Abstract
Endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) has evolved to become an indispensable tool for tissue acquisition in patients with luminal and extra luminal gastrointestinal cancers. Despite the extensive use of EUS-FNA, there still exists a wide variation in the number of samples required to ensure acquisition of diagnostic material from different kind of lesions. There are several factors that may influence the number of fine needle passes made during EUS-FNA, but the main factor seems to be the presence of a Cytopathologist during the EUS procedure. The diagnostic yield of EUS-FNA with rapid on-site evaluation (ROSE) in most studies exceeds 90%. Nevertheless, ROSE is not available in many centers. Various studies have investigated the adequate number of needle passes that should be performed if ROSE is not used. Differences exist based on the nature of the target lesion: Five to seven passes for pancreatic masses, three passes for lymphnodes, only one pass for pancreatic cystic lesions. Consider using a core biopsy needle or a 19-G FNA needle for histology could improve the diagnostic yield. Even though EUS-FNA is widely available, some patients still do not receive conclusive diagnoses upon initial EUS-FNA. One way to maximize the benefits for patients might be to centralize cases to several well-equipped, high-volume centers with experienced endosonographers that have universal availability of ROSE.
Keywords: Endoscopic ultrasound, fine needle aspiration, needle passes
INTRODUCTION
Since its introduction in the early 1990s, endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) has emerged as a safe and accurate technique for the diagnosis of various luminal and extra luminal gastrointestinal cancers. Several studies in the last years focused on technical aspects of EUS-FNA like optimal needle choice, variety of sampling methods and different way to collect specimens. However there is no one solution or answer to all our unmet needs in tissue acquisition.
The European Society of Gastrointestinal Endoscopy (ESGE) recently published recommendations on EUS-guided sampling, discussing technical issues related to maximizing the diagnostic yield of EUS-FNA including the adequate number of needle passes that is the main topic of this chapter.[1] However, this guideline may not apply in all situations and should be interpreted in the light of specific clinical situations and resource availability.
Despite the extensive use of EUS-FNA, there still exists a wide variation in the number of samples required to ensure acquisition of diagnostic material from different kind of lesions.
Factors that influence the number of fine needle passes made during EUS-FNA include type, location and sonography characteristics of the lesion; but the main factor seems to be the presence of a cytopathologist during the EUS procedure and level of cytologic expertise available. Rapid on-site evaluation (ROSE) process involves the evaluation of the direct smears obtained at the point of care in the endoscopy suite. The smears are quickly processed and examined by a light microscope in the endoscopy procedure suite with immediate direct feedback provided to the performing endosonographer. This information can assist in guiding the number of EUS-FNA passes required to obtain a final diagnosis. The immediate evaluation assists the endosonographer in knowing whether the aspirates obtained are diagnostic or non-diagnostic. When they are non-diagnostic, additional EUS-FNA can be made in an attempt to obtain cytological diagnosis. When EUS-FNA is performed without ROSE, the endosonographer does not have the pathologic information provided by an immediate assessment and is unaware if the aspirates obtained are adequate to yield a definitive diagnosis.
