Abstract
The mechanism of cisplatin resistance in cancer cells is not fully understood. Here, we show that the Akt/mTOR survival pathway plays an important role in cisplatin resistance in human ovarian cancer cells. Specifically, we found that cisplatin treatment activates the Akt/mTOR survival pathway and that inhibition of this pathway by the PI3 K inhibitor LY294002 or knockdown of Akt sensitizes ovarian cancer cells to cisplatin. Furthermore, we generated cisplatin-resistant cells and found that resistant cells express a higher level of activated Akt as compared to their cisplatin sensitive counterparts. Importantly, inhibition of Akt or mTOR sensitized resistant cells to cisplatin-induced apoptosis. Taken together, our data indicate that activation of the Akt/mTOR pathway prevents cisplatin-induced apoptosis, leading to cisplatin resistance. Therefore, our study suggests that cisplatin resistance can be overcome by targeting the Akt/mTOR survival pathway in human ovarian cancer cells.
Keywords: Cisplatin resistance, Ovarian cancer cells, Akt, mTOR, Apoptosis
1. Introduction
Cisplatin (cis-diamminedichloroplatinum), a platinum compound, has been an important chemotherapeutic drug for the treatment of many cancers, including ovarian, lung, testicular, and bladder cancers, as a single agent or in combination with other anticancer agents [1–4]. Although its biochemical mechanisms of action are not fully understood, accumulating evidence indicates that cisplatin covalently binds to the N-7 atoms of purines on DNA to form DNA adducts. Formation of DNA adducts distorts DNA conformation and inhibits replication and transcription, resulting in the activation of multiple signaling pathways to induce cell cycle arrest and apoptosis. Cisplatin is the first line chemotherapeutic agent for several cancers, particularly for patients with testicular and ovarian cancers, because some cancer cells develop acquired cisplatin resistance while other tumor cells are intrinsically resistant, cisplatin resistance limits its use in cancer patients [5,6]. The mechanisms of cisplatin resistance are not fully understood, but they are believed to be multifactorial in nature. They include insufficient DNA binding, increased detoxification, increased DNA repair, deregulated expression of transporters, and altered expression and activation of genes involved in cell death pathways, such as p53, Bcl-2, and Akt/mTOR [2,7–14]. Among these pathways, the activation of the Akt/mTOR pathway plays an important role in cisplatin resistance [13,15].
The PI3 K/Akt/mTOR signaling pathway is a prototypic survival pathway that plays a central role in diverse cellular functions, including proliferation, growth, survival, and metabolism [16,17]. Upon growth factor stimulation, PI3 K is activated to phosphorylate phosphatidylinositol-3,4-bisphosphate (PIP2), converting it to phosphatidylinsitol-3,4,5-triphosphate (PIP3). PIP3 binds to Akt and then translocates to the plasma membrane where Akt is activated by sequential phosphorylation at T308 and S473 residues [16]. Once activated, Akt can phosphorylate many substrates to exert its functions. The best-characterized substrate of Akt is the serine/threonine kinase mTOR (mammalian target of rapamycin). Akt can directly activate mTOR or indirectly activate mTOR by phosphorylating and inactivating tuberous sclerosis complex 2 (TSC2). Activated mTOR forms a complex with Raptor to activate ribosomal protein S6 kinases (S6 K) and inhibit 4E-BP, leading to increased protein translation [16]. It is established that activation of the Akt/mTOR pathway can increase cell survival and apoptosis resistance [16,17]. For example, overexpression of Akt increases drug resistance [18,19]. However, the mechanism by which the Akt/mTOR survival pathway in cisplatin resistance in ovarian cancer is not fully understood.
In this study, we demonstrate that increased activation of the Akt/mTOR pathway plays an important role in cisplatin resistance. We show that cisplatin activates the Akt/mTOR survival pathway and that blockade of its activation by the PI3 K inhibitor LY294002 or knockdown of Akt expression by siRNA sensitizes resistant cells to cisplatin. We also show that ovarian cancer cells with acquired cisplatin resistance have an increased activation of the Akt/mTOR survival pathway, as compared to their sensitive counterparts. Furthermore, inhibition of the Akt/mTOR pathway sensitizes resistant cells to cisplatin. These results suggest that increased activation of the Akt/mTOR pathway is associated with cisplatin resistance in ovarian cancer cells.
