Abstract
Background
It was hypothesized that if periodontal infections predispose low birth weights and premature birth, then such outcomes should be apparent when the mother has aggressive periodontitis (AgP).
Methods
Birth weight data were collected by questionnaire from females with AgP, their periodontally healthy siblings, and unrelated periodontally healthy women. Both prospective and retrospective birth outcome data were used. Because many of the periodontal evaluations were performed after the births, there were incomplete data regarding most of the risk factors for low birth weight. We determined associations between mothers’ periodontal diagnoses and clinical variables and the reported birth weights.
Results
There were no significant differences in mean birth weights of babies born to control subjects or AgP patients. This was true whether all the births were considered or only those reported <1 or 2 years before periodontal examination. For periodontally healthy controls, 13.2% of babies born to siblings of AgP patients and 12.8% of babies born to unrelated mothers weighed <2,500 g, whereas 9.9% of those born to mothers with generalized AgP and 10.3% of those born to mothers with localized AgP weighed <2,500 g.
Conclusions
Because of the relative rarity of AgP in the population, and attendant difficulties in performing a prospective study of its association with pregnancy outcomes, we used a compromised approach using prospective data as well as weaker retrospective data assuming that disease onset was likely before the births. Our results, within the limitations of this approach, indicate no evidence that AgP in the mother predisposes low birth weights. AgP has many unique biologic characteristics that differentiate it from chronic forms of periodontal disease, and the possible lack of its association with birth weight may be another such characteristic.
Keywords: Aggressive periodontitis; infant, low birth weight; pregnancy; preterm birth
Many recent studies have demonstrated significant associations between periodontal disease and adverse pregnancy outcomes.1,2 A variety of measures of periodontal infection, including clinical measures of periodontal probing depth (PD), progression of periodontal disease during pregnancy,3 immune responsiveness or lack thereof to periodontal microorganisms,4,5 and specific bacterial colonization,4–7 have been shown to be associated with low birth weight, premature birth, preeclampsia, and even fetal death. It has been hypothesized that systemic inflammation, attributable to periodontal infection and bacterial colonization in the absence of adequate maternal antibody responses to specific periodontal pathogens, might contribute to such adverse outcomes. Notably, other studies have failed to reproduce the associations between periodontal diseases and pregnancy outcomes. 8–13 Furthermore, although some studies14,15 have shown that treatment of periodontal disease during pregnancy is of benefit to birth outcomes,16 others have failed to show such an effect.17–19
Aggressive periodontitis (AgP), a disease with clinical onset typically in teenagers and young adults, is characterized by rapid loss of periodontal supporting tissues. It occurs in two distinct clinical patterns, including a localized form affecting first molars and incisors primarily and a generalized form that may affect all teeth.20 The prevalence of AgP in teenagers in the United States has been estimated to be between 0.1% and 2% depending on the clinical subform of the disease, race, and sex of the subject population.21 Thus, AgP, and particularly generalized aggressive periodontitis (GAgP), is a relatively rare disease.
The relative rarity of AgP mitigates against designing a practical prospective study of the impact of this severe form of periodontitis on pregnancy outcomes. Because the typical age of onset of AgP indicates a reasonably high likelihood that the presence of disease would co-occur with pregnancy, we thought it reasonable to perform an analysis using both prospective and retrospective birth outcome data in AgP based on information collected from mothers with this disease as well as their periodontally healthy (NP) female siblings and unrelated control NP subjects.
MATERIALS AND METHODS
Clinical Methods
This study was approved by the office of Research Subjects Protection for the Conduct of Human Research of Virginia Commonwealth University, Richmond, Virginia. All participants provided written informed consent prior to their participation in this study. This study was initially performed during a long-term series of studies22–25 of clinical, genetic, and biologic aspects of AgP, which included a family study of these diseases. Thus, participants in the study included patients with AgP, their NP siblings, and unrelated NP subjects. Subjects were enrolled and examined from 1976 to 2010 at the Virginia Commonwealth University Clinical Research Center for Periodontal Disease. A total of 135 localized aggressive periodontitis (LAgP) subjects, 147 GAgP subjects, and 170 NP siblings of AgP subjects were identified during this time frame. Data collection for determination of birth outcomes for these subjects began in 1994. Periodontal examinations were performed at the time of initial examination. Subsequent to, or at the time of, initial or follow-up periodontal examination, 85 LAgP subjects, 84 GAgP subjects, and 125 NP siblings completed questionnaires that asked questions about socioeconomic status and the birth weight and length of pregnancy for each of their children, if any. The number of subjects completing the questionnaire who provided information about their children is shown in Table 1.
Table 1.
