Abstract
Tissue acquisition plays a key role before treatment decision in most of oncological pathologies but also in several benign diseases. By offering tissue sampling, endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) has become an essential tool in the diagnostic processes. One of the reasons for the success of the technique is related to its excellent diagnostic performance. The diagnostic accuracy of EUS-FNA is above 80% for most of the usual indications. These performances are however dependent on some factors related to both the disease and patient's medical history but also related to medical staff expertise. Endoscopist needs to know how to reach a lesion but also how to efficiently acquire good tissue samples. This review aims to report general recommendations available in the literature for high quality EUS-FNA. Sample processing and sample interpretation also influence diagnostic accuracy of FNA. This paper includes a discussion on sample processing and benefits of the on-site pathology examination. It also provides the results reported in the literature of sample adequacy and diagnostic performance of EUS-FNA for most common indications: Pancreatic diseases, sub-mucosal lesion, mucosal thickenings, lymph nodes, cystic lesion and free fluids.
Keywords: Adequacy, diagnostic performance, endoscopic ultrasound-guided fine-needle aspiration, lymph nodes, pancreas, rapid on-site evaluation, sample quality, sub-mucosal tumor
INTRODUCTION
Performing a “good” fine-needle aspiration (FNA) during an endoscopic ultrasound (EUS) consists of obtaining tissue or liquid in order to provide a proof of disease or to help excluding one. Beside the importance for the workup of the patient, this procedure is associated with increased costs and it is of paramount importance that adequate material is obtained at the initial procedure. The success for the quest of adequate material depends on several factors [Table 1]. Many steps need to be overcome to get a helpful result. These can be divided in several categories.
Table 1.
Factors influencing performance of endoscopic ultrasound-guided fine-needle aspiration
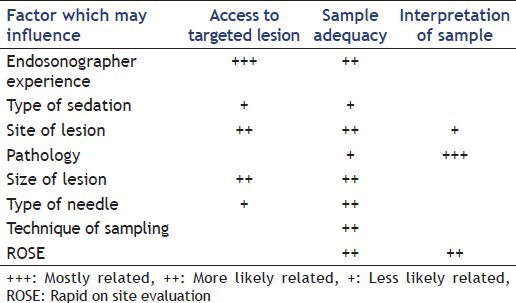
ACCESS TO THE LESION
The first step is to access the targeted lesion. It is highly dependent on the endoscopist's experience[1,2] in identifying an abnormal structure but also on some factors related to the lesion or the patient itself. Recent publications have demonstrated that the limits for accessing a lesion are currently further pushed forward. As an example, think to publication on lesion approach in case of altered anatomy[3] or for sites such as the right adrenal gland for which access was previously considered to be difficult.[4]
Once a lesion is reached and visualized, the needle insertion route has to be evaluated. Distance or vascular interposition may sometimes jeopardize the lesion access. Scope insertion of the needle in an adequate position for puncture is generally not a problem above the pylorus, the distal end of the scope being relatively straight. Problems to take out the needle tip may appear when a lesion is targeted in a trans-duodenal route. This may be overcome by shortening the scope from the second duodenum, keeping in mind that position is more unstable. Nevertheless, in daily clinical practice, the percentage of unreached lesions or needle insertion failure (into the targeted lesion), is extremely low in experienced hands.
Finally, diagnostic yield (the likelihood that a test will provide the information needed to establish a diagnosis) of EUS-FNA and accuracy, are also dependent on the way the material is processed after the extraction of the needle up to the microscope. That part is generally on the responsibility of the pathologist. However, many endoscopic unit do not benefit, in the EUS room, on the presence of pathologist for rapid on site evaluation (ROSE) of the material or even a technician to start that part of the processing.
SAMPLE QUALITY
The second challenge is retrieving adequate material from the targeted lesion with maximum productivity through performing a minimum number of passes (number of re-insertion of the needle) which reduces time and risk of the procedure. Sample quality increases the diagnostic yield of FNA and reduces the number of passes particularly when ROSE is available. It is directly related to adequacy in cellularity and inversely related to the bloodiness of the sample. Adequacy is a more direct measure of sampling performance than diagnostic yield or accuracy, which are more related to patient's outcomes. Adequacy per sample was reported by several studies and varies between 57% and 87% [Table 2]. A weak adequacy can be improved by increasing the number of passes that does however also prolong procedure time. The sampling adequacy per lesion is reported often above 90% [Table 2]. Factors influencing adequacy per lesion are numerous. Endosonographist experience, beside is influence on being able to access the lesion, have been shown to influence sampling quality by reducing number of needed pass.[1,5] Over the last decade, many papers have addressed the influence of needle choice and needle utilization on sample adequacy or bloodiness. Please refer to the dedicated articles in this special issue for a further discussion of this topic. Results concerning lesion size influence on adequacy show conflicting results.[6,7,8] A dedicated comparative study on lesion size influence on specimen adequacy and diagnostic yield showed that, for group of mixed pathology, a lower number of passes was needed to get adequate sample for lesion inferior to 25 mm compared to larger lesion.[8] Both other publications showed direct correlation between size and adequacy. This discrepancy in results may be related to the variable importance of central necrosis that increases with malignant lesion size. Inserting a needle into necrosis is generally associated with inconclusive results. This is probably the fundament of new recommendation when performing FNA on large lesion. Fanning consist in modifying angle of needle insertion into the lesion, by changing either elevator position, or great wheel rotation, in order to sample tissue from different sector of the lesion [Figure 1]. “Fanning technique” have shown to be associated with fewer passes for same diagnostic yield compared to “one direction” puncture.[9]
Table 2.