It has been 14 years since the publication of the initial reports of the evaluation of the number of passes needed to obtain a diagnostic yield and the significance of on-site cytopathology during the procedure. In an article in 2003, Klapman et al. in their study have reported that when an attending pathologist was not available for bedside interpretation, results were inconclusive in 48% of cases compared with 23% when ROSE was available.[2] During this time, many studies have examined the use of ROSE for EUS-FNA biopsy of solid masses of the pancreas and other anatomic lesions showing that the presence of a cytopathologist during EUS-FNA improves the diagnostic yield and decreases the number of inadequate or unsatisfactory samples and limits the number of passes required to establish a diagnosis.[3] The diagnostic yield of EUS-FNA with ROSE in most studies exceeds 90%.[1] Moreover, structured literature reviews and recent meta-analysis have concluded that ROSE improves diagnostic accuracy with a significant clinical impact.[4,5] ESGE technical guideline state that ROSE provides a highly reliable diagnosis with an excellent agreement with the final cytopathological diagnosis.[1]
Nevertheless, ROSE is not available in many centers due to excess time commitment, limited resources, relatively low reimbursements. By calculating a representative salary for an attending pathologist, time required for bedside interpretation and medicare compensation rates, Layfield et al. demonstrated that 40-50 USD are lost by the institution per procedures.[6] Consequently, financial consideration drive many centers to employ options other than ROSE to support EUS-FNA. In some cases, when Cytopathologist is not available, gross inspection and ROSE are performed by the endosonographer but, when compared with cytotechnician ROSE, it has been shown to be poor (in the diagnosis of malignancy 69-72% for the endosonographers vs. 89% for a cytotechnician; P = 0.001).[7] At many EUS centers, a cytology technician supports EUS-FNA during the procedure, however there have been few data published on this topic. In this field, it has been demonstrated that systematic training of technicians by the attending Cytopathologist specifically in EUS-FNA interpretation, dramatically improves their efficacy in this important role.[8]
In this setting, we have to remember that the importance of an experienced endosonographer also should not be underestimated. Diagnostic yield may vary significantly among endosonographers. A multicenter retrospective review of 1075 patients suggested that the diagnostic yield of malignancy in solid pancreatic masses could be used as benchmarks for quality performance measurement, given its high pre-test probability. A final cytologic diagnosis of malignancy was made in 71% of solid pancreatic masses and it was suggested that endoscopists with a diagnostic rate <52% likely need performance reassessment.[9] Nonetheless, it is reasonable to assume that endoscopists performing a high volume of FNAs are likely to have more success because the procedures is highly operator-dependent with significant inter operator variability.[10]
HOW MANY PASSES SHOULD BE PERFORMED IF ROSE IS NOT AVAILABLE?
Various studies have investigated the adequate number of needle passes that should be performed if ROSE is not used. Differences exist based on the nature of the target lesion. Discordant conclusion has been reached for solid masses, whereas more concordant results have been reported for lymphnodes, liver lesions and pancreatic cysts.
We are going to analyze the adequate number of needle passes to be performed to reach a good diagnostic accuracy, considering the different target lesion.
Pancreatic masses
The 22-G or 25-G needle is most commonly used for cytologic sampling of pancreatic masses. Multiple studies have compared the cytologic yield of FNA for 22-G and 25-G needles showing a similar overall diagnostic accuracy.[11,12] However there was a trend towards better performance with the 25-G needle for FNA of pancreatic head-uncinate masses. In a recent meta-analysis that compared the 25-G and 22-G needles for EUS-FNA of pancreatic messes, the diagnostic sensitivity of the 25-G needle was significantly better than that of the 22-G needle [Figure 1].[13]
Figure 1.
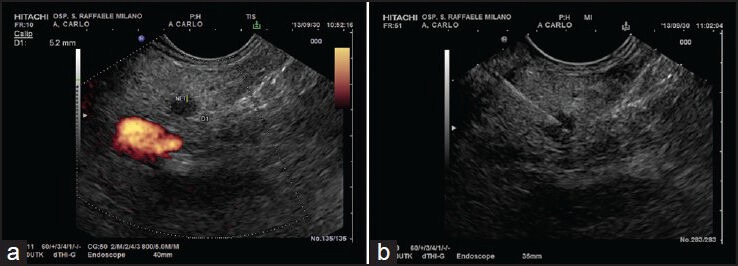
(a-b) A small hypoechoic lesion of pancreatic body. The lesion was biopsied with a 25G needle and cytology was positive for neuroendocrine tumor
The initial reports of the evaluation of the number of passes for diagnosis of pancreatic malignancies are published in the early 2000s. A study by Erickson et al. showed in a large study that without a cytopathologist in attendance, 5-6 passes should be made for pancreatic masses and that well-differentiated pancreatic adenocarcinoma required a higher number of passes as compared to moderately and poorly differentiated tumours.[14] LeBlanc et al. found that the sensitivity gradually increased from 17% for the first pass to 87% when more than seven passes were performed, concluding that during EUS-FNA at least seven passes with a 22-G needle into pancreatic masses are needed to ensure a high degree of certainty for making a correct diagnosis.[15]
A more recent prospective study, re-evaluate the optimal number of needle passes with a 25-G needle for solid pancreatic lesions, suggesting that four needle passes may be sufficient for providing adequate cellularity [Figure 2].[16]
Figure 2.
