Abstract
Like any other technique, fine needle aspiration (FNA) proficiency requires adequate experience. Although this technique is not difficult to master, formal training will allow endosonographers to achieve better results. The following article is derived in two parts: (1) To review current knowledge on endoscopic ultrasound (EUS)-FNA training, discuss the current recommendations on training guidelines, explore other training adjuncts and review the latest studies evaluating the validity of current recommendations; and (2) to provide some basic grounds on the EUS-FNA technique. EUS-FNA can be broken down into a series of steps. Proper execution of each step will make FNA easier and likely increase its diagnostic yield. Adequate positioning of the lesion in regards to the ultrasound probe is a key factor to obtain best results. The following will discuss useful tips in order to achieve maximal success rates.
Keywords: Endoscopic ultrasound, fine needle aspiration, training
INTRODUCTION
Fine needle aspiration (FNA) provides some of the most powerful information that endoscopic ultrasound (EUS) can offer. Like any other technique, proficiency requires adequate experience. EUS-FNA is not a universally difficult technique to master and can be integrated during basic training with curvilinear EUS.[1] Formal “hands-on” training will allow endosonographers to achieve better results than by self-training.[2]
Although some cases may be more technically demanding than others (e.g., sampling a 5 mm lesion deeply buried in the uncinate process), some of the easiest cases may provide tremendous information that will clearly impact management (such as FNA of a 4 cm malignant subcarinal node in the setting of non-small-cell lung cancer). The following chapter will review the current knowledge on EUS-FNA training, as well as provide some basic grounds on the EUS-FNA technique.
CURRENT KNOWLEDGE ON EUS-FNA TRAINING
Current recommendations for EUS-FNA
In 2001, the American Society for Gastrointestinal Endoscopy (ASGE) recommended that the minimum number of EUS procedures before assessing competency should include 150 supervised cases (75 being pancreatobiliary), with 50 EUS-FNA (at least 25 being pancreatic).[3] These recommendations have not been updated since. However, more recent training guidelines in other countries have been proposed. A 2011 Working Group mandated by the British Society of Gastroenterology recommended that EUS trainees should undergo 250 EUS procedures, including 80 luminal cancers, 20 subepithelial lesions and 150 pancreatobiliary cases (at least half of which are likely pancreatic adenocarcinoma). A total of at least 75 FNA should be performed, of which at least 45 are likely pancreatic adenocarcinomas.[4]
Although achieving the proper amount of cases to reach a specific “number” is an easily measurable goal, EUS-FNA proficiency cannot be strictly defined by this sole information. Cognitive aspects of the procedure are important, as well as understanding the indications/contraindications and the risks/benefits of performing the technique. Most experts recommend a 6-24 month “hands-on” training in EUS before achieving competency.[5]
Training adjuncts for EUS-FNA
Although formal hands-on training remains pivotal in acquiring EUS-FNA skills, several training adjuncts are also available. Direct observation of proficient endosonographers can provide useful information. There are also several video libraries demonstrating EUS-FNA through various websites. Non-human hands-on training models have been developed over time, such as phantoms with no animal material or models using porcine organs.[6,7,8]
Live animal porcine models, although cumbersome given the necessary resources needed to operate, seem to offer the best training experience after direct hands-on human training. Porcine models resemble human anatomy in regards to sampling lymph nodes. Prior exposition to lymph node FNA in porcine models may improve trainee performance, confidence and procedural comfort when returning to patient examinations.[9,10]
Evaluation of current EUS-FNA recommendations
As noted previously, ASGE recommendations for EUS before trainee evaluation include 150 supervised cases, including 50 EUS-FNA. Recent studies have demonstrated that the learning curve for proficiency may be much more than what was previously anticipated. A study by Eloubeidi and Tamhane showed that after 1 year of formal EUS training (including supervision of over 300 EUS and 45 EUS-FNA), the median number of passes to achieve a diagnosis significantly decreased after 100 additional FNA procedures and that complication rates decreased after 200 additional procedures.[11]
Moreover, in a study by Wani et al. evaluating the performance of 5 advanced endoscopic trainees (who had undergone between 175 and 402 EUS procedures during training) observed substantial variability in achieving competency, with only 2 trainees showing acceptable performance after 225 and 196 cases. None of the trainees achieved training guideline goals after the recommended minimal 150 procedures.[12] This study prompted some experts to argue that the volume required to obtain competency was greater than what had been previously promulgated.[13] Although this study was underpowered to specifically address EUS-FNA, further studies in this field are required to reevaluate actual training guidelines.