2. Materials and methods
2.1. Reagents and antibodies
Cisplatin and monoclonal antibody against actin were purchased from Sigma (St. Louise, MO). The PI3 K inhibitor LY294002 was obtained from LC Laboratories (Woburn, MA). Rapamycin was purchased from LKT Laboratories (St. Paul, MN). Rabbit polyclonal antibodies against total or phosphorylated Akt, GSK3β, p70S6 K, mTOR and PARP were purchased from Cell Signaling Technology (Beverly, MA).
2.2. Cell lines and culture conditions
The human ovarian cancer cell lines TOV112D, CAOV3, OV433, DOV13, SKOV3 and RMG1 were kindly provided by Dr. Samuel Mok (Brigham and Women’s Hospital, Boston, MA) [20]. All cell lines were maintained in MCDB105/M199 except CAOV3 and SKOV3, which were maintained in DMEM and RPMI1640, respectively. All media were supplemented with 10% fetal bovine serum and antibiotics. Cells were incubated at 37 °C in 5% CO2.
2.3. Establishment of cisplatin-resistant ovarian cancer cells
The ovarian cancer cell OV433 was chronically exposed to gradually increased concentrations of cisplatin starting with 0.1–1.2 µg/ml. After 6 months, acquired resistant cells were obtained. These cells were maintained in medium containing 1.2 µg/ml cisplatin, as described previously [21].
2.4. MTT assays
The MTT assay was performed as previously described [22]. Briefly, cells were left untreated or pretreated with 10 µmol/L LY294002 or 50 nM rapamycin for 30 min, and then treated with cisplatin. After incubation with MTT solution, isopropanol was added to dissolve the formazan crystals. Absorbance was measured using a Vmax Microplate Reader (Molecular Devices, Sunnyvale, CA) at a wavelength of 590 nm. Cell proliferation was calculated from the means of pooled data from three independent experiments.
2.5. Western blot analysis
Whole cell lysates were prepared as described previously [22], and protein concentration was determined using the Protein Assay Kit (Bio-Rad, Hercules, CA). Cell lysates (100 µg) were electrophoresised through 10% or 12% denaturing polyacrylamide gels and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA). The blots were probed with primary antibodies and appropriate secondary antibodies conjugated with HRP, and then developed using enhanced chemiluminescence (ECL) or ECL-plus Reagent (Amersham Pharmacia Biotech, Piscataway, NJ) according to the manufacturer’s protocol.
2.6. Knockdown of Akt by siRNA
Signalsilence® Akt siRNA and non-target siRNA were purchased from Cell Signaling Technology. siRNA transfections were performed using LipofectAMINE 2000 (Invitrogen, Carsbad, CA) according to manufacturer’s protocol. Briefly, SKOV3 cells were placed into 6-well plates. The next day, cells were transfected with Akt siRNA or non-target siRNA by using LipofectAMINE 2000. The transfected cells were harvested 3 days after transfection, and whole cell lysates were isolated for detecting Akt expression by Western blot assays. For MTT assay, transfected cells were placed into 96-well plates. The next day, cells were then treated with vehicle or 50 µg/ml cisplatin for 48 h, followed by MTT assay to determine cell proliferation [22].
3. Results
3.1. Cisplatin inhibits the growth of ovarian cancer cells
To study the mechanism of cisplatin resistance, we first examined cisplatin sensitivity in three ovarian cancer cell lines including OV433, SKOV3, and TOV112D. We treated these cells with various doses of cisplatin for 48 h and then examined growth inhibition by MTT assays. Fig. 1A shows that cisplatin inhibited the growth of all three ovarian cancer cell lines; TOV112D cells were the most sensitive line whereas SKOV3 cells were the most resistant lines while OV433 cells fell in between. These data indicates that each ovarian cancer cell line has different cisplatin sensitivity.
Fig. 1.
Role of cisplatin in inhibition of cell proliferation and activation of the Akt/mTOR survival pathway. (A) Effect of cisplatin on growth inhibition. Human ovarian cancer cell lines OV433, SKOV3, and TOV112D were treated with various concentrations of cisplatin for 48 h. Cell growth inhibition was determined by MTT assays. Cell proliferation data are expressed as percentage of untreated cells. Representative of three independent experiments. (B and C) Activation of Akt and mTOR by cisplation. SKOV3 and TOV112D cells were treated with 50 µg/ml of cisplatin (Cis) for 1, 3, 5, and 7 h. The activation of the Akt/mTOR pathway was analyzed by Western blotting with antibodies against total and phosphorylated Akt (Thr308), phosphorylated GSK3β (S9), phosphorylated p70S6 K (T389), and phosphorylated mTOR (T2448). The level of actin was used as loading control. (D) Ovarian cancer cell lines including RMG1, DOV13, CAOV3, OV433, and TOV112D were treated with 50 µg/ml of cisplatin for 7 h. Total cells were harvested, and the levels of the indicated molecules were determined by Western blotting.