Adequacy of sampling (per lesion or per pass) reported in the literature
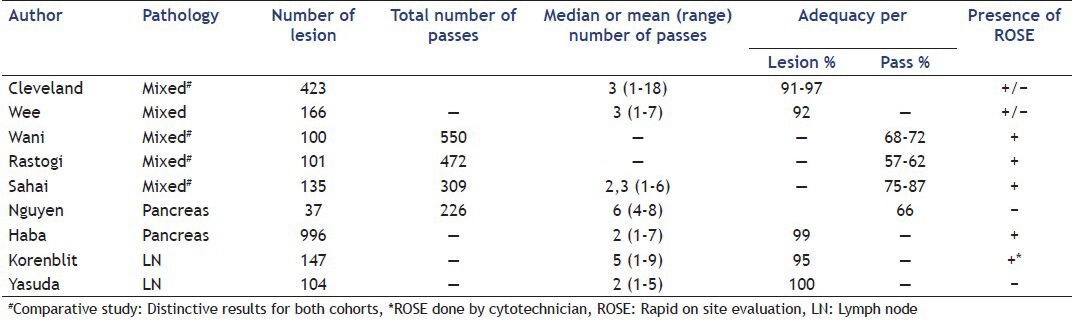
Figure 1.
Endoscopic ultrasound-guided fine-needle aspiration of pancreatic lesion using Fanning technique
The number of passes made will vary, depending on the pathology and of lesion type but also on whether immediate (or “rapid”) on site cytologic evaluation is performed. When ROSE is not available, guide lines recommend performing three needle passes for lymph nodes (LNs) and liver lesions, at least five needle passes for solid pancreatic masses and a single pass for pancreatic cysts.[10] Even when ROSE is available, it is often still practical and more efficient to take 2-3 passes more before consulting the pathology findings.
SAMPLE PROCESSING
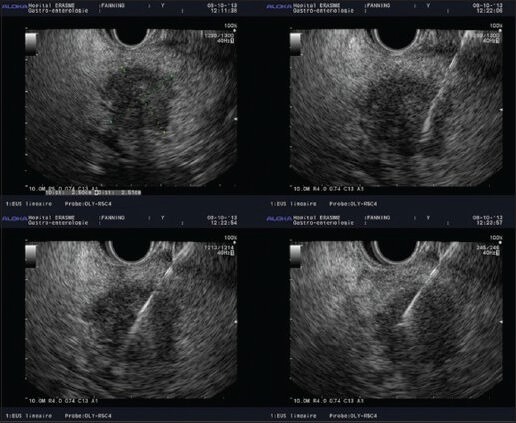
Finally, diagnostic yield (the likelihood that a test will provide the information needed to establish a diagnosis) of EUS-FNA and accuracy, are also dependent on the way the material is processed after the extraction of the needle up to the microscope. That part is generally on the responsibility of the pathologist. However, many endoscopic unit do not benefit, in the EUS room, on the presence of pathologist for ROSE of the material or even a technician to start that part of the processing. Procured material after FNA can be processed in different ways. The samples are typically extracted from the needle either by flushing air or by using the more controlled method of stylet reinsertion. The material is then put on glass slides, which are air-dried or fixed with alcohol [Figure 2]. If needed, a part of the material, from the same pass or from following ones, may be preserved for cell block. Several studies have demonstrated an advantage of combining cytological and histological methods for better accuracy.[11]
Figure 2.
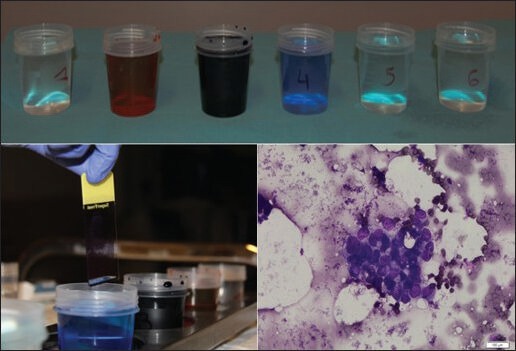
Rapid on-site evaluation sample preparation (with courtesy of Pr. Pieter Demetter, Erasme Hospital, ULB, Brussels, Belgium)
ROSE VERSUS NON-ROSE
Theoretically, the following advantages can be associated to the presence of a pathologist in the endoscopic suite during FNA:
Increase in sample processing quality from the beginning.[12]
Improvement in communication between specialty and particularly clarify for the pathologist the clinical issue of the results.
Faster information about the adequacy of the material (ROSE) [Figure 2].
Whereas the first two goals can be reached by a systematic procedure that puts in place an adequate training of the endoscopic team and perfect communication, ROSE requires an expertise generally not available within the endoscopic community. Even the expertise of cytotechnicians has been shown to be inferior to that of cytopathologists for tissue specimen adequacy assessment.[13] Intra procedural feedback on the adequacy of sampling may reduce procedure time and risk by minimizing needle passes. The potential request of the pathologist during the procedure for additional samples for immunochemistry or flow cytometry (FC) may also have a great clinical impact.
If no prospective study had compared impact of ROSE on specimen adequacy and on diagnostic yield, a lot of papers are in favor of ROSE implementation based on retrospective study. A recent metaanalysis on 34 distinct studies on EUS-FNA, with a pooled sensitivity of 88.6% for pancreatic cancer diagnosis, identified ROSE as a significant determinant of better EUS-FNA accuracy.[14] Another recent metaanalysis on five retrospective studies on pancreatic masses showed an improvement in specimen adequacy of EUS-FNA but no impact on diagnostic yield.[15] The improvement in adequacy was most convincing when ROSE was performed by pathologists and at centers where the initial adequacy rates (without ROSE) were low.
