Abstract
There are several variables that have been studied to optimize various outcomes of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) such as quality and adequacy of specimens, diagnostic yield of malignancy, accuracy and overall efficiency. Using an evidence-based approach, the objectives of this review are to discuss two key aspects of EUS-FNA: (a) Use of a stylet and (b) use of suction. Level 1 evidence available from randomized controlled trials demonstrates that the use of a stylet during EUS-FNA has no impact on the diagnostic yield of malignancy or the quality of specimens. Air flushing in a slow, controlled fashion is superior to reinsertion of a stylet to express EUS-FNA aspirates. The use of suction should be considered during EUS-FNA of pancreatic masses. However, data from a randomized controlled trial suggest that suction should not be used during EUS-FNA of lymph nodes as it increases bloodiness of specimens obtained and has no impact on the overall diagnostic yield.
Keywords: Endoscopic ultrasound, fine-needle aspiration, stylet, suction
INTRODUCTION
Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration (FNA) have become integral to the diagnosis and staging of gastrointestinal (GI) and certain non-GI malignancies.[1] The common indications for EUS and EUS-FNA include diagnosis and staging of pancreaticobiliary, esophageal, gastric and rectal malignancies and evaluation of GI subepithelial lesions and mediastinal and intraabdominal lymphadenopathy.[2,3] There are several variables that have been studied to optimize outcomes of EUS-FNA such as quality and adequacy of specimens, diagnostic yield of malignancy, accuracy and overall efficiency. These include the skill and experience of the endosonographer and the Cytopathologist, technical difficulty associated with the procedure, the diameter of the EUS-FNA needle, use of suction and stylet, number of EUS-FNA passes and presence of on-site cytopathology assessment.[4] Using an evidence-based approach, the objectives of this review are to discuss two key aspects of EUS-FNA: (a) Use of a stylet and (b) use of suction.
USE OF A STYLET DURING EUS-FNA – IS IT WORTH THE EFFORT?
All commercially available EUS-FNA needle systems include a removable stylet. It was previously believed that the use of a stylet during EUS-FNA prevents clogging of the needle lumen by GI wall tissue as the needle traverses this to reach the target lesion, which could limit the ability to aspirate cells from the target lesion.[4] Based on this theoretical belief of improving the quality of specimens obtained, the use of a stylet is routine practice for some endosonographers during EUS-FNA. The stylet may also be useful for expression of EUS-FNA aspirates from needles.
Evidence-based approach
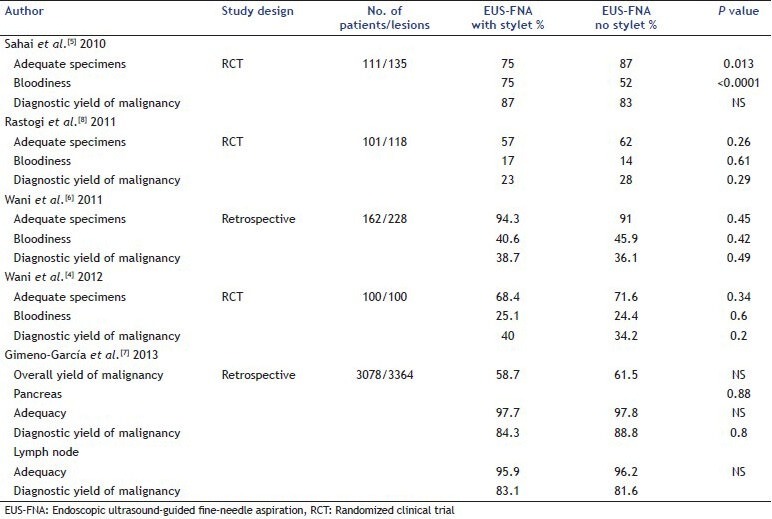
The following studies have evaluated the role of a stylet during EUS-FNA assessing endpoints of specimen quality (cytologic characteristics such as adequacy, cellularity, contamination, amount of blood) and diagnostic yield of malignancy [Table 1]. Sahai et al. in their study compared the yield of malignancy and quality of specimens obtained during EUS-FNA with and without stylet. A total of 135 lesions in 111 patients were sampled by a single endosonographer using a 22 gauge needle with a systematic assignment of with and without stylet passes in a 1:2 ratio. All slides in this study were read by a single, blinded Cytopathologist for the amount of blood, adequacy and presence of malignancy. Proportion of adequate samples were lower (75% vs. 87%, P = 0.013) and proportion of blood samples higher (75% vs. 52%, P < 0.0001) in the with stylet group compared with the without stylet passes. Thus, in this study, the use of stylet was associated with an inferior sample quality. In 46 lesions where an equal number of passes were made with and without stylet, the yield for malignancy was similar between the two techniques.