Periodontal Measures in Subject Groups
| PI | GI | PD | AL | 2% | 5% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diagnosis | n | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| NP, unrelated | 90 | 0.45 | 0.36 | 0.67 | 0.31 | 2.06 | 0.26 | 0.18 | 0.22 | 3.4 | 5.8 | 0 | 0 |
| NP, siblings | 45 | 0.77 | 0.46 | 0.95 | 0.40 | 2.04 | 0.31 | 0.14 | 0.16 | 3.4 | 4.4 | 0 | 0 |
| LAgP | 50 | 0.67 | 0.37 | 0.95 | 0.40 | 2.40 | 0.38 | 0.61 | 0.44 | 15.2 | 11.1 | 2.9 | 3.4 |
| GAgP | 80 | 1.22 | 0.53 | 1.46 | 0.50 | 3.76 | 1.01 | 2.81 | 1.55 | 61.4 | 22.0 | 23.2 | 20.3 |
2% = percentage of sites with < 2 mm AL; 5% = percentage of sites with > 5 mm AL.
The mothers provided a comprehensive health history and received a complete periodontal examination. Data on smoking history and racial category were determined by self-report. All subjects were systemically healthy as determined by history. The periodontal examination included PD, attachment loss (AL), plaque index (PI),26 gingival index (GI),27 and bleeding on probing (BOP).28 Measurements of PD and AL were recorded to the nearest 1 mm with measures between 1 mm probe markings rounded down to the nearest millimeter. Measurements were performed at six sites per tooth (mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual, and disto-lingual).
The definitions of AgP for the subjects in this report conform to current diagnostic guidelines for these diseases29 with the additional restriction that all cases of GAgP conform to previous publications25 from our group in which the criterion of ‘‘early onset’’ of disease was invoked. The diagnostic groupings were defined as follows: 1) NP subjects with no evidence of AL other than facial recession and no periodontal pockets >3 mm. 2) GAgP subjects had a history of disease onset <35 years of age and presented with ≥8 teeth with ≥5 mm AL at interproximal sites; ≥3 of the affected teeth were not first molars and incisors. 3) LAgP subjects had a history of disease onset <30 years of age and presented with ≥2 teeth with ≥4 mm AL (one of which was a first molar) and no >2 additional teeth with AL that were not first molars or incisors.
The age of onset of AL was routinely verified through dental records and radiographs throughout the course of the studies22–25 from which this population was drawn. Furthermore, we routinely recalled and reexamined as many of these subjects as was possible to verify the diagnosis of AgP and assess progressive disease, and administered the survey form to assess birth outcomes during these follow-up examinations. The cases where female siblings of subjects displayed AL but could not be determined by follow-upexaminations to have AgP were not included in the analyses.
Statistical Analyses
Examination of the racial composition of the subjects completing the birth outcomes questionnaire revealed that 98.2% were either black or white, so data analyses were restricted to these two racial groups. Data were analyzed by analysis of variance. The model used for the analysis was a mixed model with a random error term for the mother. Adding the random error term allows the model to take into account correlation of observations among babies of the same mother. The latter was necessary because there were multiple births for many of the mothers. The response variable was birth weight (in grams). The fixed terms modeled in the analyses were as follows: 1) race; 2) periodontitis group (GAgP, LAgP, and two non-periodontitis control groups); 3) interaction between patient group and race; 4) smoker status (current, never, former); 5) GI, PI, PD, and AL; 6) months between examination and birth; and 7) mother’s age at birth. The previous model was done using statistical software.‡ When we evaluated the frequency of low-birth-weight infants, a similar analysis was done with generalized estimating equation (GEE) methods for the binary outcome of <2,500 or greater. In the GEE models(doneusing a function in the statistical software§), the terms in the model were restricted to race, smoking, mother’s age at birth, and participant group to allow convergence.
RESULTS
Data regarding birth weights and the length of pregnancy were collected by survey from 80 females with GAgP, 50 females with LAgP, and 135 NP female control subjects who were either siblings of the AgP subjects or unrelated. Table 1 illustrates the mean values of the periodontal variables for the mothers in the four groups. The data are characteristic of the groups.