3.2. Cisplatin activates the Akt/mTOR survival pathway
It has been reported that the Akt/mTOR is upregulated in ovarian cancer [13]. Because this pathway plays a role in cell survival and chemoresistance, it is possible that activation of this pathway contributes to cisplatin resistance. To test this possibility, we treated SKOV3 and TOV112D cells with cisplatin for various time points, and then examined the activation of the Akt/mTOR signaling pathway. As shown in Fig. 1B and C, the levels of phosphorylated Akt were increased as early as 3 h and lasted up at least 7 h in these cells after cisplatin treatment. Such activation was not due to an increase in the total Akt protein, which remained constant (Fig. 1B and C). Importantly, mTOR, a downstream signaling molecule of Akt, and p70S6 K, downstream signaling molecules of mTOR, were also activated by cisplatin although their activation was not closely correlated with Akt activation (Fig. 1B and C). For example, phosphorylated mTOR was detected during 1–5 h treatment in TOV112D cells while such activation was only detected in one hour following cisplatin treatment in SKOV3 cells. Furthermore, we showed that activation of these molecules is also detected in three more ovarian cancer cell lines (i.e., RMG1, DOV13, and CAOV3 cells) (Fig. 1D). Thus, our results suggest that activation of the Akt/mTOR pathway is a common event in ovarian cancer cell lines and that this pathway may be important in cisplatin resistance.
3.3. Blockage of Akt activation enhances cisplatin-induced cell death
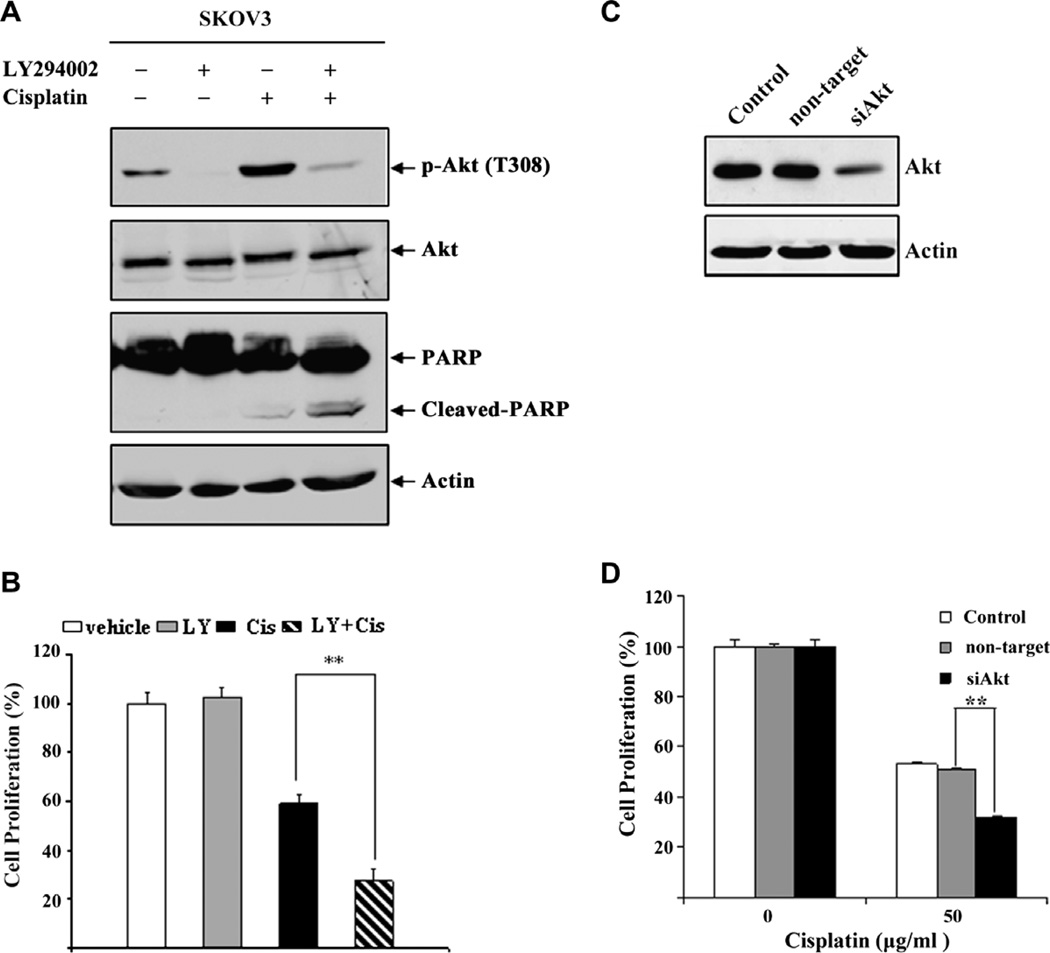
It is established that the Akt/mTOR pathway plays an important role in cell survival and chemoresistance. Therefore, activation of the Akt/mTOR survival pathway could counteract cisplatin-induced cell death, leading to cisplatin resistance. To test this, we treated SKOV3 cells with the PI3 K inhibitor LY294002 and then examined the effect of Akt inhibition on cisplatin-induced apoptosis. As shown in Fig. 2A, cisplatin treatment caused Akt phosphorylation in SKOV3 cells, which was abolished by LY294002, showing that Akt activation is PI3 K dependent. Similarly, we also showed that activation of Akt is blocked by LY294002 in OV433 cells (Supplementary Fig. 1A). Importantly, inhibition of Akt phosphorylation by LY294002 significantly increased cisplatin-induced PARP cleavage as compared to cells treated with cisplatin or LY294002 alone (Fig. 2A). Furthermore, we showed that combined treatment of cisplatin and LY294002 significantly inhibits cell proliferation as compared to either agent alone (Fig. 2B). Similar results were obtained with OV433 cells (Supplementary Fig. 1B). These results clearly indicate that the activation of Akt plays an important role in cisplatin resistance in cancer cells.