[5] A retrospective study that compared the use of the stylet during EUS-FNA during two different time periods demonstrated no difference between the two techniques with regards to cellularity, contamination, amount of blood, adequacy of specimen and diagnostic yield of malignancy.[6] Similar results were noted by Gimeno-García et al. in a retrospective study that included 3078 patients (3364 EUS-FNA procedures – 1483 passes with a stylet and 1881 passes without a stylet). There was no difference between the two groups with regards to sample adequacy and final diagnosis of malignancy.[7] Rastogi et al. compared the two techniques in a randomized controlled trial that included 101 patients with 118 lesions (two passes with and two passes without a stylet in a randomized sequence). There was no difference in the diagnostic yield of malignancy between the two groups (23% with stylet vs. 28% without stylet, P = 0.29). Similarly, there was no difference between the two groups with regards to cellularity (P = 0.98), adequacy of specimens (P = 0.26), contamination (P = 0.92), or a significant amount of blood (P = 0.61).[8] Wani et al. compared the diagnostic yield of malignancy of EUS-FNA specimens obtained with and without a stylet during EUS-FNA in a prospective, single-blinded, randomized controlled trial. Cytopathologic parameters such as adequacy, amount of blood and cellularity were compared using the standardized criteria by Cytopathologists blinded to the stylet arm. A total of 100 patients were included; 275 passes were made with and 275 without a stylet. There was no difference in the diagnostic yield of malignancy between the two groups (P = 0.2). On a lesion based analysis, the passes from without stylet group diagnosed 13% higher number of malignant lesions compared with the passes from the with stylet arm. There was no difference in the cytological characteristics such as adequacy of specimens (P = 0.34), amount of blood (P = 0.6), cellularity (P = 0.8), number of cells (P = 0.25) and contamination (P = 0.31). Similar results were noted in a sub-group analysis evaluating these endpoints for the lesion site (pancreas, lymph node and others).[4]
Table 1.
Summary of studies evaluating the role of a stylet during EUS-FNA of solid lesions
There is limited data evaluating the ideal technique for expressing EUS-FNA aspirates. A recent randomized controlled trial evaluated whether expressing aspirates from the needle by reinserting the stylet was more effective than by air flushing. This study showed that bloodiness was in fact greater in the group of samples in which the stylet was reinserted to express aspirates compared with the group that underwent air flushing (odds ratio [OR] 1.16; 95% confidence interval [CI]: 1.03-1.3). There were no differences between the two groups with regards to the number of diagnostic samples, overall accuracy, cellularity and air-drying artifact.[9]
Recommendations
Level 1 evidence available from randomized controlled trials demonstrates that the use of a stylet during EUS-FNA has no impact on the diagnostic yield of malignancy or the quality of specimens (cytologic characteristics). Air flushing in a slow, controlled fashion is superior to reinsertion of a stylet to express EUS-FNA aspirates. The traditional technique of reinserting the stylet to express EUS-FNA aspirates may be required only in cases where the aspirates cannot be expelled due to clotting or drying. In addition, the use of a stylet during EUS-FNA is labor intensive, increases the procedure time as the stylet needs to be withdrawn after puncturing the lesion and then carefully reinserted through the needle before each pass and it increases the risk of accidental needle stick injuries.