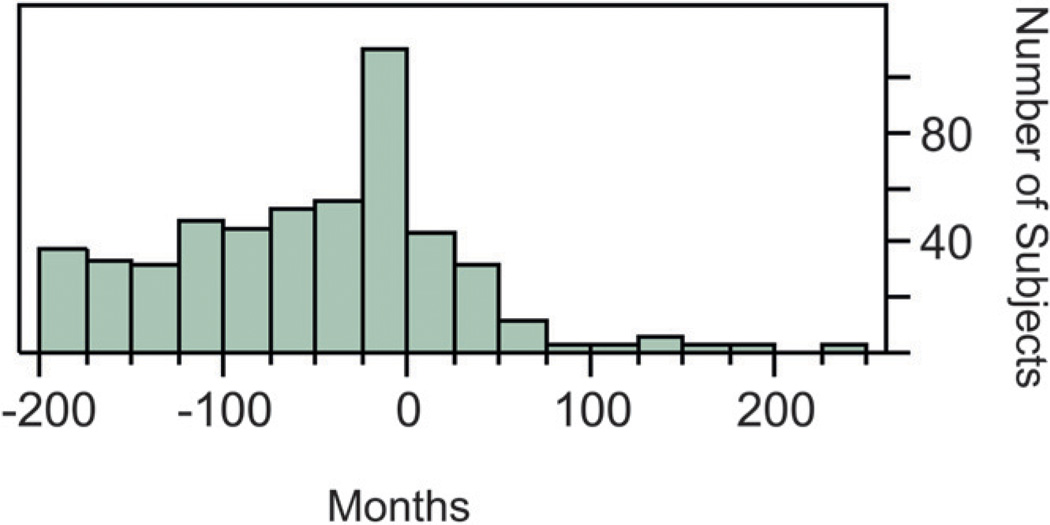
Demographic variables describing the subjects are shown in Table 2. The time between periodontal examination and the child’s birth was less for the AgP groups. The distribution of the time between the periodontal examination and the birth of the child is shown in Figure 1. For all births, 32% of the periodontal examinations were performed either before birth or within 2 years after birth.
Table 2.
Demographic Variables in Subject Groups
| Variable | GAgP | LAgP | NP (Siblings) | NP (Unrelated) |
|---|---|---|---|---|
| Mean age at diagnosis ± SD | 16.4 ± 2.9 | 15.5 ± 1.9 | 18.3 ± 5.4 | 20.0 ± 7.2 |
| Mean age at child’s birth ± SD | 24.2 ± 5.5 | 25.0 ± 5.6 | 23.5 ± 5.4 | 26.0 ± 5.6 |
| Race (% black) | 72 | 82 | 68 | 52 |
| Smoking history (% for current, former, never) | 55, 1, 44 | 24, 2, 75 | 40, 8, 52 | 29, 1, 71 |
| Family income (% for < $25,000, $25,000 to $40,000) | 82, 13 | 71, 24 | 65, 24 | 59, 25 |
| Periodontal therapy received (%) | 39.5% | 64.9% | N/A | N/A |
| Months pregnant ± SD | 8.9 ± 0.3 | 8.9 ± 0.5 | 8.8 ± 0.7 | 8.7 ± 1.1 |
N/A = not applicable.
Figure 1.
Months between periodontal examination and the birth of the child. Negative numbers indicate that the birth preceded the periodontal examination.
The mean birth weights for the four groups are shown in Table 3. Despite significant clinical differences between the AgP groups and the NP subjects, there were no significant differences in the birth weights. To ensure that the relative birth weights were not biased by the amount of time that had elapsed since the births and that periodontal clinical data collected close to the time of birth was not different from data collected several years after birth, we calculated mean birth weights for subsets of the groups according to the interval between birth and periodontal assessment. Mean birth weights for the diagnostic groups were equivalent regardless of the time between birth and periodontal examination (Table 3). This was further substantiated by analysis of covariance, where the number of months between birth and periodontal examination and whether the examination was performed before or after the birth, was used as a covariate in an analysis of the effects of periodontal diagnosis on birth weight (P = 0.9).
Table 3.
Birth Weight in Groups Examined at Different Time Intervals in Relation to Time of Birth
| Birth Weight (Grams)* |
|||
|---|---|---|---|
| Timing of Mother’s Periodontal Examination Relative to Child’s Birth |
n | Mean | SD |
| All examinations | |||
| NP, unrelated | 180 | 3,229.6 | 845.0 |
| NP, siblings | 106 | 3,163.9 | 715.4 |
| LAgP | 97 | 3,252.9 | 636.7 |
| GAgP | 223 | 3,244.6 | 581.9 |
| Examinations before birth only | |||
| NP, unrelated | 12 | 3,401.9 | 584.8 |
| NP, siblings | 19 | 3,166.2 | 716.0 |
| LAgP | 23 | 3,347.7 | 626.0 |
| GAgP | 39 | 3,179.5 | 621.6 |
| Examinations before birth and within 1 year after birth | |||
| NP, unrelated | 24 | 3,210.6 | 876.2 |
| NP, siblings | 32 | 3,189.3 | 603.7 |
| LAgP | 54 | 3,229.1 | 783.8 |
| GAgP | 53 | 3,191.7 | 637.5 |
| Examinations before birth and within 2 years after birth | |||
| NP, unrelated | 33 | 3,258.5 | 823.1 |
| NP, siblings | 37 | 3,193.5 | 612.2 |
| LAgP | 60 | 3,263.5 | 752.1 |
| GAgP | 65 | 3,234.0 | 635.5 |
No statistical differences were noted among groups.