Fig. 2.
Inhibition and knockdown of Akt activity increases cisplatin-induced cell death. (A) Effect of LY294002 on Akt activation. SKOV3 cells were treated with vehicle (DMSO), 20 µM LY294002, 50 µg/ml cisplatin, or their combination. After 7 h treatment, total cell were harvested, and the levels of p-Akt, Akt and PARP were determined by Western blot analysis. Actin was used as a loading control. (B) Effect of LY294002 on growth inhibition. SKOV3 cells were serum starved for 24 h, and then treated with 20 µM LY294002, 50 µg/ml cisplatin or their combination. After 2 days, cell proliferation was determined by MTT assays. (C) Akt knockdown. SKOV3 cells were transfected with Akt or control siRNAs. After 3 days, total protein was extracted for assaying the levels of total Akt protein by Western blot analysis. Actin was used as a loading control. (D) Effect of Akt knockdown on cell proliferation. SKOV3 cells were plated at 6 × 105 per well in six-well plates. The next day, cells were transfected with Akt or control siRNAs using LipofectAMINE 2000. After 2 days, cells were treated with 50 µg/ml cisplatin or DMSO for 48 h, and cell proliferation was determined by MTT assays. Data are expressed as percentage of untreated cells. Representative of three independent experiments. *P < 0.01, statistical significance.
3.4. Knockdown of Akt sensitizes ovarian cancer cell SKOV3 to cisplatin
We showed that treatment with the PI3 K inhibitor LY294002 increased cisplatin-induced apoptosis and growth inhibition. Because LY294002 may inhibit other kinases, the results obtained with LY294002 may not completely reflect the role of Akt in cisplatin resistance. To investigate the direct role of Akt activation in cisplatin resistance, we used siRNA to knock down Akt expression and then examined the effect of Akt knockdown on cisplatin- induced growth inhibition. We transfected SKOV3 cells with siRNA against Akt (Akt 1/2/3) or control siRNA and showed that Akt was efficiently knocked down in Akt siRNA transfected cells, as compared to cells transfected with control siRNA (Fig. 2C). Importantly, we showed that knockdown of Akt increased cisplatin-induced growth inhibition as compared to the same cells transfected with control siRNA (Fig. 2D). Thus, our results indicate that while cisplatin can cause apoptosis, it also activates the Akt survival pathway to counteract cisplatin-induced apoptosis.