Future prospective studies should focus on the potential benefit of ROSE for situations with lower initial sample adequacy (i.e., pancreatic mass in chronic pancreatitis (CP), sub-mucosal tumor [SMT]); this approach ought limit the issue of pathologist availability in some institution to challenging situation.
PERFORMANCE OF EUS-FNA ACCORDING TYPE OF PATHOLOGY
Adequacy of sample, but more generally diagnostic accuracy, has been reported with variable results for the different indications of EUS-FNA. In the next chapter we are going to classify the evidence we have according to site of different organs as well as different pathological conditions. Comparisons of technique performance between different reports has to be done with caution due to variable categorization of “suspicious” and “atypical” pathological interpretation for calculation of performance results.
Solid lesions
Pancreas
Pancreatic masses
A review of 28 studies on EUS-FNA performance in pancreatic mass reports a good median accuracy for pancreatic neoplasia of 88% (65-96%) despite a low median negative predictive value (NPV) of 72% with a wide range from 16% to 92%.[16] A recent paper on 996 pancreatic lesion identified several factors to be related to EUS-FNA accuracy for cancer detection within an Asian population using multivariate analysis.[11] These were histological final diagnosis, location of lesion, lesion size, availability of on-site cytopathological evaluation and endosonographer experience. The diagnostic accuracy for neuroendocrine tumor (NET) was in this paper significantly lower than for adenocarcinoma (ADC) (76.8% vs. 92.7% respectively). Another cause of lowered accuracy within occidental populations is the presence of underground CP. This is a consequence of reduced ability to identify suspicious mass between inflammatory pseudomasses and calcifications, but also the more difficult cytological evaluation of pancreatic tissue in the setting of chronic inflammation. In a prospective series of 207 patients, EUS-guided FNA had a sensitivity 89% in those with a focal pancreatic lesion with normal surrounding parenchyma, whereas the sensitivity in patients with CP was significantly lower (54%).[17] In another retrospective cohort trial, a false positive rate of 11% ADC in pancreatic lesion was reported with all of those cases in a context of CP.[18]
New techniques including contrast-enhanced EUS and elastosonoendoscopy may be of some interest to increase this low NPV of EUS-FNA particularly in CP. This will be discussed elsewhere in this journal. In addition, in order to increase the FNA accuracy for cancer identification additional techniques can be used. The advantage of fanning was already discussed and was demonstrated in a prospective comparative study on patients with pancreatic mass.[9] Suspicious LNs or suspected liver metastasis detected during a EUS for suspected pancreatic cancer have to be our first step for puncture. Firstly because it confirms a more advanced pathology but also because adequacy per sample seems to be higher.[19] Type of sedation may also influence diagnostic yield of pancreatic EUS-FNA. In a retrospective study on 371 patients it was shown that using general anesthesia (either with or without intubation) increased significantly the rate of cytological diagnosis compared to conscious sedation.[20] Finally, in cases with a high suspicion of cancer and previous inconclusive or negative FNA findings, studies have shown a benefit in increasing final number of true positive results when procedure is repeated.[21,22]
Pancreatic NET
As already, mentioned, diagnostic accuracy for NET could be lower than for ADC as reported at least in two large series (68% and 77%).[11,23] In a series of 68 patients with confirmed NET after surgery, the sensitivity of EUS-FNA for the diagnosis was 87% (95% CI: 76-93%). The sensitivity of EUS-FNA was similar for patients with functional and non-functional NET. However, the sensitivity of EUS-FNA was higher for malignant NET.[24]
Pancreatic metastasis
Clinically apparent metastases to the pancreas are not exceptional: 0.73% in series over 2000 pancreatic masses.[25] While lung cancer was previously the principal site of origin for these lesions, renal carcinoma now seems to become a frequent diagnosis in EUS-FNA series.[26] Metastatic renal cell carcinoma of clear cell type within the pancreas raises a number of difficult differential diagnostic considerations and is easily confused with ductal carcinoma of the pancreas. Renal cell carcinoma can be present in the pancreas as a solitary mass and it also may demonstrate its initial metastasis only years after the initial diagnosis of the primary tumor. This further increases the diagnostic challenge.[25] Reports dealing with the FNA diagnostic yield are limited but on a small series, the EUS-FNA sampling adequacy seems to be similar compared to primitive tumors of the pancreas.[27]
Autoimmune pancreatitis (AIP)
Data on EUS-FNA or FNB in patients with AIP are limited. The FNA sample needs to contain a micro-core enabling the pathologist to identify criteria for AIP. In fact, the diagnosis of AIP is based on a cluster of clinical, radiological and histological arguments as described in the International Consensus Diagnostic Criteria.[28] Several papers have reported, using retrospective studies, that the sensitivity for AIP ranges from 43% to 80% for standard FNA.[29,30] However, if non-suspected, FNA of pancreatic mass in a context of AIP often leads to atypical cytopathological interpretation but also non-rarely to suspicion for malignant process.[31]
SMT