We then determined the frequencies of reported low birth weight (defined as <2,500 g) within the diagnostic categories (Table 4). There were no statistically significant differences between groups for preterm birth. This was true whether we considered all the births or only those reported <1 or 2 years before periodontal examination. The percentage of low birth weight ranged from 9.9% to 17.3% (P = 0.91). The lowest proportion of preterm births occurred in the GAgP group.
Table 4.
Frequency of Low Birth Weight (<2,500 g) in Subject Groups
| Timing of Mother’s Periodontal Evaluation Relative to Child’s Birth |
n | n for Low Birth Weight* | % of Low Birth Weight |
|---|---|---|---|
| All examinations | |||
| NP, unrelated | 180 | 23 | 12.8 |
| NP, siblings | 106 | 14 | 13.2 |
| LAgP | 97 | 10 | 10.3 |
| GAgP | 223 | 22 | 9.9 |
| Examinations before birth and within 1 year after birth | |||
| LAgP | 52 | 9 | 17.3 |
| GAgP | 53 | 7 | 13.2 |
| Examinations before birth and within 2 years after birth | |||
| LAgP | 60 | 9 | 15.0 |
| GAgP | 65 | 8 | 12.3 |
No statistical differences were noted among groups.
Because of the frequently reported differences in adverse pregnancy outcomes between white and black individuals in the United States, we further examined birth weight within these racial groups. As seen in Table 5, birth weight was significantly influenced by race (P = 0.001) but not by periodontal condition, smoking habits, mother’s age, or the interval between periodontal examination and birth. In addition, because our participant cohort contained a significant number of young subjects who could be at increased risk for preterm low birth weight, we compared birth weights of babies born to teenagers (≤19 years) to those born to mothers >19 years old. Teenage subjects had significantly smaller babies (P = 0.009) than non-teenagers. However, analyses of birth weights between diagnostic groups that included teenage status as a covariate failed to alter the observation that no difference in birth weights between diagnostic groups could be observed (P = 0.89).
Table 5.
Birth Weight in Subject Groups by Race
| Birth Weight (g)* |
|||
|---|---|---|---|
| Race | n | Mean | SD |
| NP, unrelated | 171 | 3,317 | 656 |
| Black | 91 | 3,183 | 655 |
| White | 80 | 3,468 | 627 |
| NP, related | 103 | 3,163 | 612 |
| Black | 72 | 3,109 | 657 |
| White | 31 | 3,291 | 477 |
| LAgP | 95 | 3,250 | 642 |
| Black | 80 | 3,218 | 650 |
| White | 15 | 3,421 | 590 |
| GAgP | 219 | 3,213 | 577 |
| Black | 159 | 3,079 | 506 |
| White | 60 | 3,573 | 604 |
No significant differences were noted for birth weight between diagnostic categories (P > 0.9). Black subjects had significantly lower birth weight (< 0.001) than white subjects. Analyses were corrected for age of the mother, time between birth, and dental examination and smoking.
Finally, we examined the reported birth outcomes in the most severely affected GAgP subjects compared to the NP subjects by restricting the AgP subjects to those with >50% of their teeth with >5 mm AL. No differences were noted in birth weight (P = 0.47) among the GAgP subjects and the two NP groups.
DISCUSSION
The data indicate that there is no significant influence of AgP on birth outcomes. In some respects, this result is surprising given the recent literature1,2 indicating that periodontal disease is associated with, and possibly a risk factor for, preterm-birth and low birth weight. AgP is a relatively severe form of periodontal disease that is most apparent in teenagers and young adults. This engendered our hypothesis that, because periodontal disease is considered to be a likely risk factor for adverse pregnancy outcome, we would readily see differences in birth outcome among patients with early-onset severe disease and healthy individuals. In fact, our expectation was that this group would experience more severe adverse pregnancy outcomes than any other periodontal diagnostic group, which was not the case.
Numerous studies have been performed that demonstrate the validity and accuracy of maternal recall of their infants’ birth weights.30–35 These studies uniformly show that maternal recall of their infants’ birth weight generally has a correlation with information from birth certificates of >0.9, this remains accurate even 30 years after the child’s birth, and varies only slightly with mother’s educational level. In a study of >46,000 females in Tennessee in which reported birth weights were compared to birth records, only 1.1% of births could be misclassified as either low birth weight or preterm.32 Thus, it is highly likely that the birth weights reported in this study were accurately reported with a small and acceptable level of error.