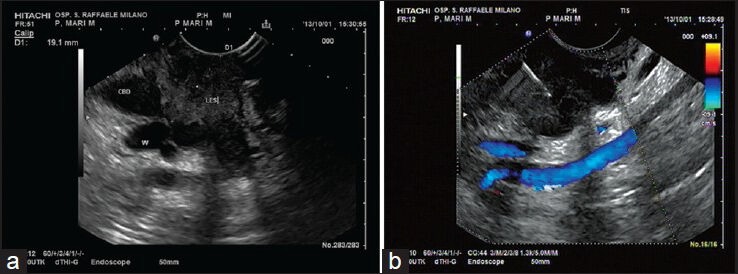
(a-b) A focal hypoechoic lesion (les) of pancreatic head conditioning dilation of both common bile duct (CBD) and pancreatic duct (W). The FNA performed with a 25G needle was positive for pancreatic adenocarcinoma
Turner et al. reported in a large cohort of 559 patients with a pancreatic mass (including pancreatic adenocarcinoma and pancreatic neuroendocrine tumors) that diagnostic accuracy of about 80% could be obtained with only 2-3 needle passes.[17] However ROSE was available in about 44% of cases and when the authors analyzed the population excluding patients with neuroendocrine tumors (40 patients), immediate cytopathologic evaluation significantly influenced the diagnosis (P = 0.0001). In another retrospective study a high yield with a mean of 1.88 needle passes was described, in which material was obtained with a 22-G needle and evaluated at first for the presence of small tissue core samples that were placed in formalin for histopathological examination and the rest of the material was sent for cytopathological analysis.[18] The authors showed that in pancreatic mass lesions, with only a few needle passes, adequate material for both histology and cytology can be gained in about 90% of lesions. However when they compared the histology evaluation alone with cytology evaluation alone, histology did not yield better overall sensitivity.
Several studies in the last years focused on technical aspects of EUS-core biopsy needle in order to obtain more tissue. A fine needle biopsy (FNB) specimen contains core tissue with better preservation of cellular architecture than an aspirate. Therefore, in general, a FNB specimen should have greater diagnostic accuracy and provides more tissue for ancillary testing than a typical FNA sample. In this setting, FNB may offset the limitations of FNA wherein the diagnostic sensitivity is incumbent on the availability of an onsite cytopathologist. Two prospective studies on EUS-guided biopsy of pancreatic masses have shown that by using a dedicated core biopsy needle or a 19-G needle, a satisfactory histological assessment can be made in about 90% of patients.[19,20]
However, the number of dedicated passes that one has to carry out in order to obtain adequate material is unclear. Indirect evidence suggests that for the evaluation of pancreatic masses, a single pass with a 19-G needle is adequate for diagnosis in 85% of cases.[19,21]
Without a Cytopathologist in endoscopic room, combining EUS-FNA cytology and histology significantly increases the sensitivity for malignancy diagnosis compared with cytology or histology alone (89.9% vs. 68.1% for cytology [P = 0.007] and 60% for histology P < 0.0001).[18] ESGE guidelines suggest implementation of this technique into routine practice.[1]
In conclusion, if on-site cytopathology service is unavailable, the following measures must be considered: Perform at least five to seven passes for pancreatic masses; rather than sampling the same area over and over, adapt the “fanning” technique to procure better quality specimens; consider using a core biopsy needle or a 19-G FNA needle for histology; develop open and frequent communication with the cytopathologist.[22]
Pancreatic cystic lesions (PCL)
There are many suggested algorithms on the management of PCL. Once a PCL is identified, the key clinical issue becomes diagnosis of the cyst type, at the least categorizing the cyst as benign, premalignant or malignant, to guide subsequent management. EUS-FNA can be valuable because of its ability to evaluate viscosity, cytology, chemistry and tumor markers. As such, the American Society of Gastrointestinal Endoscopy support the use of FNA for pancreatic cyst diagnosis.[23]
Much emphasis is placed on the size and the morphology of the PCLs. Cyst size is often the most important determinant of success for cyst aspiration and acquisition of adequate fluid for analysis.