3.5. Ovarian cancer cells with acquired cisplatin resistance express a higher level of phosphorylated Akt
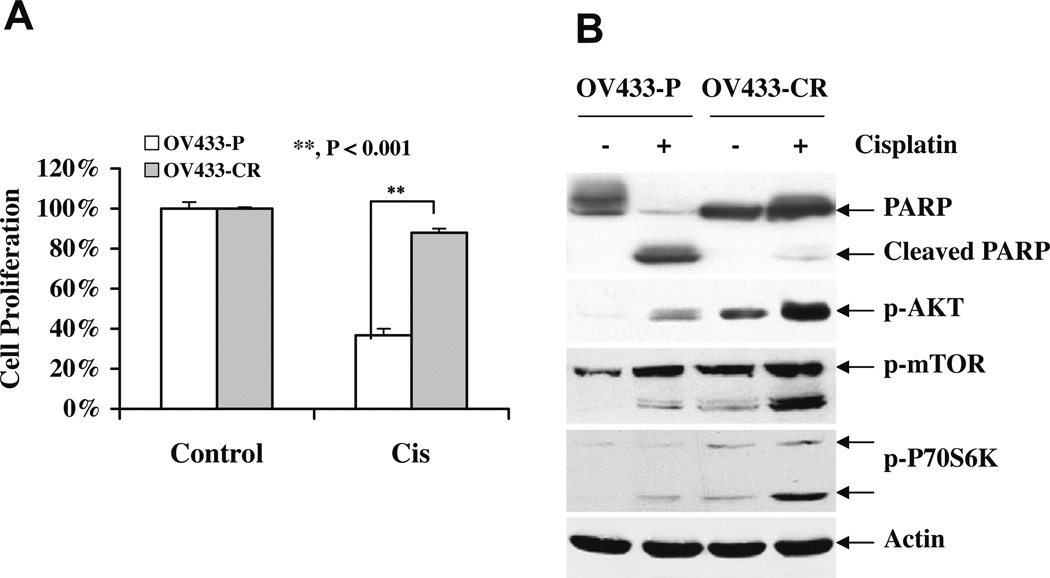
It is established that most ovarian cancer patients initially respond to cisplatin, but the majority of responsive patients relapse due to the development of acquired resistance [5,6]. To determine if Akt plays a role in acquired cisplatin resistance, we established a cisplatin-resistant ovarian cancer cell line OV433 named OV433-CR by exposing the parental OV433 (OV433-P) cells to gradually increased concentrations of cisplatin starting from 0.1 to 1.2 µg/ml for over 6 months. As shown in Fig. 3A, OV433-CR cells were much more resistant than OV433-P cells to cisplatin. To maintain the acquired resistance to cisplatin, we routinely cultured OV433-CR in medium containing 1.2 µg/ml cisplatin. Importantly, OV433-CR cells had higher levels of activated Akt than OV433-P cells in the absence and presence of cisplatin treatment (Fig. 3B). Furthermore, increased activation of mTOR and its downstream target p70S6 K was also detected in OV433-CR cells over OV433-P cells. These data clearly show that sustained or chronic exposure to cisplatin results in increased activation of the Akt/mTOR pathway, suggesting that elevated activation of the Akt/mTOR axis may contribute to acquired cisplatin resistance.
Fig. 3.
Akt/mTOR activation in cisplatin-resistant cells. (A) Cisplatin sensitivity. OV433 cells were chronically exposed to gradually increased concentrations of cisplatin starting from 0.1 to 0.8 µg/ml for over 6 months. Both parental (P) and cisplatin-resistant (CR) cells were treated with cisplatin (50 µM) for 24 h, and cisplatin sensitivity was determined by MTT assays. Data are representative of three independent experiments. **P < 0.001, statistically significant. (B) Activation of the Akt/mTOR pathway by cisplatin. OV433-P and -CR cells were treated with cisplatin (50 µM) for 24 h. Total protein was extracted, and the levels of PARP, phosphorylated Akt, mTOR, and p70S6 K were determined by Western blot analysis. Actin was used as a loading control.