Finally, some training centers may not offer minimal training requirements during a 1-year curriculum. A 2006 study of 25 United States-based gastrointestinal programs offering advanced EUS training showed that the average number of procedures varied considerably (median 200; range: 50-1100), with only 48% of fellows meeting the minimum number of procedures as recommended by the ASGE.[14]
In conclusion, formal training in EUS-FNA is recommended in order to maximize proficiency. Although animal training may provide useful starting tips, human “hands-on” training is required to achieve best results. Current training guidelines on the number of recommended supervised EUS-FNA vary between countries (50 in the US, 75 in the UK), but at least half of those should be performed on pancreatic lesions. Further studies are needed to evaluate if current minimal training thresholds should be upgraded.
BASIC TECHNIQUE OF EUS-FNA
EUS-FNA can be broken down into a series of steps. Proper execution of each step will make FNA easier and likely increase its diagnostic yield. The following will address the basic principles for properly achieving EUS-FNA of solid lesions. More precise topics such as suction, use of the stylet, number of passes and type of needle to use will be discussed elsewhere.
Identifying and characterizing the lesion
The indication for performing EUS-FNA should be clear and the endoscopy suite adequately prepared. The information obtained by the FNA should have a reasonable chance of being clinically useful. Not all solid lesions need to undergo FNA and potential risks need to be weighed with the benefits of obtaining tissue. If there is any doubt, this issue should be addressed with the referring physician before the procedure (or even during the procedure), if necessary.
When faced with the possibility of performing FNA on multiple sites, one should focus on the lesion likely to provide the most relevant information first. For instance, in the setting of a pancreatic head mass with suspicious liver nodules, FNA of the liver lesions may provide a positive cytologic diagnosis and confirm that the patient is not a surgical candidate.
Positioning the echoendoscope as straight as possible
Whenever possible, the echoendoscope should be straight. This makes needle movement easier and reduces the risks of damage to the accessory channel during insertion of the needle into the scope.
Most pancreatic lesions (including pancreatic head/uncinate lesion) can also be sampled with the scope in a straight position. The scope should be passed into the second duodenum and then withdrawn into a “short” position. By withdrawing the scope toward the duodenal bulb, most pancreatic head lesions can be accessed and punctured. However, when withdrawn further, this position will become unstable and the scope will slip into the stomach. Lesions near the pancreatic genu are often difficult to FNA with this withdrawal technique since they often become visible just at the moment that the position becomes unstable.
For these lesions (and any other lesions that cannot be accessed with the scope in a straight position), it is necessary to assume a “long” position, with the scope in the bulb or pre-pyloric region. This position will also provide a mechanical advantage when trying to puncture very firm lesions in the pancreatic head region.
Inserting the needle into the scope
If possible, the needle should be inserted into the scope with the scope in a straight position. One should never use excessive force to push the sheath past an excessive bend, as this could result in perforation of the inner lining of the biopsy channel. Instead, the echoendoscope should be withdrawn into a straight configuration before attempting to re-insert the needle system completely.
For lesions needing access from the second duodenum, the needle should be inserted into the scope only after the scope has been placed into the second duodenum. In order to decrease duodenal laceration, the duodenal sweep should not be negotiated with the needle and/or sheath protruding from the biopsy channel.
The rubber cap covering the operating channel must be removed before inserting the needle system. Once the needle is fully inserted into the echoendoscope, the base of the needle should be luer-locked to the operating channel. The needle sheath should then be adjusted so that it protrudes just beyond the elevator and only minimally seen on the endoscopic view [Figure 1]. A needle sheath that is too long will limit precise movements with the needle.
Figure 1.
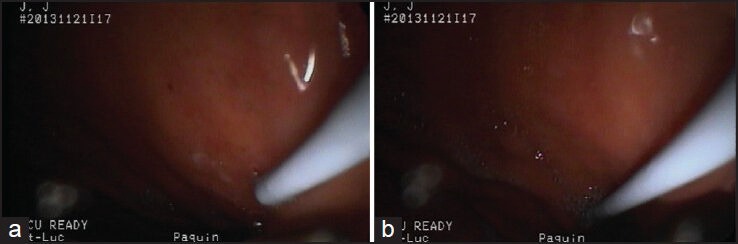
The needle sheath should be minimally seen in the endoscopic view. (a) Correct distance; (b) Excessively long distance
In some cases, the lesion to target may be less visible once the needle is in position; this can be explained either by artifact induced by the needle and/or sheath or by reducing the coupling between the gut wall and the ultrasound probe. Reducing the length of the sheath and applying suction to reduce air artifact between the ultrasound probe and the gut wall may help correct the problem. When using stiff, large caliber needles, increased rigidity of the scope may also alter its angulation, provoking less adequate images.
