalence across the deprivation categories. This is not unexpected given that deprived patients have higher case fatality rates and shorter survival times when they do develop heart failure as shown in our study and another Scottish study that examined survival in 66 547 patients admitted to hospital with heart failure between 1986 and 1995.7
Our finding that socially deprived individuals with heart failure have less ongoing contact with their general practitioners is novel and worrying, particularly as the limited data in this discipline suggest that socially deprived patients with heart failure have a worse functional status.17,18 The obvious questions raised by this novel example of Tudor-Hart's inverse care relation are twofold: why does this occur and what consequences might ensue?19
Several potential factors may contribute to these lower consultation rates. Firstly, the behaviour of deprived groups when ill is substantially different, fatalism is more common, and non-professionals are often consulted for healthcare advice.8 Secondly, socially deprived patients may seek care in hospital emergency rooms rather than attend their primary care physicians—this pattern has been observed for other cardiovascular and respiratory illnesses characterised by intermittent acute exacerbations.20,21 Thirdly, general practitioners may fail to offer regular follow up care. However, this is argued against by the comparable rates of prescribing across the social class spectrum.
Although we do not know what consequences may ensue from less frequent follow up with general practitioners for socioeconomically deprived patients with heart failure, other studies suggest that this lack of contact may have important consequences for patients with chronic diseases. For example, patients with heart failure or asthma who are not seen regularly after hospital discharge are more likely to be readmitted.6,22 Moreover, a retrospective analysis of administrative data from Canada found improved survival rates in patients with heart failure who were regularly followed by a physician compared with those without ongoing contact (P Kaul, personal communication, 27 July 2003). Finally, there is a wealth of evidence that closer follow up of patients with heart failure (either by physicians or by specially trained nurses) leads to better outcomes.23 It is thus intriguing to speculate whether disease management programmes specifically targeting more deprived individuals with heart failure would improve their prognosis.
We did not find any evidence of socioeconomic bias in these general practitioners' prescribing patterns. Although we had no information on patient adherence, an earlier study in Scotland did not find any differences in compliance with diuretics between deprived and affluent patients with heart failure.5
Our study describes the experience of primary care with heart failure from a nationally representative sample of general practices. However, there are some limitations to the data. Firstly, we do not have any independent confirmation of heart failure diagnoses nor data on disease severity. This should not, however, detract from our research question as it seems unlikely that individual clinicians would apply different case definitions or diagnostic thresholds in patients of differing socioeconomic status. Secondly, we do not have any data on potential confounders related to cardiovascular risk factors, and these may vary substantially across socioeconomic gradients. Although this may account for the observed differences in incidence, it does not explain the socioeconomic gradients in contacts after diagnosis. Thirdly, we used postcode sector based as a proxy for individual socioeconomic status; the Carstairs deprivation index is well accepted and has been validated.24 Finally, counting only those drugs that had been prescribed at least twice over 12 months may have increased data quality at the cost of underestimating prescribing rates. However, we were interested in chronic prescribing, and if there was any underestimation it is unlikely to have varied differentially between deprivation categories.
In conclusion, we have described the substantial burden of heart failure in primary care. Although the incidence of heart failure diagnosed in general practice is significantly higher in socioeconomically deprived individuals, the subsequent follow up is significantly less frequent. Although there was no evidence of socioeconomic bias in prescribing by the general practitioners in our study, it is likely that socioeconomic status may play more of a part in countries with healthcare systems that are not publicly funded (for example, the United States, where lower income is negatively correlated with being listed for cardiac transplantation).25 Regardless, our study has eliminated the prescribing bias hypotheses commonly cited as a potential explanation for the socioeconomic gradients in heart failure. Indeed, our data raise another potentially important explanation, that socioeconomically deprived patients may have poorer outcomes because they have less ongoing contact with their general practitioner. Further studies are required to determine why such patients are followed less closely. Once the mechanisms behind these socioeconomic gradients are better understood, programmes can be devised for optimal outcomes of all patients, irrespective of social class.
What is already known on this topic
Socioeconomic deprivation is associated with more frequent admissions to hospital and higher mortality in patients with heart failure
These excess risks are independent of age, sex, comorbidities, disease severity, and treatment adherence
These excess risks may arise because of differences in how socioeconomically deprived patients are managed by general practitioners
What this study adds
Socioeconomically deprived individuals are 44% more likely to develop heart failure
Once heart failure develops, these individuals have 23% less ongoing contact with their general practitioner
General practitioner prescribing does not differ between affluent and socioeconomically deprived individuals
Contributors: FMcA, NM, and JMcM designed the study. FMcA coordinated the study, analysed the data, and led the writing team. NM and CS contributed to the study design, data analysis, and report drafting. SS, KMacI, JC, AR, and SC helped analyse and interpret the data and draft the report. MK contributed to the study design and extracted the data. All investigators contributed to the final report. JMcM and FMcA will act as guarantors.
Funding: FMcA is supported by the Canadian Institutes of Health Research and the Alberta Heritage Foundation for Medical Research, SS is supported by the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia and NM is funded by the British Heart Foundation.
Competing interest: None declared.
Ethical approval: Not required.