Walsh et al. conducted a study to determine whether cyst size or cyst location predicted success of cyst fluid collection and analysis.[24] It was concluded that successful aspiration of cysts was independent of cyst location in the pancreas and that the larger the cyst, the larger number of diagnostic variables (cytology, carcinoembryonic antigen, amylase) were able to be obtained. The investigators stated that a minimum cyst size of 1.5 cm was needed to successfully result in at least one variable with an 84% success rate. The authors agree with this threshold of 1.5 cm and endorse FNA of pancreatic cysts 1.5 cm or larger. FNA should be performed with a 19-G or 22-G needle; only one pass should be performed and the cyst fluid should be aspirated until the cyst collapses, to prevent infectious complications [Figure 3].[25]
Figure 3.
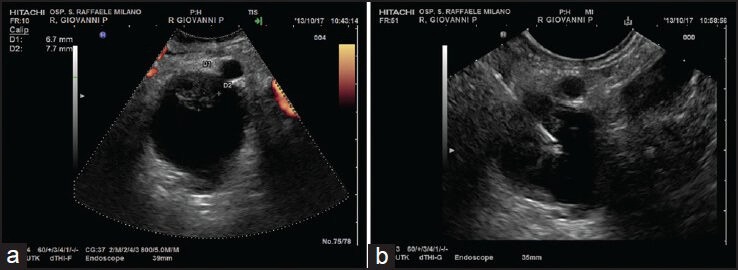
(a-b) A cystic lesion of the body of the pancreas with mural nodule targeted. FNA was performed with a 22G needle
If focal nodules, thick septations and adjacent masses are present, they should be targeted for aspiration and cytologic examination.[26] For pancreatic cyst with solid component, repeat needle passes might be considered in attempts to increase the diagnostic yield. In a recent multicenter Asian study, considering pancreatic cysts with a solid component, the diagnostic yield of EUS-FNA increased significantly from 44% with one pass to 78% with more than one pass (P = 0.01). In the absence of a solid component, the diagnostic yield was 29% with one pass and was not significantly different from diagnostic yield obtained with more than one pass.[27]
Lymph nodes
EUS-FNA allows accurate determination of the nature of lymph nodes of unknown origin. Lymphnodes generally required a lower number of needle passes to obtain an adequate diagnostic accuracy. Different studies agreed that three needle passes were sufficient.[14,15,28]
In a series of 104 patients with mediastinal and/or abdominal lymphadenopathy of unknown origin, a specimen adequate for histopathological evaluation was obtained in all cases using a 19-G needle. Among 50 patients with a diagnosis of lymphoma, subtyping was possible in 88% of cases.[1,29]
Submucosal tumors
EUS is currently the most accurate imaging technique for submucosal lesions.[30] Some studies were performed to determine how many passes are needed for accurate diagnosis. In submucosal tumors, the accuracy of EUS-FNA increased gradually with each consequent pass to reach a plateau after the fourth pass [Figure 4].[1]
Figure 4.