Adjusting the projected needle path with the lesion
Once the scope is in position, fine movements with the controls and gentle pushing or pulling of the scope must be performed to bring the lesion into the best position that will allow an easy puncture. The lesion should be positioned slightly to the upper center-left part of the screen, immediately underneath the ultrasound probe [Figure 2]. This optimal positioning will only require minimal up/down tip deflection and minimal elevator movement in order to achieve proper alignment with the needle path and the lesion. Lesions that are more difficult to center should be positioned slightly more rightward, in a way that will permit puncture with more elevator deflection [Figure 3].
Figure 2.
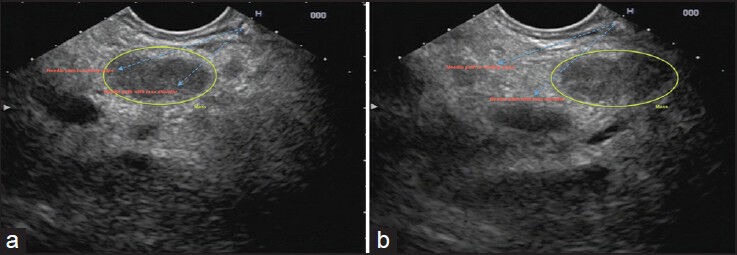
Positioning of a lesion prior to endoscopic ultrasound-guided fine needle aspiration. (a) The lesion is in the center underneath the probe, slightly to the left and within the natural path of the needle and the elevator path; (b) The lesion is not optimally positioned
Figure 3.
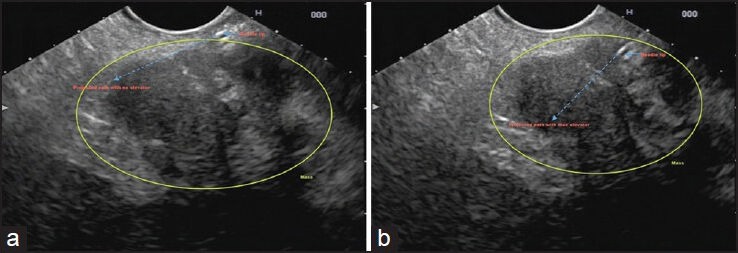
Using elevator to optimize needle path when the lesion in suboptimally positioned. (a) The lesion is slightly off toward the right of the screen and is not within the adequate needle trajectory; (b) The elevator permits re-alignment of the needle path within the mass
Once proper position is achieved, locking the up/down dial will increase scope stability and will allow the left thumb to manipulate the elevator dial without losing position.
Puncturing the lesion
Once the needle and the lesion are properly aligned, tissue sampling may begin. The screw securing the movable portion of the needle system should be unscrewed to allow forward thrusting of the needle. The fixed component of the needle should be grasped by the right palm of your hand and held together by the 4th and 5th fingers. This will allow maximal needle stability. The thumb and index finger should then grasp the movable portion of the needle and begin slow forward thrusting of the needle [Figure 4].
Figure 4.