The adequacy of cytological material sampled with EUS-FNA in SMT is known to be lower than for puncture of pancreatic mass or LNs. Other sampling techniques are therefore recommended as valuable alternatives.[10] Based on cytologic examination and immunohistochemical staining (IHC) recent studies reported a wide spectrum of diagnostic yield ranging from 34% to 90%.[10] When cases of IHC failure but with conclusive cytologic or histologic examination are added, diagnostic yield rose above 70%. The largest recent retrospective study on upper gastrointestinal (GI) tract SMT perfectly illustrates these results showing a sensitivity of only 45% when diagnosis rely on IHC and increased by 37% when diagnosis is based only on pathological aspect.[32] However, as in other papers, when sampling is adequate for pathological interpretation, diagnostic accuracy is excellent (95%). Several factors that could increase sampling adequacy were reported: Larger size, gastric location and heterogeneous aspect of the lesion.[32,33]
Other current abdominal solid masses
EUS-FNA of adrenal glands is generally used as a confirmation procedure of metastatic disease of lung cancer even if a recent study suggests a more systematic use as its accuracy for the diagnosis of adrenal metastasis is at least as good as computed tomography (CT) or positron emission tomography-CT (PET-CT).[4] Access to the right adrenal gland seems to be less a problem than previously thought.[4] In a study of 85 patients with lung cancer and a left adrenal mass, inadequate or inconclusive sample was reported in only 5.9% of cases and EUS-FNA sensitivity and NPV were 86% and 70% respectively.[34]
EUS-FNA of liver parenchyma have been demonstrated to be safe in cases of unobstructed bile duct.[35] In this context, solid liver lesions may be adequately sampled by EUS-FNA as suggested by a prospective study on 41 patients with contributory results of 98%.[36] Combining cytology and histology led to a sensitivity and NPV for the diagnosis of malignancy of 94% and 78%, respectively. Similar results are reported in retrospective studies (inconclusive sample 4-9%; sensitivity for malignancy 82-94%) which show also that EUS-FNA may detect malignancy in patients with previously negative US or CT examinations.[35,37] A recent report also suggests the systematic use of EUS-FNA in hepatocellular carcinoma detection in high risk subject before liver transplantation.[38]
LNs
LNs are certainly the structure that is most frequently targeted by endosonographists using FNA. Sampling of a LN is generally gainful, firstly because it may have a great impact on patient management, possibly changing disease stage, but also because, compared to FNA on masses, the adequacy of LN FNA is known to be very high with fewer passes.[39] As a function of the study and categorization of “suspicious” result, diagnostic samples are reported in 91-100% of performed procedures.[8,40,41,42] While the presence of a pathologist on site may improve sample adequacy in a retrospective study,[40] no correlation between LN characteristics and cellularity of samples was reported.
LN of unknown origin and lymphoma
Several retrospective studies addressed the question of EUS-FNA diagnostic accuracy for LN of unknown origin. In three retrospective studies, totalizing 476 patients with mediastinal or upper abdominal LN of unknown origin, adequate specimens were reported in 87-100% of cases and accuracy for correct diagnosis in 85-98%.[42,43,44] Prevalence of malignant disease was between 37% and 50% with a large variability for the lymphoma proportion in these series (8-46%).
The diagnostic of neoplasia in the case of lymphoma is already difficult when based on cytological specimens alone. One report has described the yield of EUS-FNA for lymphoma when combined with FC and immunocytochemistry (IC). Overall sensitivity, specificity and accuracy of the EUS-FNA cytology with FC/IC were 74%, 93% and 81%, respectively.[45]
However, sub-classification of the lymphomas on the basis of the cytological findings remained difficult. When focusing on micro-core specimen obtained through the use of 19G needless, EUS-FNA offers the ability to classify the lymphomas in 88% of cases (in a series of 104 patients out of which 48 patients were diagnosed with lymphoma). If possible, current guidelines suggest reserving one specimen for FC, which has been reported to significantly increase the yield for lymphoma diagnosis.[46]
Mediastinal LN in lung cancer
The advent of EUS-guided sampling procedures such as EUS-FNA and endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) has led to significant advances in the mediastinal diagnosis and staging of lung cancer. Endoscopic techniques such as EBUS-TBNA are showing sensitivities exceeding that of mediastinoscopy.[47] The challenge is to choose the most appropriate endoscopic procedures to accurately stage lung cancer in the most cost-effective manner. A partial answer was given by a prospective study of 120 patients with suspected resectable lung cancer on CT. The accuracy of the combined approach using EUS-FNA and EBUS-TBNA was significantly higher than that of PET-CT (90.0% vs. 73.6%; P < 0.0001).[48] A recent meta-analysis analyzing eight studies confirmed a high sensitivity of 0.86 and specificity of 1.00 for EBUS-TBNA plus EUS-FNA in mediastinal nodal staging of lung cancer.[49] Nevertheless, the current guidelines do not recommend EUS-FNA or EBUS-TBNA in negative mediastinal PET-CT for patients with resectable lung cancer.[50] EUS-FNA in restaging purposes after adjuvant chemo/radiation therapy is also not recommended due to a low NPV in exclusion of persistent mediastinal metastases.[51]
Sarcoidosis and tuberculosis (TB)
Mediastinal lymphadenopathy may indicate diseases such as TB or sarcoidosis and it is often difficult to establish a diagnosis when standard medical work-up is inconclusive. In the case of sarcoidosis, this is in particular the case at beginning of the disease when anomalies are mainly limited to mediastinal LN (stages I and II of the disease).
Three prospective studies reported the role of EUS-FNA in mediastinal lymphadenopathy with negative endoscopic investigations and no radiological evidence of lung cancer or other malignancies on CT.[52,53,54] In all three of these series the adequacy of sampling was excellent, ranging from 93% to 98%.