3.6. Inhibition of Akt/mTOR activation enhances cisplatin-induced cell death in resistant cells
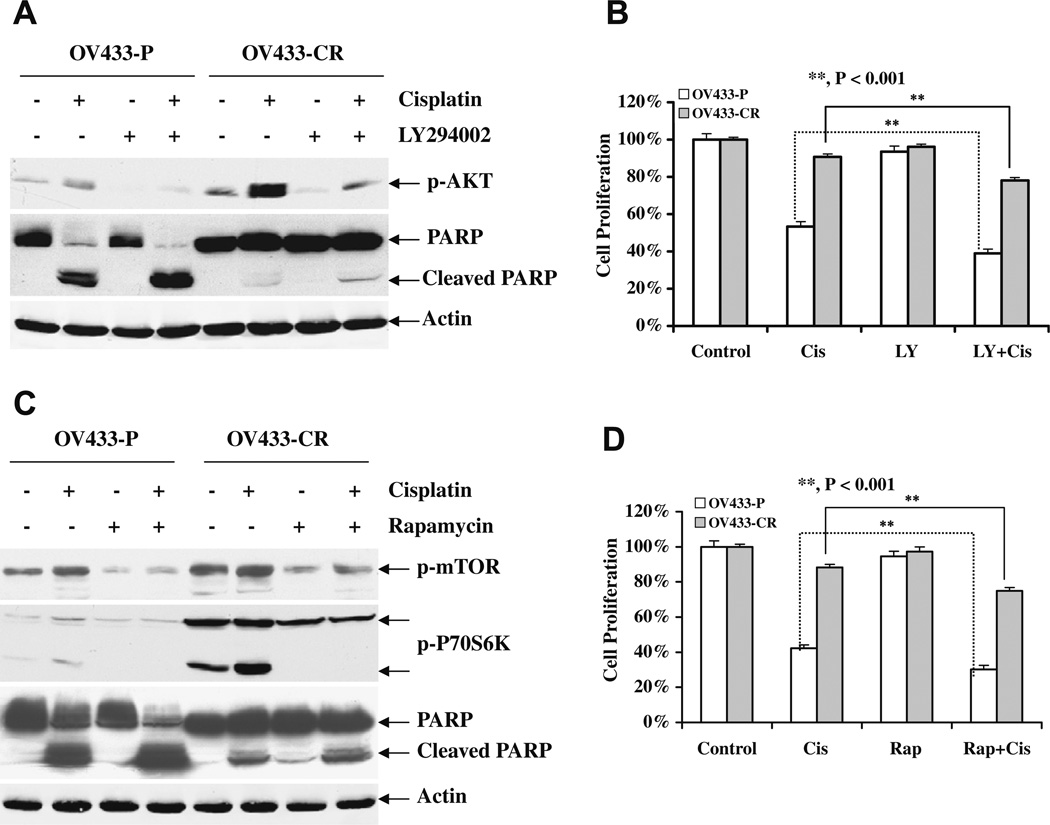
To further understand the role of the activation of the Akt/mTOR axis in acquired cisplatin resistance, we first treated OV433-P and OV433-CR cells with cisplatin in the presence and absence of the Akt inhibitor LY294002, and Akt activation and PARP cleavage were determined. As shown in Fig. 4A, LY294002 effectively inhibited Akt activation in the presence and absence of cisplatin treatment. Furthermore, we showed that LY284002 enhances cisplatin-induced apoptosis in both OV433-P and -CR cells. Importantly, LY284002 enhanced cisplatin-induced growth inhibition in OV433-CR cells (Fig. 4B), which suggests that Akt indeed plays a role in cisplatin resistance. To determine whether mTOR is involved in acquired cisplatin resistance, we treated OV433-P and -CR cells with the mTOR inhibitor rapamycin and then determined the effect of rapamycin on cisplatin-induced cell death. As expected, rapamycin inhibited phosporylation of mTOR and its downstream target p70S6 K (Fig. 4C). Furthermore, rapamycin increased cisplatin-induced PARP cleavage in both parental and resistant OV433 cells. More importantly, rapamycin enhanced cisplatin-induced growth inhibition in OV433-CR cells (Fig. 4D), Taken together, our data suggest that the activation of the Akt/mTOR axis is indeed involved in cisplatin resistance including acquired cisplatin resistance.
Fig. 4.
Effect of Akt/mTOR inhibition on cisplatin sensitivity and apoptosis in OV433 cells. (A), Increased cisplatin-induced apoptosis by Akt inhibition. OV433-P and -CR cells were treated with cisplatin (50 µM) in the presence or absence of LY294002 (10 µM) for 24 h. Total protein was extracted, and Akt phosphorylation and PARP cleavage were determined by Western blot analysis. (B) Akt inhibition increased cisplatin-induced growth inhibition. Both OV433-P and -CR cells were treated as described in (A) and growth inhibition was determined by MTT assays. (C) Increased cisplatin-induced apoptosis by mTOR inhibition. OV433-P and -CR cells were treated with cisplatin (50 µM) in the presence or absence of rapamycin (50 nM) for 24 h. Total protein was extracted, and the levels of PARP, phosphorylated mTOR and p70S6 K were determined by Western blot analysis. (D) mTOR inhibition increased cisplatin-induced growth inhibition. Both OV433-P and -CR cells were treated as described in (C) and growth inhibition was determined by the MTT assay. Data are representative of three independent experiments. **P < 0.001, statistically significant. Cis, cisplatin; LY, LY294002; Rap, rapamycin.