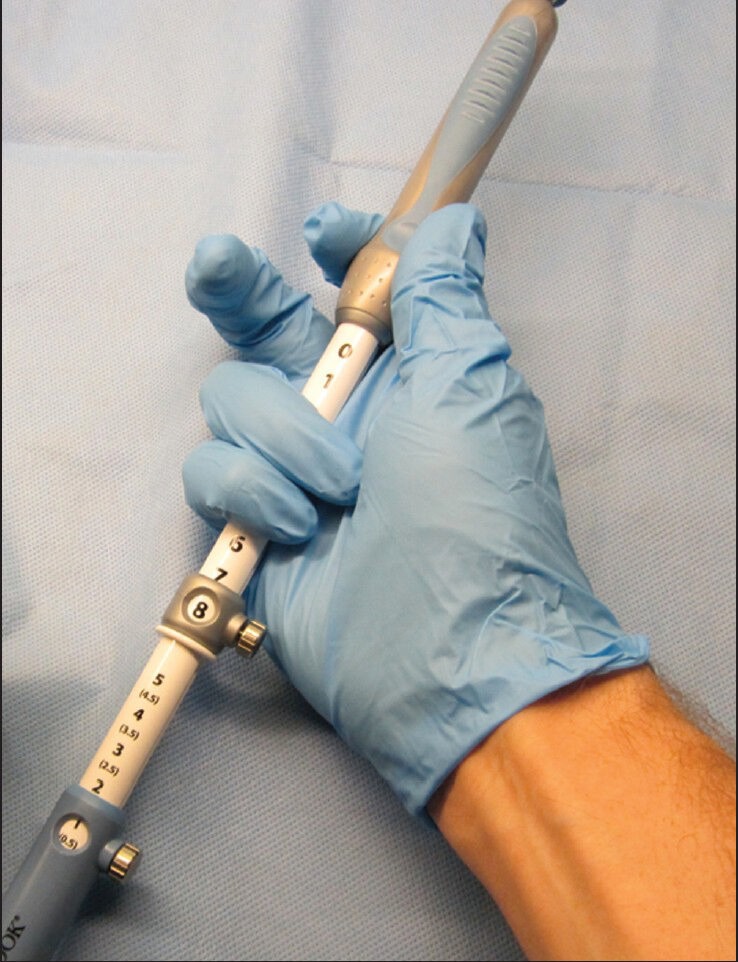
Adequate holding of the needle shaft prior to performing fine need aspiration. The shaft is held in the palm of the right hand and grasped by the 4th and 5th fingers. The thumb and index finger hold the moveable portion of the needle system and are ready to begin forward thrusting
As the needle is deployed, one should always ensure that the needle is properly seen advancing into the patient. If no needle movement is seen after it has been deployed for 1 cm, the needle should be withdrawn back into the sheath and inspected to ensure that there is no major bend in the needle that would prevent proper ultrasound-guided deployment.
Once the needle is seen approaching the gut wall, final repositioning of the elevator is performed in order to ensure proper alignment with the lesion. In order to minimize risks of puncturing other vital structures such as vessels or ducts, one should try to limit the distance needed to travel by the needle in order to sample the lesion. Applying power Doppler prior to performing the FNA can also uncover small vessels that were not easily seen and may help to reorient the path in order to minimize post-puncture bleeding.
If the needle tip can no longer be visualized once the lesion has been punctured, this is likely the result of excess bending of the needle (usually caused by torquing of the scope in areas more difficult to access, such as the liver hilar region). One should immediately stop advancing the needle if proper visualization is lost. Gentle torquing of the scope in a left and rightward fashion may uncover the needle if it is positioned slightly off the ultrasound field. If the needle is still not properly seen, the needle must be withdrawn back into the sheath and the needle assembly should be removed from the scope and the needle straightened if needed. The puncture can then be re-attempted.
Once the needle is in the lesion and the tip properly seen, the needle is moved back and forth several times into the lesion, with adequate force to produce cell shearing. If the elevator was used during the puncture of the needle, relaxing the elevator back to its neutral position will permit easier back-and-forth movement into the lesion. Care should be taken to ensure that the needle does not exit the confines of the lesion during sampling. As noted earlier, specific details regarding use of the stylet, the number of passes to perform and the use of suction will be addressed elsewhere.
Withdrawing the needle and processing the aspirate
Once sufficient sampling is produced, the needle is fully withdrawn back into the sheath and the locking device should be screwed back on to prevent accidental needle exit during sample processing.
To avoid clotting in the needle, the specimen should be expressed from the needle as quickly as possible. The aspirate can be expelled on a glass slide for thin-prep smears in the setting of on-site cytopathology. Subsequent samples can be expelled in 50% ethanol for cell block, formalin for pathology, or flow cytometry medium (discussed elsewhere).
Preparing the needle for subsequent passes
The same needle can be used multiple times for several passes. A needle change may be required if it malfunctions or if the needle tip becomes too blunt (manifesting itself by increased difficulty to puncture the lesion). The needle can be rinsed with normal saline before the next pass if the prior aspirate was bloody.
If the needle is bent, it must be straightened before reinsertion into the scope. Failure to properly unbend the needle will result in needle deflection out of the ultrasound plane during subsequent deployments, resulting in loss of needle tip visualization during forward thrusting. This situation is often encountered in the setting of a very firm lesion or if the pass was performed in the duodenal bulb or sweep. To straighten the needle, push it completely out of the sheath to expose it. Use your fingers to manually redress it. An alcohol swab can then be used to clean the outer surface of the needle.