In a cohort of 60 patients with a majority of TB (endemic areas), EUS-FNA showed accuracy for correct diagnosis of 93% based only on cytopathological examination.[52]
The accuracy was also excellent[53] for sarcoidosis with a difference between disease stages I (92%) and II (77%).[54] However, in order to confirm the non-caseating granulomas, a good sensitivity of 86% was acquired only after four needle passes which seems to be a bit more than for other LN diseases.[54]
GI tract lesions
The role of EUS-FNA in evaluating lesions adjacent to the upper GI tract wall is well established. However, this tool is underused in evaluating GI tract lesions, possibly due to insufficient experience and under recognized value of this procedure.
Esophageal and gastric wall thickening
Collective data from previous studies showed a low sensitivity for cancer diagnosis in EUS-FNA for wall thickening for which the sensitivity was around 60%.[10] On the other side, mural trucut biopsies offered higher sensitivity which had been shown in a recent series of 31 patients (esophageal wall [n = 10] and gastric wall [n = 21]), with a sensitivity of 85% for malignant wall thickness. In this case, the sample adequacy was 90%.[55] A similar sensitivity was found in a small group of patients (n = 11) using 19G needles with focus on histological sampling.[56]
Rectal lesions
FNA was proven to be of limited additional value to EUS workup in pre-operative staging of rectal cancer.[57] In contrast, EUS-FNA allowed diagnosing the loco-regional recurrence with anaccuracy above 90%.[58,59]
Pancreatic cystic lesions
The current guidelines advise against the use of mediastinal cyst puncture due to the high risk of iatrogenic infection. Consequently, we limit the following section on pancreatic cyst FNA.[10]
Indication of diagnostic FNA of pancreatic cystic lesion is a debatable matter. If it could change patient management, European guidelines suggest to limit FNA to lesions larger than 2 cm and to perform cytopathological examination and dosage of tumoral markers (i.e., carcinoembryonic antigen [CEA]). However, even if not related to cyst size, a report on 128 pancreatic cyst EUS-FNAs showed that if often technically successful, cytological classifying diagnosis and chemical analysis was obtained in only in 34% and 53% of the cases respectively.[60]
Beyond sample adequacy limitation, accuracy of the technique to distinguish mucinous lesion from pseudocyst and serous cystadenoma will mainly depend on the threshold chosen for the interpretation of chemical analysis results. Up to now, CEA is considered one of the best discriminatory markers for mucinous cystic neoplasm diagnosis. In a pooled analysis of 332 patients from 11 studies, accuracy and NPV of 79% and 75% were obtained for detecting mucinous lesion with CEA concentration in cyst fluid above 800 ng/ml. In the same paper, combined sensitivities of cytologic examination of cyst fluid were reported as 38.3%, 45.4% and 47.7% for serous cystadenoma, mucinous cystadenoma and cystic cancer respectively.[61]
Free abdominal fluid
EUS have a high sensitivity and offer an easy access to collect free abdominal fluid even when available in limited quantity.[62] The efficiency of cytological and histological analyses for spined samples after EUS-guided paracenteses was demonstrated in a retrospective series on 101 patients. It showed a diagnostic accuracy for peritoneal carcinomatosis of 96% and a sensitivity of 80%, including presence of neoplastic cells in samples of small volume.[63]
The role of EUS-guided FNA in the diagnosis of peritoneal carcinomatosis has not been well studied. Few case reports showed the usefulness of EUS-FNA of peritoneal nodules. A recent case series studied 12 patients with undiagnosed ascites. Ten were identified with peritoneal deposits. Cytological examination of EUS-FNA samples from these deposits revealed neoplastic process in six patients and inflammatory cells in 4, out of which 2 with positive polymerase chain reaction for TB.[64]
CONCLUSION
If used by experienced teams (including endosonographist and pathologist), EUS-FNA is a valuable addition to the diagnostic toolset for mediastinal and peri-gastric pathologies particularly within the neoplastic field. Our performance review demonstrated that: (1) The probability to obtain adequate samples is excellent for most common targets provided that an adequate number of passes are executed if ROSE is not available; and (2) The diagnostic accuracy is above 80% for most of the usual indications of the technique.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Eloubeidi MA, Tamhane A. EUS-guided FNA of solid pancreatic masses: A learning curve with 300 consecutive procedures. Gastrointest Endosc. 2005;61:700–8. doi: 10.1016/s0016-5107(05)00363-9. [DOI] [PubMed] [Google Scholar]
- 2.Mertz H, Gautam S. The learning curve for EUS-guided FNA of pancreatic cancer. Gastrointest Endosc. 2004;59:33–7. doi: 10.1016/s0016-5107(03)02028-5. [DOI] [PubMed] [Google Scholar]