When there is a lack of a statistically significant difference between groups, this result can be real or attributable to a lack of statistical power. Because this report describes a cohort of subjects identified during a family study of genetic risk, we did not develop a formal power analysis before creating this report. However, we did perform a power analysis after the completion of data collection for this study. Because there were multiple children per mother and ≈50% of the variance was explained by a random error term, we based this power analysis on a sample size of the 135 mothers in the two periodontally healthy groups and the 80 GAgP mothers. This approach provided a conservative power estimate. At 80% power, we could have found a difference of 238 g between the children of the healthy mothers and the children of the AgP mothers. We did not do a power analysis for the prevalence of low-birth-weight babies, but the prevalence of low-birth-weight babies in the AgP group was 9.9%, which was ≈3% lower than the NP mothers. The number of mothers in our study is similar to that in a report by Offenbacher et al.36 in which the authors were investigating the relationships between periodontal disease and birth outcomes. In that report, the authors were able to find statistically significant differences in both birth weight and the prevalence of low-birth-weight babies between NP mothers (n = 201) and mothers with moderate-to-severe chronic periodontal disease (n = 45). Thus, there appears to be adequate statistical power in the current study to find meaningful differences between the groups.
Although there are obvious inherent weaknesses associated with the design of a study in which retrospective data are used and in which many of the periodontal assessments are performed several years after pregnancy, this approach is more feasible for examination of birth outcomes in AgP than a prospective study. Aggressive forms of periodontitis are relatively rare conditions,21 and a prospective study with adequate numbers of subjects would be impractical. The retrospective approach takes advantage of the fact that AgP, and particularly GAgP, is known to have onset and progression during the late teenage years and early 20s, when females would be most likely to be having children. The mean maternal age for birth of the children reported on in this study is 23.5 to 26 years, depending on the group, and it is reasonable to assume that the periodontal condition of most of the AgP subjects had developed by that time and that disease was present at the time of birth. Additionally, it is obvious that the control subjects did not have periodontitis at the time of the birth of their children. Most importantly, sufficient numbers of subjects were examined within 1 or 2 years of the births of their children to ensure that the relationships between periodontal status and birth weights reported herein are likely to be valid.
We included two NP control groups in this study. It is established that AgP is a familial condition that likely has a significant underlying genetic etiology.23,37,38 Thus, we considered it imperative to include an NP group of mothers who were first-degree relatives of the subjects with AgP. In this way, we would be able to differentiate between familial effects on birth outcomes that could be specific to AgP families and comparative effects to non-family NP subjects. However, there were no apparent differences in birth outcomes among the groups.
Data regarding periodontal treatment was self-reported by the AgP subjects on the same questionnaire used for reports of pregnancy outcomes. However, we do not have systematic data regarding the extent, nature, or outcome of the therapy. We performed an analysis of reported birth weight as a function of whether the individual reported receiving therapy. The results indicate that there was no difference in birth weight in the diagnostic for subjects receiving or not receiving therapy (P = 0.46).
The clinical periodontal ‘‘exposure’’ that is required to influence pregnancy outcomes is not entirely clear. Many of the studies that demonstrate associations between periodontal disease and preterm birth or low birth weight note that measures of PD, AL, or GI and BOP are the important clinical variables that impact pregnancy.3,39–42 Examination of these variables in our groups demonstrates significantly greater PI, GI, BOP, and PD measures in the GAgP group than in the other clinic group (all P <0.0001 compared to all other groups) without impact on pregnancy outcome. We also considered the possibility that only the most severely affected GAgP subjects might demonstrate significant effects of their periodontal condition on birth outcomes, so we compared the pregnancy outcomes for the most severely affected mothers with controls. Again, there was no significant effect. It is important to note, however, that these variables were measured in some of the mothers several years after the reported births (Table 1) and may not reflect the clinical measures that would have been observed during pregnancy.
We were concerned that the population studied herein might not be reflective of the population in general with respect to pregnancy outcomes, so we obtained data reporting the mean birth weights in the Richmond, Virginia area by race for the years 1997 to 2007. These data indicated that mean birth weights were very similar in the population in general (blacks, 3,047.0 ± 661.2 g; whites, 3,338.5 ± 583.9 g) to those in our subjects (Tables 3 and 5). Thus, it is unlikely that the population studied is unique with respect to pregnancy outcomes.
There are ample data in the literature indicating that AgP subjects demonstrate unique biologic characteristics with respect to inflammatory and immunologic responsiveness and genetic susceptibility.25,43–46 In this regard, an explanation for our failure to detect an influence of AgP on pregnancy outcomes could relate to fundamental biologic differences between AgP and gingivitis or chronic periodontitis. For example, inflammation is a hallmark of preterm labor,47,48 and aberrations in the systemic inflammatory response, such as impaired neutrophil function or alterations in T-cell regulatory profiles that control inflammatory responses, may provide a more favorable environment for pregnancy in AgP subjects. Additionally, we and others have noted differences in the microbial flora of AgP compared to chronic periodontitis and gingivitis49 that could account for variable pathogenic potential with respect to effects on pregnancy outcomes.