ROLE OF SUCTION DURING EUS-FNA
The role of suction during EUS-FNA is unclear and not standardized. The proposed mechanism for the use of suction to improve the diagnostic yield during EUS-FNA is by holding the tissue against the cutting edge of the needle as it is moved through the target lesion and drawing up cells. EUS-FNA without suction uses the fine-needle capillary sampling technique to achieve the same result. Although some endosonographers routinely use suction during EUS-FNA or tailor the use of suction based on on-site cytopathology evaluation, others have abandoned the use of suction during EUS-FNA of solid lesions and reported high a diagnostic yield with this technique.[10]
Evidence-based approach
The following studies evaluated the role of suction during EUS-FNA (pancreatic masses, lymph nodes, others). A recent randomized controlled trial by Lee et al. compared diagnostic yield and cytologic characteristics during EUS-FNA of pancreatic masses with and without suction. EUS-FNA was performed using a 22- or 25-gauge needle and suction was applied using a 10-ml syringe. A total of 81 patients and 324 EUS-FNA passes were included in the final analysis. Samples in the suction group were associated with higher diagnostic yield (72.8% vs. 58.6%, P = 0.001), cellularity (OR: 2.12, 95% CI: 1.3-3.3, P < 0.001), and bloodiness (OR: 1.46, 95% CI: 1.2-1.6, P < 0.001) compared with samples obtained without suction. Overall, samples in the suction group had higher accuracy (82.4% vs. 72.1%, P = 0.005) compared with those obtained without suction.[9]
In a heterogeneous study group, Puri et al. compared EUS-FNA with and without suction in randomized controlled trial that included 52 patients with solid lesions (19% pancreatic lesions, 66% lymph nodes, 15% adrenal lesions) [Table 2]. EUS-FNA with suction was associated with increased number of pathology slides with no difference in the bloodiness of each sample. The use of suction was associated with a higher sensitivity (85.7% vs. 66.7%).[11] On the other hand, a study that included 53 patients (23 pancreatic masses, 26 lymph nodes, 3 subepithelial lesions and 1 liver lesion) showed that there was no difference in quality and diagnostic accuracy of specimens obtained with and without suction.[12]
Table 2.
Summary of randomized controlled trials evaluating the role of suction during EUS-FNA of solid lesions
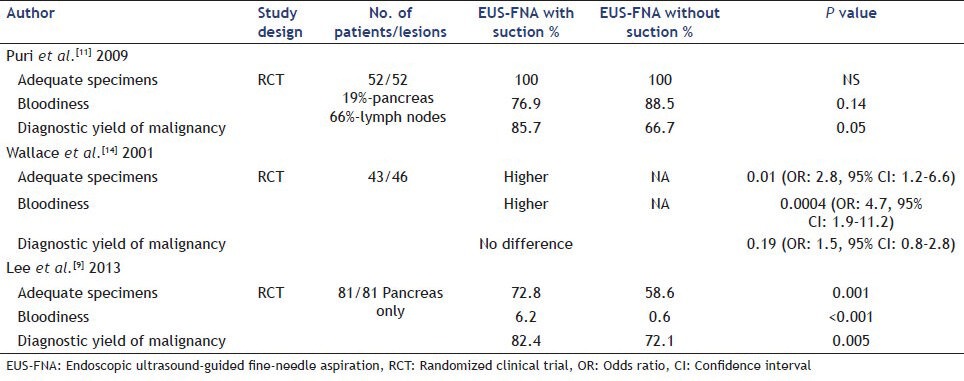
Bhutani et al. first described the use of suction and reported that continuous suction using a 10-ml syringe rather than intermittent suction provided optimal cellularity in EUS-FNA of mediastinal lymph nodes.[13] A subsequent randomized controlled trial evaluated the effect of suction in 43 patients with 46 lymph nodes during EUS-FNA. Although the use of suction was associated with an increase in cellularity, it had no impact on obtaining a correct diagnosis and was in fact associated with excessive bloodiness (OR: 4.7, 95% CI: 1.9-11.2).[14]
Recommendations
Based on level 1 evidence, the use of suction should be considered during EUS-FNA of pancreatic masses. However, data from a randomized controlled trial suggest that suction should not be used during EUS-FNA of lymph nodes as it increases bloodiness of specimens obtained and has no impact on the overall diagnostic yield.