- 3.Wilson JA, Hoffman B, Hawes RH, et al. EUS in patients with surgically altered upper GI anatomy. Gastrointest Endosc. 2010;72:947–53. doi: 10.1016/j.gie.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 4.Uemura S, Yasuda I, Kato T, et al. Preoperative routine evaluation of bilateral adrenal glands by endoscopic ultrasound and fine-needle aspiration in patients with potentially resectable lung cancer. Endoscopy. 2013;45:195–201. doi: 10.1055/s-0032-1325988. [DOI] [PubMed] [Google Scholar]
- 5.Harewood GC, Wiersema LM, Halling AC, et al. Influence of EUS training and pathology interpretation on accuracy of EUS-guided fine needle aspiration of pancreatic masses. Gastrointest Endosc. 2002;55:669–73. doi: 10.1067/mge.2002.123419. [DOI] [PubMed] [Google Scholar]
- 6.Sahai AV, Schembre D, Stevens PD, et al. A multicenter U.S. experience with EUS-guided fine-needle aspiration using the Olympus GF-UM30P choendoscope Safety and effectiveness. Gastrointest Endosc. 1999;50:792–6. doi: 10.1016/s0016-5107(99)70160-4. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqui AA, Brown LJ, Hong SK, et al. Relationship of pancreatic mass size and diagnostic yield of endoscopic ultrasound-guided fine needle aspiration. Dig Dis Sci. 2011;56:3370–5. doi: 10.1007/s10620-011-1782-z. [DOI] [PubMed] [Google Scholar]
- 8.Jhala NC, Jhala D, Eltoum I, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy: A powerful tool to obtain samples from small lesions. Cancer. 2004;102:239–46. doi: 10.1002/cncr.20451. [DOI] [PubMed] [Google Scholar]
- 9.Bang JY, Hebert-Magee S, Trevino J, et al. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012;76:321–7. doi: 10.1016/j.gie.2012.03.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumonceau JM, Polkowski M, Larghi A, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011;43:897–912. doi: 10.1055/s-0030-1256754. [DOI] [PubMed] [Google Scholar]
- 11.Haba S, Yamao K, Bhatia V, et al. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J Gastroenterol. 2013;48:973–81. doi: 10.1007/s00535-012-0695-8. [DOI] [PubMed] [Google Scholar]
- 12.Alsohaibani F, Girgis S, Sandha GS. Does onsite cytotechnology evaluation improve the accuracy of endoscopic ultrasound-guided fine-needle aspiration biopsy? Can J Gastroenterol. 2009;23:26–30. doi: 10.1155/2009/194351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen YP, Maple JT, Zhang Q, et al. Reliability of gross visual assessment of specimen adequacy during EUS-guided FNA of pancreatic masses. Gastrointest Endosc. 2009;69:1264–70. doi: 10.1016/j.gie.2008.08.030. [DOI] [PubMed] [Google Scholar]
- 14.Hébert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology. 2013;24:159–71. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt RL, Witt BL, Matynia AP, et al. Rapid on-site evaluation increases endoscopic ultrasound-guided fine-needle aspiration adequacy for pancreatic lesions. Dig Dis Sci. 2013;58:872–82. doi: 10.1007/s10620-012-2411-1. [DOI] [PubMed] [Google Scholar]
- 16.Hartwig W, Schneider L, Diener MK, et al. Preoperative tissue diagnosis for tumours of the pancreas. Br J Surg. 2009;96:5–20. doi: 10.1002/bjs.6407. [DOI] [PubMed] [Google Scholar]
- 17.Fritscher-Ravens A, Brand L, Knöfel WT, et al. Comparison of endoscopic ultrasound-guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. Am J Gastroenterol. 2002;97:2768–75. doi: 10.1111/j.1572-0241.2002.07020.x. [DOI] [PubMed] [Google Scholar]
- 18.Siddiqui AA, Kowalski TE, Shahid H, et al. False-positive EUS-guided FNA cytology for solid pancreatic lesions. Gastrointest Endosc. 2011;74:535–40. doi: 10.1016/j.gie.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 19.Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–90. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 20.Ootaki C, Stevens T, Vargo J, et al. Does general anesthesia increase the diagnostic yield of endoscopic ultrasound-guided fine needle aspiration of pancreatic masses? Anesthesiology. 2012;117:1044–50. doi: 10.1097/ALN.0b013e31826e0590. [DOI] [PubMed] [Google Scholar]
- 21.Eloubeidi MA, Varadarajulu S, Desai S, et al. Value of repeat endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic cancer. J Gastroenterol Hepatol. 2008;23:567–70. doi: 10.1111/j.1440-1746.2007.05119.x. [DOI] [PubMed] [Google Scholar]
- 22.Tadic M, Kujundzic M, Stoos-Veic T, et al. Role of repeated endoscopic ultrasound-guided fine needle aspiration in small solid pancreatic masses with previous indeterminate and negative cytological findings. Dig Dis. 2008;26:377–82. doi: 10.1159/000177025. [DOI] [PubMed] [Google Scholar]
- 23.Turner BG, Cizginer S, Agarwal D, et al. Diagnosis of pancreatic neoplasia with EUS and FNA: A report of accuracy. Gastrointest Endosc. 2010;71:91–8. doi: 10.1016/j.gie.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 24.Pais SA, Al-Haddad M, Mohamadnejad M, et al. EUS for pancreatic neuroendocrine tumors: A single-center, 11-year experience. Gastrointest Endosc. 2010;71:1185–93. doi: 10.1016/j.gie.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Layfield LJ, Hirschowitz SL, Adler DG. Metastatic disease to the pancreas documented by endoscopic ultrasound guided fine-needle aspiration: A seven-year experience. Diagn Cytopathol. 2012;40:228–33. doi: 10.1002/dc.21564. [DOI] [PubMed] [Google Scholar]