CONCLUSIONS
The data from this study fail to demonstrate an impact of AgP on birth weight. Although a study that incorporates a significant amount of retrospective data has some clear weaknesses compared to an entirely prospective study, the typical age of onset and progression of AgP, and especially GAgP, would strengthen the interpretation of the result because it is likely that the majority of the AgP mothers were affected by the disease at the time of their childrens’ births.
ACKNOWLEDGMENTS
The work in this publication was supported by Grants P60 002256 and RO1 DE018125 from the National Institutes of Health, Bethesda, Maryland. The authors thank Ms. Kimberly Hollaway and Ms. Luanne Norville, Clinical Research Center for Periodontal Disease, Virginia Commonwealth University School of Dentistry, for their skillful clinical management of the volunteers who participated in this study, and Mr. Brian Bush, Department of Biostatistics, Virginia Commonwealth University, for expert database management.
Footnotes
JMP software, SAS, Cary, NC.
PROC GENMOD function, SAS.
The authors report no conflicts of interest related to this study.
REFERENCES
- 1.Matevosyan NR. Periodontal disease and perinatal outcomes. Arch Gynecol Obstet. 2011;283:675–686. doi: 10.1007/s00404-010-1774-9. [DOI] [PubMed] [Google Scholar]
- 2.Vergnes JN, Sixou M. Preterm low birth weight and maternal periodontal status: A meta-analysis. Am J Obstet Gynecol. 2007;196:135.e1-e7. doi: 10.1016/j.ajog.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 3.Offenbacher S, Boggess KA, Murtha AP, et al. Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol. 2006;107:29–36. doi: 10.1097/01.AOG.0000190212.87012.96. [DOI] [PubMed] [Google Scholar]
- 4.Jared H, Boggess KA, Moss K, et al. Fetal exposure to oral pathogens and subsequent risk for neonatal intensive care admission. J Periodontol. 2009;80:878–883. doi: 10.1902/jop.2009.080642. [DOI] [PubMed] [Google Scholar]
- 5.Lin D, Moss K, Beck JD, Hefti A, Offenbacher S. Persistently high levels of periodontal pathogens associated with preterm pregnancy outcome. J Periodontol. 2007;78:833–841. doi: 10.1902/jop.2007.060201. [DOI] [PubMed] [Google Scholar]
- 6.Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intraamniotic inflammation leading to preterm birth. J Clin Microbiol. 2009;47:38–47. doi: 10.1128/JCM.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokoyama M, Hinode D, Yoshioka M, et al. Relationship between Campylobacter rectus and periodontal status during pregnancy. Oral Microbiol Immunol. 2008;23:55–59. doi: 10.1111/j.1399-302X.2007.00391.x. [DOI] [PubMed] [Google Scholar]
- 8.Ebersole JL, Novak MJ, Michalowicz BS, et al. Systemic immune responses in pregnancy and periodontitis: Relationship to pregnancy outcomes in the Obstetrics and Periodontal Therapy (OPT) Study. J Periodontol. 2009;80:953–960. doi: 10.1902/jop.2009.080464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michalowicz BS, Hodges JS, Novak MJ, et al. Change in periodontitis during pregnancy and the risk of pre-term birth and low birthweight. J Clin Periodontol. 2009;36:308–314. doi: 10.1111/j.1600-051X.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Novak MJ, Novak KF, Hodges JS, et al. Periodontal bacterial profiles in pregnant women: Response to treatment and associations with birth outcomes in the Obstetrics and Periodontal Therapy (OPT) Study. J Periodontol. 2008;79:1870–1879. doi: 10.1902/jop.2008.070554. [DOI] [PubMed] [Google Scholar]
- 11.Wimmer G, Pihlstrom BL. A critical assessment of adverse pregnancy outcome and periodontal disease. J Clin Periodontol. 2008;35(Suppl. 8):380–397. doi: 10.1111/j.1600-051X.2008.01284.x. [DOI] [PubMed] [Google Scholar]
- 12.Vettore MV, Leão AT, Leal Mdo C, Feres M, Sheiham A. The relationship between periodontal disease and preterm low birthweight: Clinical and microbiological results. J Periodontal Res. 2008;43:615–626. doi: 10.1111/j.1600-0765.2007.01027.x. [DOI] [PubMed] [Google Scholar]
- 13.Vettore MV, Leal M, Leão AT, da Silva AM, Lamarca GA, Sheiham A. The relationship between periodontitis and preterm low birthweight. J Dent Res. 2008;87:73–78. doi: 10.1177/154405910808700113. [DOI] [PubMed] [Google Scholar]