CONCLUSION
EUS-FNA is a powerful clinical tool, enabling the endosonographer to document malignant lesions and obtain cytological specimens that would otherwise require more invasive procedures to properly sample. Adequate positioning of the lesion in regards to the ultrasound probe is a key factor to obtain best results. In most cases, the scope should be positioned in a straight fashion in order to maximize the ease of proper needle deployment. Although challenging at times, this technique is relatively straightforward if the lesion is sufficiently large and the above conditions have been met.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Coté GA, Hovis CE, Kohlmeier C, et al. Training in EUS-guided fine needle aspiration: Safety and diagnostic yield of attending supervised, trainee-directed FNA from the onset of training. Diagn Ther Endosc 2011. 2011:378–540. doi: 10.1155/2011/378540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nayar M, Joy D, Wadehra V, et al. Effect of dedicated and supervised training on achieving competence in EUS-FNA of solid pancreatic lesions. Scand J Gastroenterol. 2011;46:997–1003. doi: 10.3109/00365521.2011.579158. [DOI] [PubMed] [Google Scholar]
- 3.Eisen GM, Dominitz JA, Faigel DO, et al. Guidelines for credentialing and granting privileges for endoscopic ultrasound. Gastrointest Endosc. 2001;54:811–4. doi: 10.1016/s0016-5107(01)70082-x. [DOI] [PubMed] [Google Scholar]
- 4.Meenan J, Harris K, Oppong K, et al. Service provision and training for endoscopic ultrasound in the UK. Frontline Gastroenterol. 2011;2:188–94. doi: 10.1136/fg.2010.004101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polkowski M, Larghi A, Weynand B, et al. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline. Endoscopy. 2012;44:190–206. doi: 10.1055/s-0031-1291543. [DOI] [PubMed] [Google Scholar]
- 6.Sorbi D, Vazquez-Sequeiros E, Wiersema MJ. A simple phantom for learning EUS-guided FNA. Gastrointest Endosc. 2003;57:580–3. doi: 10.1067/mge.2003.141. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda K, Tajiri H, Hawes RH. How shall we experience EUS and EUS-FNA before the first procedure? The development of learning tools. Dig Endosc. 2004;16:236–9. [Google Scholar]
- 8.Bussen D, Sailer M, Fuchs KH, et al. A teaching model for endorectal ultrasound-guided biopsy and drainage of pararectal tumors. Endoscopy. 2004;36:217–9. doi: 10.1055/s-2004-814251. [DOI] [PubMed] [Google Scholar]
- 9.Fritscher-Ravens A, Cuming T, Dhar S, et al. Endoscopic ultrasound-guided fine needle aspiration training: Evaluation of a new porcine lymphadenopathy model for in vivo hands-on teaching and training, and review of the literature. Endoscopy. 2013;45:114–20. doi: 10.1055/s-0032-1325931. [DOI] [PubMed] [Google Scholar]
- 10.Barthet M. Endoscopic ultrasound teaching and learning. Minerva Med. 2007;98:247–51. [PubMed] [Google Scholar]
- 11.Eloubeidi MA, Tamhane A. EUS-guided FNA of solid pancreatic masses: A learning curve with 300 consecutive procedures. Gastrointest Endosc. 2005;61:700–8. doi: 10.1016/s0016-5107(05)00363-9. [DOI] [PubMed] [Google Scholar]
- 12.Wani S, Coté GA, Keswani R, et al. Learning curves for EUS by using cumulative sum analysis: Implications for American Society for Gastrointestinal Endoscopy recommendations for training. Gastrointest Endosc. 2013;77:558–65. doi: 10.1016/j.gie.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 13.Sedarat A, Kochman ML. Competency in EUS: Is it the curriculum, test, trainer, or trainee? Gastrointest Endosc. 2013;77:566–7. doi: 10.1016/j.gie.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 14.Azad JS, Verma D, Kapadia AS, et al. Can U.S. GI fellowship programs meet American Society for Gastrointestinal Endoscopy recommendations for training in EUS? A survey of U.S. GI fellowship program directors. Gastrointest Endosc. 2006;64:235–41. doi: 10.1016/j.gie.2006.04.041. [DOI] [PubMed] [Google Scholar]