CONCLUSIONS
Using an evidence-based approach, this review summarizes the available data on the use of a stylet and suction during EUS-FNA. Based on level 1 evidence, the use of a stylet during EUS-FNA is not recommended as it has no impact on the diagnostic yield and quality of specimens obtained. While data on use of suction are contradictory, the use of suction during EUS-FNA of lymph nodes is not recommended. Recent data suggest that suction during EUS-FNA of pancreatic masses may improve the diagnostic yield of malignancy and should be standard practice if confirmed by other centers in future randomized controlled trials.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Erickson RA. EUS-guided FNA. Gastrointest Endosc. 2004;60:267–79. doi: 10.1016/s0016-5107(04)01529-9. [DOI] [PubMed] [Google Scholar]
- 2.ASGE Standards of Practice Committee. Early DS, Ben-Menachem T, et al. Appropriate use of GI endoscopy. Gastrointest Endosc. 2012;75:1127–31. doi: 10.1016/j.gie.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Dumonceau JM, Polkowski M, Larghi A, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2011;43:897–912. doi: 10.1055/s-0030-1256754. [DOI] [PubMed] [Google Scholar]
- 4.Wani S, Early D, Kunkel J, et al. Diagnostic yield of malignancy during EUS-guided FNA of solid lesions with and without a stylet: A prospective, single blind, randomized, controlled trial. Gastrointest Endosc. 2012;76:328–35. doi: 10.1016/j.gie.2012.03.1395. [DOI] [PubMed] [Google Scholar]
- 5.Sahai AV, Paquin SC, Gariépy G. A prospective comparison of endoscopic ultrasound-guided fine needle aspiration results obtained in the same lesion, with and without the needle stylet. Endoscopy. 2010;42:900–3. doi: 10.1055/s-0030-1255676. [DOI] [PubMed] [Google Scholar]
- 6.Wani S, Gupta N, Gaddam S, et al. A comparative study of endoscopic ultrasound guided fine needle aspiration with and without a stylet. Dig Dis Sci. 2011;56:2409–14. doi: 10.1007/s10620-011-1608-z. [DOI] [PubMed] [Google Scholar]
- 7.Gimeno-García AZ, Paquin SC, Gariépy G, et al. Comparison of endoscopic ultrasonography-guided fine-needle aspiration cytology results with and without the stylet in 3364 cases. Dig Endosc. 2013;25:303–7. doi: 10.1111/j.1443-1661.2012.01374.x. [DOI] [PubMed] [Google Scholar]
- 8.Rastogi A, Wani S, Gupta N, et al. A prospective, single-blind, randomized, controlled trial of EUS-guided FNA with and without a stylet. Gastrointest Endosc. 2011;74:58–64. doi: 10.1016/j.gie.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Lee JK, Choi JH, Lee KH, et al. A prospective, comparative trial to optimize sampling techniques in EUS-guided FNA of solid pancreatic masses. Gastrointest Endosc. 2013;77:745–51. doi: 10.1016/j.gie.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Bang JY, Ramesh J, Trevino J, et al. Objective assessment of an algorithmic approach to EUS-guided FNA and interventions. Gastrointest Endosc. 2013;77:739–44. doi: 10.1016/j.gie.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Puri R, Vilmann P, Săftoiu A, et al. Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol. 2009;44:499–504. doi: 10.1080/00365520802647392. [DOI] [PubMed] [Google Scholar]
- 12.Storch IM, Sussman DA, Jorda M, et al. Evaluation of fine needle aspiration vs. fine needle capillary sampling on specimen quality and diagnostic accuracy in endoscopic ultrasound-guided biopsy. Acta Cytol. 2007;51:837–42. doi: 10.1159/000325857. [DOI] [PubMed] [Google Scholar]
- 13.Bhutani MS, Suryaprasad S, Moezzi J, et al. Improved technique for performing endoscopic ultrasound guided fine needle aspiration of lymph nodes. Endoscopy. 1999;31:550–3. doi: 10.1055/s-1999-125. [DOI] [PubMed] [Google Scholar]
- 14.Wallace MB, Kennedy T, Durkalski V, et al. Randomized controlled trial of EUS-guided fine needle aspiration techniques for the detection of malignant lymphadenopathy. Gastrointest Endosc. 2001;54:441–7. doi: 10.1067/mge.2001.117764. [DOI] [PubMed] [Google Scholar]