4. Discussion
In this study, we show that cisplatin activates the Akt/mTOR survival pathway. We also show that inhibition of the Akt/mTOR pathway increases cisplatin-induced apoptosis. Furthermore, we find that the activation of the Akt/mTOR pathway is elevated in acquired cisplatin-resistant ovarian cancer cell line OV433. Importantly, inhibition of the Akt/mTOR activation increases apoptosis in acquired cisplatin-resistant cells. Thus, our findings suggest that targeting the Akt/mTOR may overcome cisplatin resistance in ovarian cancer cells.
Previous studies suggested that overexpression of Akt and its upstream regulator PI3 K increased drug resistance [19,23], but the underlying mechanism is not fully understood. In this study, we showed that while cisplatin induces apoptosis in cancer cells, it also activates the Akt/mTOR survival pathway, which may counteract cisplatin-induced apoptosis, leading to cisplatin resistance. Furthermore, we found that the Akt/mTOR pathway is activated in four ovarian cancer cell lines tested (Fig. 1), suggesting that the activation of the Akt/mTOR pathway may be a common event in ovarian cancer cells. Consistent with the role of Akt/mTOR in inhibiting cisplatin-induced apoptosis, we found that inhibition of Akt activation by its pharmacological inhibitor LY294002 or knockdown of its expression by siRNA sensitizes ovarian cancer cells to cisplatin (Fig. 2 and Supplementary Fig. 1). Thus, these data suggest that activation of the Akt/mTOR survival pathway plays an important role in cisplatin resistance in ovarian cancer cells.
It is established that Akt exerts its biological functions by phosphorylating its downstream substrates including mTOR. Phosphorylated mTOR becomes activated, leading to an increase in protein translation and cell survival [16]. In addition to activation of Akt, we found that cisplatin treatment can cause mTOR phosphorylation and subsequent activation (Fig. 1). This suggests that cisplatin may activate the Akt/mTOR axis to counteract cisplatin-induced apoptosis, leading to cisplatin resistance. Consistent with role of mTOR in Akt activation by cisplatin, we showed that the mTOR inhibitor rapamycin enhances cisplatin-induced apoptosis (Fig. 4), which mimics inhibition of Akt activity. Thus, we believe that cisplatin induces the Akt/mTOR axis to counteract cisplatin-induced apoptosis, leading to chemoresistance.
Cisplatin is the first line chemotherapeutic agent for patients with ovarian cancers, because some cancer cells develop acquired cisplatin resistance while other tumor cells are intrinsically resistant, cisplatin resistance limits its use in ovarian cancer patients. For example, 70% of ovarian cancer patients initially respond to cisplatin, but the majority of responsive patients relapse due to the development of acquired resistance [5,6]. Therefore, it is critical for understanding the mechanism of acquired cisplatin resistance. To this end, we established cisplatin-resistant OV433 cells and found that phosphorylation of Akt, mTOR, and p70S6 K is elevated in cisplatin-resistant cells as compared to the parental cells (Fig. 3), which suggests that the Akt/mTOR pathway is overactivated. More importantly, we showed that inhibition of either Akt or mTOR by their phamarcological inhibitors increases cisplatin-induced-apoptosis and growth inhibition (Fig. 4), which further validates the role of the Akt/mTOR axis in acquired cisplatin resistance. Collectively, these data indicate that increased activation of the Akt/mTOR survival pathway contributes acquired cisplatin resistance, which can be therapeutically targeted for improvement of cisplatin efficacy.
In summary, we demonstrate that cisplatin activates the Akt/mTOR survival pathway. We also demonstrate that inhibition of the Akt/mTOR survival pathway sensitizes ovarian cancer cells to cisplatin. More importantly, we show that the Akt/mTOR survival pathway is also increasingly activated in ovarian cancer cells with acquired cisplatin resistance and that inhibition of the Akt/mTOR pathway increased cisplatin-induced cell death in resistant cells. Therefore, our findings suggest that targeting the Akt/mTOR survival pathway could overcome cisplatin resistance in ovarian cancer cells.
Supplementary Material
Acknowledgments
We thank Dr. Samuel Mok for providing ovarian cancer cell lines. This work was supported by NIH Grant R01 CA100073 and Gail Purtan Ovarian Cancer Research Fund.
Footnotes
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbrc.2010.03.029.