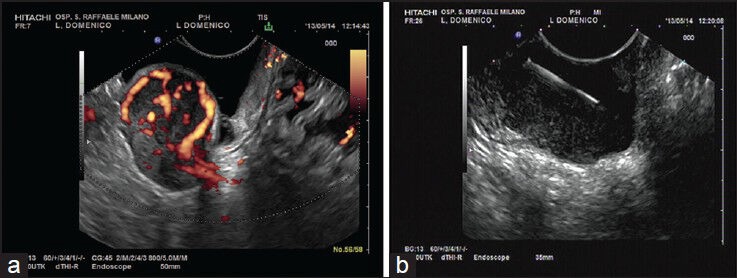
(a-b) A submucosal lesion of the gastric wall. Eus findings were suggestive for gastrointestinal stromal tumour, confirmed by cytology
In a large retrospective study a total of 112 patients underwent EUS-FNA for evaluation of upper gastrointestinal subepithelial lesions. EUS-FNA sampling was diagnostic in 61.6% and suspicious in 22%, leading to an overall diagnostic yield of 83.9%. This high diagnostic yield was obtained with a mean of 5.3 FNA passes. However all procedures were performed with in-room cytopathology assistance.[31] In a Japanese study on 141 patients, a mean of 2.5 ± 0.7 passes with 22-G needle were performed, with an overall rate of sample adequacy of 83% that was significantly better for lesions >2 cm than for those with a smaller diameter. Even in this study the slides were reviewed immediately by the cytopathologist to ensure specimens adequacy.[32] Multivariate analysis showed that a heterogeneous echo pattern of the lesion was the only independent predictor for obtaining a sufficient sample by EUS-FNA (P = 0.002). Other factors such as the size of the mass, number of needle passes were not significant.
Comparative study are lacking, but studies that used EUS-FNA histology alone or combined with EUS-FNA cytology reported a higher diagnostic yield than studies that relied only on cytopathological preparations.[1]
Miscellaneous and liver lesions
The number of needle passes recommended for miscellaneous and liver lesions are similar to those recommended by different authors for pancreatic masses and lymphnodes, respectively.[1]
LeBlanc et al. found that for miscellaneous lesions the sensitivity of EUS-FNA increased from 33% up to 92% after seven passes and did not change with additional passes.[15] For liver lesions, Erickson et al. suggested a good diagnostic accuracy with 2-3 needle passes, a number which is in agreement with other studies.[1,14]
Concluding, it is interesting to analyze a recent survey to better understand the potential difference between the results of published studies and daily clinical practice that was highlighted in a most interesting survey of 161 participants at the 13th international live course of EUS held in Amsterdam. A two page questionnaire was developed for the study. It contained 30 questions that pertained to demographics and the current practice for EUS-FNA of responders, including sampling technique, sample processing, cytopathological diagnosis and sensitivity of EUS-FNA for the diagnosis of solid mass lesions. About 57% of the participants answered the questions and 37.7% of the endosonographers reported a sensitivity for the diagnosis of solid mass lesions >80%. Self-reported sensitivity of EUS was 60-80% in further 37.7% of respondents and only <60% in about 25%. Low EUS-FNA sensitivity was associated with unavailability of ROSE, few needle passes, absence of micro core isolation and low hospital caseload.[33] Very similar results were reported in a survey of 142 EUS centers in Germany. The self-reported diagnostic yield of EUS-FNA was assessed to be >75% in only 48% of hospitals and lower than 50% in 15%.[34]
We can state, as literature has shown, that even though EUS-FNA is widely available nationally and internationally, some patients still do not receive conclusive diagnoses upon initial EUS-FNA. One way to maximize the benefits for patients might be to centralize cases to several well-equipped, high-volume centers with experienced endosonographers that have universal availability of ROSE.[35,36]
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Polkowski M, Larghi A, Weynand B, et al. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190–206. doi: 10.1055/s-0031-1291543. [DOI] [PubMed] [Google Scholar]
- 2.Klapman JB, Logrono R, Dye CE, et al. Clinical impact of on-site cytopathology interpretation on endoscopic ultrasound-guided fine needle aspiration. Am J Gastroenterol. 2003;98:1289–94. doi: 10.1111/j.1572-0241.2003.07472.x. [DOI] [PubMed] [Google Scholar]