References
- 1.Smith GD, Carroll D, Rankin S, Rowan D. Socioeconomic differentials in mortality: evidence from Glasgow graveyards. BMJ 1995;305: 1554-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alter DA, Naylor CD, Austin P, Tu JV. Effects of socioeconomic status on access to invasive cardiac procedures and on mortality after acute myocardial infarction. N Engl J Med 1999;341: 1359-67. [DOI] [PubMed] [Google Scholar]
- 3.Kaplan GA, Keil JE. Socioeconomic factors and cardiovascular disease: a review of the literature. Circulation 1993;88: 1973-98. [DOI] [PubMed] [Google Scholar]
- 4.MacLeod MCM, Finlayson AR, Pell JP, Findlay IN. Geographic, demographic, and socio-economic variations in the investigation and management of coronary heart disease in Scotland. Heart 1999;81: 252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Struthers AD, Anderson G, Donnan PT, MacDonald T. Social deprivation increases cardiac hospitalisations in chronic heart failure independent of disease severity and diuretic non-adherence. Heart 2000;83: 12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Philbin EF, Dec GW, Jenkins PL, DiSalvo TG. Socioeconomic status as an independent risk factor for hospital readmission for heart failure. Am J Cardiol 2001;87: 1367-71. [DOI] [PubMed] [Google Scholar]
- 7.MacIntyre K, Capewell S, Stewart S, Chalmers JWT, Boyd J, Finlayson A, et al. Evidence of improving prognosis in heart failure. Trends in case fatality in 66 547 patients hospitalized between 1986 and 1995. Circulation 2000;102: 1126-31. [DOI] [PubMed] [Google Scholar]
- 8.Blair AS, Lloyd-Williams F, Mair FS. What do we know about socioeconomic status and congestive heart failure? A review of the literature. J Fam Pract 2002;51: 169. [PubMed] [Google Scholar]
- 9.Stewart S, MacIntyre K, MacLeod MMC, Bailey AEM, Capewell S, McMurray JJV. Trends in hospitalization for heart failure in Scotland, 1990-1996. An epidemic that has reached its peak? Eur Heart J 2001;22: 209-17. [DOI] [PubMed] [Google Scholar]
- 10.Stewart S, Jenkins A, Buchan S, McGuire A, Capewell S, McMurray JJV. The current cost of heart failure to the National Health Service in the UK. Eur J Heart Failure 2002;4: 361-71. [DOI] [PubMed] [Google Scholar]
- 11.Milne RM, Taylor MW, Taylor RJ. Audit of populations in general practice: the creation of a national resource for the study of morbidity in Scottish general practice. J Epidemiol Community Health 1998;52(Suppl 1): S20-4. [PubMed] [Google Scholar]
- 12.O'Neil M, Payne C, Read J. Read codes version 3: a user led terminology. Methods Inf Med 1995;34: 187-92. [PubMed] [Google Scholar]
- 13.Carstairs V, Morris R. Deprivation and health in Scotland. Aberdeen, Scotland: Aberdeen University Press, 1991.
- 14.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, et al. Predictors of congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol 2000;35: 1628-37. [DOI] [PubMed] [Google Scholar]
- 15.Eriksson H, Svardsudd K, Larsson B, Ohlson LO, Tibblin G, Welin L, et al. Risk factors for heart failure in the general population: the study of men born in 1913. Eur Heart J 1989;10: 647-56. [DOI] [PubMed] [Google Scholar]
- 16.Osler M, Gerdes LU, Davidsen M, Bronnum-Hansen H, Madsen M, Jorgensen T, et al. Socioeconomic status and trends in risk factors for cardiovascular diseases in the Danish MONICA population. J Epidemiol Community Health 2000;54: 108-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clarke SP, Frasure-Smith N, Lesperance F, Bourassa MG. Psychosocial factors as predictors of functional status at 1 year in patients with left ventricular dysfunction. Res Nurs Health 2000;23: 290-300. [DOI] [PubMed] [Google Scholar]
- 18.Woolf SH, Rothemich SF, Johnson RE, Marsland DW. The functional status of inner-city primary care patients. Diminished function in a family practice population and its potential determinants. J Fam Pract 1998;47(4): 312-5. [PubMed] [Google Scholar]
- 19.Tudor-Hart J. The inverse care law. Lancet 1971;I: 405-12. [DOI] [PubMed] [Google Scholar]
- 20.Blatchford O, Capewell S, Murray S, Blatchford M. Emergency medical admissions in Glasgow: general practices vary despite adjustments for age, sex, and deprivation. Br J Gen Pract 1999;49: 551-4. [PMC free article] [PubMed] [Google Scholar]
- 21.Ciccine G. Social class, mode of admission, severity of illness, and hospital mortality: an analysis with “all patient refined DRG” of discharges from Molinette hospitals in Turin. Epidemiologia e Prevanzione 1999;223: 188-96. [PubMed] [Google Scholar]
- 22.Sin DD, Bell NR, Svenson LW, Man SFP. The impact of follow-up physician visits on emergency readmissions for patients with asthma and chronic obstructive pulmonary disease: a population-based study. Am J Med 2002;112: 120-5. [DOI] [PubMed] [Google Scholar]
- 23.McAlister FA, Lawson FME, Teo KK, Armstrong PW. A systematic review of randomized trials of disease management programs in heart failure. Am J Med 2001;110: 378-84. [DOI] [PubMed] [Google Scholar]
- 24.Woodward M. Small area statistics as markers for personal social status in the Scottish heart health study. J Epidemiol Community Health 1996;50: 570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coughlin SS, Halabi S, Metayer C. Barriers to cardiac transplantation in idiopathic dilated cardiomyopathy: the Washington DC dilated cardiomyopathy study. J Nat Med Assoc 1998;90: 342-8. [PMC free article] [PubMed] [Google Scholar]