- 14.Jeffcoat M, Parry S, Sammel M, Clothier B, Catlin A, Macones G. Periodontal infection and preterm birth: Successful periodontal therapy reduces the risk of preterm birth. BJOG. 2011;118:250–256. doi: 10.1111/j.1471-0528.2010.02713.x. [DOI] [PubMed] [Google Scholar]
- 15.Jeffcoat MK, Hauth JC, Geurs NC, et al. Periodontal disease and preterm birth: Results of a pilot intervention study. J Periodontol. 2003;74:1214–1218. doi: 10.1902/jop.2003.74.8.1214. [DOI] [PubMed] [Google Scholar]
- 16.López NJ, Smith PC, Gutierrez J. Periodontal therapy may reduce the risk of preterm low birth weight in women with periodontal disease: A randomized controlled trial. J Periodontol. 2002;73:911–924. doi: 10.1902/jop.2002.73.8.911. [DOI] [PubMed] [Google Scholar]
- 17.Michalowicz BS, Durand R. Maternal periodontal disease and spontaneous preterm birth. Periodontol 2000. 2007;44:103–112. doi: 10.1111/j.1600-0757.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- 18.Macones GA, Parry S, Nelson DB, et al. Treatment of localized periodontal disease in pregnancy does not reduce the occurrence of preterm birth: Results from the Periodontal Infections and Prematurity Study (PIPS) Am J Obstet Gynecol. 2010;202:147.e1–8. doi: 10.1016/j.ajog.2009.10.892. [DOI] [PubMed] [Google Scholar]
- 19.Boggess KA. Treatment of localized periodontal disease in pregnancy does not reduce the occurrence of preterm birth: Results from the Periodontal Infections and Prematurity Study (PIPS) Am J Obstet Gynecol. 2010;202:101–102. doi: 10.1016/j.ajog.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Armitage GC. Development of a classification system for periodontal diseases and conditions. Northwest Dent. 2000;79:31–35. [PubMed] [Google Scholar]
- 21.Löe H, Brown LJ. Early onset periodontitis in the United States of America. J Periodontol. 1991;62:608–616. doi: 10.1902/jop.1991.62.10.608. [DOI] [PubMed] [Google Scholar]
- 22.Diehl SR, Wu T, Michalowicz BS, et al. Quantitative measures of aggressive periodontitis show substantial heritability and consistency with traditional diagnoses. J Periodontol. 2005;76:279–288. doi: 10.1902/jop.2005.76.2.279. [DOI] [PubMed] [Google Scholar]
- 23.Marazita ML, Burmeister JA, Gunsolley JC, Koertge TE, Lake K, Schenkein HA. Evidence for autosomal dominant inheritance and race-specific heterogeneity in early-onset periodontitis. J Periodontol. 1994;65:623–630. doi: 10.1902/jop.1994.65.6.623. [DOI] [PubMed] [Google Scholar]
- 24.Schenkein HA, Best AM, Brooks CN, et al. Anticardiolipin and increased serum adhesion molecule levels in patients with aggressive periodontitis. J Periodontol. 2007;78:459–466. doi: 10.1902/jop.2007.060305. [DOI] [PubMed] [Google Scholar]
- 25.Schenkein HA, Koertge TE, Brooks CN, Sabatini R, Purkall DE, Tew JG. IL-17 in sera from patients with aggressive periodontitis. J Dent Res. 2010;89:943–947. doi: 10.1177/0022034510369297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silness J, Löe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 27.Löe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–551. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 28.Muhlemann HR, Son S. Gingival sulcus bleeding: A leading symptom in initial gingivitis. Helv Odont Acta. 1971;15:107–113. [PubMed] [Google Scholar]
- 29.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 30.Tilley BC, Barnes AB, Bergstralh E, et al. A comparison of pregnancy history recall and medical records. Implications for retrospective studies. Am J Epidemiol. 1985;121:269–281. doi: 10.1093/oxfordjournals.aje.a113997. [DOI] [PubMed] [Google Scholar]
- 31.Seidman DS, Slater PE, Ever-Hadani P, Gale R. Accuracy of mothers’ recall of birthweight and gestational age. Br J Obstet Gynaecol. 1987;94:731–735. doi: 10.1111/j.1471-0528.1987.tb03717.x. [DOI] [PubMed] [Google Scholar]
- 32.Gayle HD, Yip R, Frank MJ, Nieburg P, Binkin NJ. Validation of maternally reported birth weights among 46,637 Tennessee WIC program participants. Public Health Rep. 1988;103:143–147. [PMC free article] [PubMed] [Google Scholar]