- 3.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, et al. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–10. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 4.Hébert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology. 2013;24:159–71. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Yang R, Lu Y, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesion: A systematic review. J Cancer Res Clin Oncol. 2012;138:1433–41. doi: 10.1007/s00432-012-1268-1. [DOI] [PubMed] [Google Scholar]
- 6.Layfield LJ, Bentz JS, Gopez EV. Immediate on-site interpretation of fine-needle aspiration smears: A cost and compensation analysis. Cancer. 2001;93:319–22. doi: 10.1002/cncr.9046. [DOI] [PubMed] [Google Scholar]
- 7.Savoy AD, Raimondo M, Woodward TA, et al. Can endosonographers evaluate on-site cytologic adequacy? A comparison with cytotechnologists. Gastrointest Endosc. 2007;65:953–7. doi: 10.1016/j.gie.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Petrone MC, Arcidiacono PG, Carrara S, et al. Does cytotechnician training influence the accuracy of EUS-guided fine-needle aspiration of pancreatic masses? Dig Liver Dis. 2012;44:311–4. doi: 10.1016/j.dld.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Savides TJ, Donohue M, Hunt G, et al. EUS-guided FNA diagnostic yield of malignancy in solid pancreatic masses: A benchmark for quality performance measurement. Gastrointest Endosc. 2007;66:277–82. doi: 10.1016/j.gie.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Weston BR, Bhutani MS. Optimizing diagnostic yield for EUS-Guided sampling of solid pancreatic lesions: A technical review. Gastroenterol Hepatol (N Y) 2013;9:352–63. [PMC free article] [PubMed] [Google Scholar]
- 11.Fabbri C, Polifemo AM, Luigiano C, et al. Comparative study of EUS-guided 25 gauge needle versus EUS-guided 22 gauge needle for FNA in patients with solid pancreatic masses: Preliminary results. Dig Liver Dis. 2009;41:S60–1. [Google Scholar]
- 12.Siddiqui UD, Rossi F, Rosenthal LS, et al. EUS-guided FNA of solid pancreatic masses: A prospective, randomized trial comparing 22-gauge and 25-gauge needles. Gastrointest Endosc. 2009;70:1093–7. doi: 10.1016/j.gie.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 13.Madhoun MF, Wani SB, Rastogi A, et al. The diagnostic accuracy of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of solid pancreatic lesions: A meta-analysis. Endoscopy. 2013;45:86–92. doi: 10.1055/s-0032-1325992. [DOI] [PubMed] [Google Scholar]
- 14.Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–90. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 15.LeBlanc JK, Ciaccia D, Al-Assi MT, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475–81. doi: 10.1016/s0016-5107(03)02863-3. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki R, Irisawa A, Bhutani MS, et al. Prospective evaluation of the optimal number of 25-gauge needle passes for endoscopic ultrasound-guided fine-needle aspiration biopsy of solid pancreatic lesions in the absence of an onsite cytopathologist. Dig Endosc. 2012;24:452–6. doi: 10.1111/j.1443-1661.2012.01311.x. [DOI] [PubMed] [Google Scholar]
- 17.Turner BG, Cizginer S, Agarwal D, et al. Diagnosis of pancreatic neoplasia with EUS and FNA: A report of accuracy. Gastrointest Endosc. 2010;71:91–8. doi: 10.1016/j.gie.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Möller K, Papanikolaou IS, Toermer T, et al. EUS-guided FNA of solid pancreatic masses: High yield of 2 passes with combined histologic-cytologic analysis. Gastrointest Endosc. 2009;70:60–9. doi: 10.1016/j.gie.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 19.Iglesias-Garcia J, Poley JW, Larghi A, et al. Feasibility and yield of a new EUS histology needle: Results from a multicenter, pooled, cohort study. Gastrointest Endosc. 2011;73:1189–96. doi: 10.1016/j.gie.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 20.Varadarajulu S, Bang JY, Hebert-Magee S. Assessment of the technical performance of the flexible 19-gauge EUS-FNA needle. Gastrointest Endosc. 2012;76:336–43. doi: 10.1016/j.gie.2012.04.455. [DOI] [PubMed] [Google Scholar]