- 26.El Hajj II, LeBlanc JK, Sherman S, et al. Endoscopic ultrasound-guided biopsy of pancreatic metastases: A large single-center experience. Pancreas. 2013;42:524–30. doi: 10.1097/MPA.0b013e31826b3acf. [DOI] [PubMed] [Google Scholar]
- 27.Hijioka S, Matsuo K, Mizuno N, et al. Role of endoscopic ultrasound and endoscopic ultrasound-guided fine-needle aspiration in diagnosing metastasis to the pancreas: A tertiary center experience. Pancreatology. 2011;11:390–8. doi: 10.1159/000330536. [DOI] [PubMed] [Google Scholar]
- 28.Shimosegawa T, Chari ST, Frulloni L, et al. International consensus diagnostic criteria for autoimmune pancreatitis: Guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352–8. doi: 10.1097/MPA.0b013e3182142fd2. [DOI] [PubMed] [Google Scholar]
- 29.Iwashita T, Yasuda I, Doi S, et al. Use of samples from endoscopic ultrasound-guided 19-gauge fine-needle aspiration in diagnosis of autoimmune pancreatitis. Clin Gastroenterol Hepatol. 2012;10:316–22. doi: 10.1016/j.cgh.2011.09.032. [DOI] [PubMed] [Google Scholar]
- 30.Kanno A, Ishida K, Hamada S, et al. Diagnosis of autoimmune pancreatitis by EUS-FNA by using a 22-gauge needle based on the International Consensus Diagnostic Criteria. Gastrointest Endosc. 2012;76:594–602. doi: 10.1016/j.gie.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Holmes BJ, Hruban RH, Wolfgang CL, et al. Fine needle aspirate of autoimmune pancreatitis (lymphoplasmacytic sclerosing pancreatitis): Cytomorphologic characteristics and clinical correlates. Acta Cytol. 2012;56:228–32. doi: 10.1159/000336135. [DOI] [PubMed] [Google Scholar]
- 32.Mekky MA, Yamao K, Sawaki A, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010;71:913–9. doi: 10.1016/j.gie.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 33.Watson RR, Binmoeller KF, Hamerski CM, et al. Yield and performance characteristics of endoscopic ultrasound-guided fine needle aspiration for diagnosing upper GI tract stromal tumors. Dig Dis Sci. 2011;56:1757–62. doi: 10.1007/s10620-011-1646-6. [DOI] [PubMed] [Google Scholar]
- 34.Schuurbiers OC, Tournoy KG, Schoppers HJ, et al. EUS-FNA for the detection of left adrenal metastasis in patients with lung cancer. Lung Cancer. 2011;73:310–5. doi: 10.1016/j.lungcan.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 35.tenBerge J, Hoffman BJ, Hawes RH, et al. EUS-guided fine needle aspiration of the liver: Indications, yield, and safety based on an international survey of 167 cases. Gastrointest Endosc. 2002;55:859–62. doi: 10.1067/mge.2002.124557. [DOI] [PubMed] [Google Scholar]
- 36.Hollerbach S, Willert J, Topalidis T, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy of liver lesions: Histological and cytological assessment. Endoscopy. 2003;35:743–9. doi: 10.1055/s-2003-41593. [DOI] [PubMed] [Google Scholar]
- 37.DeWitt J, LeBlanc J, McHenry L, et al. Endoscopic ultrasound-guided fine needle aspiration cytology of solid liver lesions: A large single-center experience. Am J Gastroenterol. 2003;98:1976–81. doi: 10.1111/j.1572-0241.2003.07638.x. [DOI] [PubMed] [Google Scholar]
- 38.Singh P, Erickson RA, Mukhopadhyay P, et al. EUS for detection of the hepatocellular carcinoma: Results of a prospective study. Gastrointest Endosc. 2007;66:265–73. doi: 10.1016/j.gie.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 39.LeBlanc JK, Ciaccia D, Al-Assi MT, et al. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475–81. doi: 10.1016/s0016-5107(03)02863-3. [DOI] [PubMed] [Google Scholar]
- 40.Cleveland P, Gill KR, Coe SG, et al. An evaluation of risk factors for inadequate cytology in EUS-guided FNA of pancreatic tumors and lymph nodes. Gastrointest Endosc. 2010;71:1194–9. doi: 10.1016/j.gie.2010.01.029. [DOI] [PubMed] [Google Scholar]
- 41.Wee E, Lakhtakia S, Gupta R, et al. Endoscopic ultrasound guided fine-needle aspiration of lymph nodes and solid masses: Factors influencing the cellularity and adequacy of the aspirate. J Clin Gastroenterol. 2012;46:487–93. doi: 10.1097/MCG.0b013e31824432cb. [DOI] [PubMed] [Google Scholar]
- 42.Yasuda I, Tsurumi H, Omar S, et al. Endoscopic ultrasound-guided fine-needle aspiration biopsy for lymphadenopathy of unknown origin. Endoscopy. 2006;38:919–24. doi: 10.1055/s-2006-944665. [DOI] [PubMed] [Google Scholar]
- 43.Korenblit J, Anantharaman A, Loren DE, et al. The role of endoscopic ultrasound-guided fine needle aspiration (eus-fna) for the diagnosis of intra-abdominal lymphadenopathy of unknown origin. J Interv Gastroenterol. 2012;2:172–6. doi: 10.4161/jig.23742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coe A, Conway J, Evans J, et al. The yield of EUS-FNA in undiagnosed upper abdominal adenopathy is very high. J Clin Ultrasound. 2013;41:210–3. doi: 10.1002/jcu.22013. [DOI] [PubMed] [Google Scholar]