- 33.Olson JE, Shu XO, Ross JA, Pendergrass T, Robison LL. Medical record validation of maternally reported birth characteristics and pregnancy-related events: A report from the Children’s Cancer Group. Am J Epidemiol. 1997;145:58–67. doi: 10.1093/oxfordjournals.aje.a009032. [DOI] [PubMed] [Google Scholar]
- 34.Tomeo CA, Rich-Edwards JW, Michels KB, et al. Reproducibility and validity of maternal recall of pregnancy- related events. Epidemiology. 1999;10:774–777. [PubMed] [Google Scholar]
- 35.Walton KA, Murray LJ, Gallagher AM, Cran GW, Savage MJ, Boreham C. Parental recall of birthweight: A good proxy for recorded birthweight? Eur J Epidemiol. 2000;16:793–796. doi: 10.1023/a:1007625030509. [DOI] [PubMed] [Google Scholar]
- 36.Offenbacher S, Lieff S, Boggess KA, et al. Maternal periodontitis and prematurity. Part I: Obstetric outcome of prematurity and growth restriction. Ann Periodontol. 2001;6:164–174. doi: 10.1902/annals.2001.6.1.164. [DOI] [PubMed] [Google Scholar]
- 37.Stabholz A, Soskolne WA, Shapira L. Genetic and environmental risk factors for chronic periodontitis and aggressive periodontitis. Periodontol 2000. 2010;53:138–153. doi: 10.1111/j.1600-0757.2010.00340.x. [DOI] [PubMed] [Google Scholar]
- 38.de Carvalho FM, Tinoco EM, Govil M, Marazita ML, Vieira AR. Aggressive periodontitis is likely influenced by a few small effect genes. J Clin Periodontol. 2009;36:468–473. doi: 10.1111/j.1600-051X.2009.01410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khader Y, Al-shishani L, Obeidat B, et al. Maternal periodontal status and preterm low birth weight delivery: A case-control study. Arch Gynecol Obstet. 2009;279:165–169. doi: 10.1007/s00404-008-0696-2. [DOI] [PubMed] [Google Scholar]
- 40.Gomes-Filho IS, Cruz SS, Rezende EJ, et al. Exposure measurement in the association between periodontal disease and prematurity/low birth weight. J Clin Periodontol. 2007;34:957–963. doi: 10.1111/j.1600-051X.2007.01141.x. [DOI] [PubMed] [Google Scholar]
- 41.Boggess KA, Lieff S, Murtha AP, Moss K, Beck J, Offenbacher S. Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstet Gynecol. 2003;101:227–231. doi: 10.1016/s0029-7844(02)02314-1. [DOI] [PubMed] [Google Scholar]
- 42.Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, Hauth JC. Periodontal infection and preterm birth: Results of a prospective study. J Am Dent Assoc. 2001;132:875–880. doi: 10.14219/jada.archive.2001.0299. [DOI] [PubMed] [Google Scholar]
- 43.Schenkein HA, Barbour SE, Tew JG. Cytokines and inflammatory factors regulating immunoglobulin production in aggressive periodontitis. Periodontol 2000. 2007;45:113–127. doi: 10.1111/j.1600-0757.2007.00214.x. [DOI] [PubMed] [Google Scholar]
- 44.Diehl SR, Wu T, Burmeister JA, et al. Evidence of a substantial genetic basis for IgG2 levels in families with aggressive periodontitis. J Dent Res. 2003;82:708–712. doi: 10.1177/154405910308200910. [DOI] [PubMed] [Google Scholar]
- 45.Barbour SE, Ishihara Y, Fakher M, et al. Monocyte differentiation in localized juvenile periodontitis is skewed toward the dendritic cell phenotype. Infect Immun. 2002;70:2780–2786. doi: 10.1128/IAI.70.6.2780-2786.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang JB, Quinn SM, Rausch M, Gunsolley JC, Schenkein HA, Tew JG. Hyper-immunoglobulin G2 production by B cells from patients with localized juvenile periodontitis and its regulation by monocytes. Infect Immun. 1996;64:2004–2009. doi: 10.1128/iai.64.6.2004-2009.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vrachnis N, Vitoratos N, Iliodromiti Z, Sifakis S, Deligeoroglou E, Creatsas G. Intrauterine inflammation and preterm delivery. Ann N Y Acad Sci. 2010;1205:118–122. doi: 10.1111/j.1749-6632.2010.05684.x. [DOI] [PubMed] [Google Scholar]
- 48.Genc MR, Ford CE. The clinical use of inflammatory markers during pregnancy. Curr Opin Obstet Gynecol. 2010;22:116–121. doi: 10.1097/GCO.0b013e3283374ac8. [DOI] [PubMed] [Google Scholar]
- 49.Moore WE, Moore LV. The bacteria of periodontal diseases. Periodontol 2000. 1994;5:66–77. doi: 10.1111/j.1600-0757.1994.tb00019.x. [DOI] [PubMed] [Google Scholar]