- 21.Ramesh J, Bang JY, Hebert-Magee S, et al. Multi-center randomized trial comparing the 19G and 25G needles for EUS-guided FNA of solid pancreatic mass lesions. Gastrointest Endosc. 2013;77:1022. doi: 10.1016/j.gie.2012.03.1392. (Abstract supplement) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varadarajulu S, Jhala NC. Cytopathology: A dying art or something that a gastroenterologist should know? Gastrointest Endosc. 2012;76:397–9. doi: 10.1016/j.gie.2012.04.461. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson BC, Baron TH, Adler DG, et al. ASGE guideline: The role of endoscopy in the diagnosis and the management of cystic lesions and inflammatory fluid collections of the pancreas. Gastrointest Endosc. 2005;61:363–70. doi: 10.1016/s0016-5107(04)02779-8. [DOI] [PubMed] [Google Scholar]
- 24.Walsh RM, Zuccaro G, Dumot JA, et al. Predicting success of endoscopic aspiration for suspected pancreatic cystic neoplasms. JOP. 2008;9:612–7. [PubMed] [Google Scholar]
- 25.Lee LS, Saltzman JR, Bounds BC, et al. EUS-guided fine needle aspiration of pancreatic cysts: A retrospective analysis of complications and their predictors. Clin Gastroenterol Hepatol. 2005;3:231–6. doi: 10.1016/s1542-3565(04)00618-4. [DOI] [PubMed] [Google Scholar]
- 26.Samarasena JB, Nakai Y, Chang KJ. Endoscopic ultrasonography-guided fine-needle aspiration of pancreatic cystic lesions: A practical approach to diagnosis and management. Gastrointest Endosc Clin N Am. 2012;22:169–85. doi: 10.1016/j.giec.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Lim LG, Lakhtakia S, Ang TL, et al. Factors determining diagnostic yield of endoscopic ultrasound guided fine-needle aspiration for pancreatic cystic lesions: A multicentre Asian study. Dig Dis Sci. 2013;58:1751–7. doi: 10.1007/s10620-012-2528-2. [DOI] [PubMed] [Google Scholar]
- 28.Wee E, Lakhtakia S, Gupta R, et al. Endoscopic ultrasound guided fine-needle aspiration of lymph nodes and solid masses: Factors influencing the cellularity and adequacy of the aspirate. J Clin Gastroenterol. 2012;46:487–93. doi: 10.1097/MCG.0b013e31824432cb. [DOI] [PubMed] [Google Scholar]
- 29.Yasuda I, Tsurumi H, Omar S, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy for lymphadenopathy of unknown origin. Endoscopy. 2006;38:919–24. doi: 10.1055/s-2006-944665. [DOI] [PubMed] [Google Scholar]
- 30.Hwang JH, Rulyak SD, Kimmey MB American Gastroenterological Association Institute. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology. 2006;130:2217–28. doi: 10.1053/j.gastro.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 31.Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009;69:1218–23. doi: 10.1016/j.gie.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 32.Mekky MA, Yamao K, Sawaki A, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010;71:913–9. doi: 10.1016/j.gie.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 33.Dumonceau JM, Koessler T, van Hooft JE, et al. Endoscopic ultrasonography-guided fine needle aspiration: Relatively low sensitivity in the endosonographer population. World J Gastroenterol. 2012;18:2357–63. doi: 10.3748/wjg.v18.i19.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dietrich CF, Jenssen C. Endoscopic ultrasound-guided sampling in gastroenterology: European society of gastrointestinal endoscopy technical guidelines. Endosc Ultrasound. 2013;2:117–22. doi: 10.7178/eus.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki R, Lee JH, Krishna SG, et al. Repeat endoscopic ultrasound-guided fine needle aspiration for solid pancreatic lesions at a tertiary referral center will alter the initial inconclusive result. J Gastrointestin Liver Dis. 2013;22:183–7. [PubMed] [Google Scholar]
- 36.Collins BT, Murad FM, Wang JF, et al. Rapid on-site evaluation for endoscopic ultrasound-guided fine-needle biopsy of the pancreas decreases the incidence of repeat biopsy procedures. Cancer Cytopathol. 2013;121:518–24. doi: 10.1002/cncy.21340. [DOI] [PubMed] [Google Scholar]