- 45.Ribeiro A, Vazquez-Sequeiros E, Wiersema LM, et al. EUS-guided fine – needle aspiration combined with flow cytometry and immunocytochemistry in the diagnosis of lymphoma. Gastrointest Endosc. 2001;53:485–91. doi: 10.1067/mge.2001.112841. [DOI] [PubMed] [Google Scholar]
- 46.Khashab M, Mokadem M, DeWitt J, et al. Endoscopic ultrasound-guided fine-needle aspiration with or without flow cytometry for the diagnosis of primary pancreatic lymphoma - A case series. Endoscopy. 2010;42:228–31. doi: 10.1055/s-0029-1243859. [DOI] [PubMed] [Google Scholar]
- 47.Gu P, Zhao YZ, Jiang LY, et al. Endobronchial ultrasound-guided transbronchial needle aspiration for staging of lung cancer: A systematic review and meta-analysis. Eur J Cancer. 2009;45:1389–96. doi: 10.1016/j.ejca.2008.11.043. [DOI] [PubMed] [Google Scholar]
- 48.Ohnishi R, Yasuda I, Kato T, et al. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal nodal staging of lung cancer. Endoscopy. 2011;43:1082–9. doi: 10.1055/s-0030-1256766. [DOI] [PubMed] [Google Scholar]
- 49.Zhang R, Ying K, Shi L, et al. Combined endobronchial and endoscopic ultrasound-guided fine needle aspiration for mediastinal lymph node staging of lung cancer: A meta-analysis. Eur J Cancer. 2013;49:1860–7. doi: 10.1016/j.ejca.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 50.De Leyn P, Bedert L, Delcroix M, et al. Tracheotomy: Clinical review and guidelines. Eur J Cardiothorac Surg. 2007;32:412–21. doi: 10.1016/j.ejcts.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 51.von Bartheld MB, Versteegh MI, Braun J, et al. Transesophageal ultrasound-guided fine-needle aspiration for the mediastinal restaging of non-small cell lung cancer. J Thorac Oncol. 2011;6:1510–5. doi: 10.1097/JTO.0b013e31821e1a64. [DOI] [PubMed] [Google Scholar]
- 52.Puri R, Vilmann P, Sud R, et al. Endoscopic ultrasound-guided fine-needle aspiration cytology in the evaluation of suspected tuberculosis in patients with isolated mediastinal lymphadenopathy. Endoscopy. 2010;42:462–7. doi: 10.1055/s-0029-1244133. [DOI] [PubMed] [Google Scholar]
- 53.Fritscher-Ravens A, Ghanbari A, Topalidis T, et al. Granulomatous mediastinal adenopathy: Can endoscopic ultrasound-guided fine-needle aspiration differentiate between tuberculosis and sarcoidosis? Endoscopy. 2011;43:955–61. doi: 10.1055/s-0031-1271110. [DOI] [PubMed] [Google Scholar]
- 54.von Bartheld MB, Veseliç-Charvat M, Rabe KF, et al. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of sarcoidosis. Endoscopy. 2010;42:213–7. doi: 10.1055/s-0029-1243890. [DOI] [PubMed] [Google Scholar]
- 55.Thomas T, Kaye PV, Ragunath K, et al. Endoscopic-ultrasound-guided mural trucut biopsy in the investigation of unexplained thickening of esophagogastric wall. Endoscopy. 2009;41:335–9. doi: 10.1055/s-0029-1214470. [DOI] [PubMed] [Google Scholar]
- 56.Larghi A, Verna EC, Ricci R, et al. EUS-guided fine-needle tissue acquisition by using a 19-gauge needle in a selected patient population: A prospective study. Gastrointest Endosc. 2011;74:504–10. doi: 10.1016/j.gie.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 57.Harewood GC, Wiersema MJ, Nelson H, et al. A prospective, blinded assessment of the impact of preoperative staging on the management of rectal cancer. Gastroenterology. 2002;123:24–32. doi: 10.1053/gast.2002.34163. [DOI] [PubMed] [Google Scholar]
- 58.Sasaki Y, Niwa Y, Hirooka Y, et al. The use of endoscopic ultrasound-guided fine-needle aspiration for investigation of submucosal and extrinsic masses of the colon and rectum. Endoscopy. 2005;37:154–60. doi: 10.1055/s-2004-826152. [DOI] [PubMed] [Google Scholar]
- 59.Hünerbein M, Totkas S, Moesta KT, et al. The role of transrectal ultrasound-guided biopsy in the postoperative follow-up of patients with rectal cancer. Surgery. 2001;129:164–9. doi: 10.1067/msy.2001.110428. [DOI] [PubMed] [Google Scholar]
- 60.de Jong K, Poley JW, van Hooft JE, et al. Endoscopic ultrasound-guided fine-needle aspiration of pancreatic cystic lesions provides inadequate material for cytology and laboratory analysis: Initial results from a prospective study. Endoscopy. 2011;43:585–90. doi: 10.1055/s-0030-1256440. [DOI] [PubMed] [Google Scholar]
- 61.van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: A pooled analysis. Gastrointest Endosc. 2005;62:383–9. doi: 10.1016/s0016-5107(05)01581-6. [DOI] [PubMed] [Google Scholar]
- 62.DeWitt J, LeBlanc J, McHenry L, et al. Endoscopic ultrasound-guided fine-needle aspiration of ascites. Clin Gastroenterol Hepatol. 2007;5:609–15. doi: 10.1016/j.cgh.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 63.Wardeh R, Lee JG, Gu M. Endoscopic ultrasound-guided paracentesis of ascitic fluid: A morphologic study with ultrasonographic correlation. Cancer Cytopathol. 2011;119:27–36. doi: 10.1002/cncy.20123. [DOI] [PubMed] [Google Scholar]
- 64.Rana SS, Bhasin DK, Srinivasan R, et al. Endoscopic ultrasound-guided fine needle aspiration of peritoneal nodules in patients with ascites of unknown cause. Endoscopy. 2011;43:1010–3. doi: 10.1055/s-0031-1271111. [DOI] [PubMed] [Google Scholar]