References
- 1.Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J. Clin. Oncol. 1999;17:409–422. doi: 10.1200/JCO.1999.17.1.409. [DOI] [PubMed] [Google Scholar]
- 2.Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 3.Boeckman HJ, Trego KS, Turchi JJ. Cisplatin sensitizes cancer cells to ionizing radiation via inhibition of nonhomologous end joining. Mol. Cancer Res. 2005;3:277–285. doi: 10.1158/1541-7786.MCR-04-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarkaria JN, Schwingler P, Schild SE, Grogan PT, Mladek AC, Mandrekar SJ, Tan AD, Kobayashi T, Marks RS, Kita H, Miller RC, Limper AH, Leof EB. Phase I trial of sirolimus combined with radiation and cisplatin in non-small cell lung cancer. J. Thorac. Oncol. 2007;2:751–757. doi: 10.1097/JTO.0b013e3180cc2587. [DOI] [PubMed] [Google Scholar]
- 5.Tummala MK, McGuire WP. Recurrent ovarian cancer. Clin. Adv. Hematol. Oncol. 2005;3:723–736. [PubMed] [Google Scholar]
- 6.Agarwal R, Kaye SB. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat. Rev. Cancer. 2003;3:502–516. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 7.Johnson SW, Shen D, Pastan I, Gottesman MM, Hamilton TC. Cross-resistance, cisplatin accumulation, and platinum-DNA adduct formation and removal in cisplatin-sensitive and -resistant human hepatoma cell lines. Exp. Cell Res. 1996;226:133–139. doi: 10.1006/excr.1996.0211. [DOI] [PubMed] [Google Scholar]
- 8.Cullen KJ, Newkirk KA, Schumaker LM, Aldosari N, Rone JD, Haddad BR. Glutathione S-transferase pi amplification is associated with cisplatin resistance in head and neck squamous cell carcinoma cell lines and primary tumors. Cancer Res. 2003;63:8097–8102. [PubMed] [Google Scholar]
- 9.Turchi JJ. Nitric oxide and cisplatin resistance: no easy answers. Proc. Natl. Acad. Sci. USA. 2006;103:4337–4338. doi: 10.1073/pnas.0601001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dabholkar M, Vionnet J, Bostick-Bruton F, Yu JJ, Reed E. Messenger RNA levels of XPAC and ERCC1 in ovarian cancer tissue correlate with response to platinum-based chemotherapy. J. Clin. Invest. 1994;94:703–708. doi: 10.1172/JCI117388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perego P, Giarola M, Righetti SC, Supino R, Caserini C, Delia D, Pierotti MA, Miyashita T, Reed JC, Zunino F. Association between cisplatin resistance and mutation of p53 gene and reduced bax expression in ovarian carcinoma cell systems. Cancer Res. 1996;56:556–562. [PubMed] [Google Scholar]
- 12.Beale PJ, Rogers P, Boxall F, Sharp SY, Kelland LR. BCL-2 family protein expression and platinum drug resistance in ovarian carcinoma. Br. J. Cancer. 2000;82:436–440. doi: 10.1054/bjoc.1999.0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altomare DA, Wang HQ, Skele KL, De Rienzo A, Klein-Szanto AJ, Godwin AK, Testa JR. AKT and mTOR phosphorylation is frequently detected in ovarian cancer and can be targeted to disrupt ovarian tumor cell growth. Oncogene. 2004;23:5853–5857. doi: 10.1038/sj.onc.1207721. [DOI] [PubMed] [Google Scholar]
- 14.Kuo MT, Chen HH, Song IS, Savaraj N, Ishikawa T. The roles of copper transporters in cisplatin resistance. Cancer Metastasis Rev. 2007;26:71–83. doi: 10.1007/s10555-007-9045-3. [DOI] [PubMed] [Google Scholar]
- 15.Fraser M, Bai T, Tsang BK. Akt promotes cisplatin resistance in human ovarian cancer cells through inhibition of p53 phosphorylation and nuclear function. Int. J. Cancer. 2008;122:534–546. doi: 10.1002/ijc.23086. [DOI] [PubMed] [Google Scholar]
- 16.Hay N. The Akt-mTOR tango and its relevance to cancer. Cancer Cell. 2005;8:179–183. doi: 10.1016/j.ccr.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Plastaras JP, Dorsey JF, Carroll K, Kim SH, Birnbaum MJ, El-Deiry WS. Role of PI3K/Akt signaling in TRAIL- and radiation-induced gastrointestinal apoptosis. Cancer Biol. Ther. 2008;7:2047–2053. doi: 10.4161/cbt.7.12.7570. [DOI] [PubMed] [Google Scholar]
- 18.Nesterov A, Lu X, Johnson M, Miller GJ, Ivashchenko Y, Kraft AS. Elevated AKT activity protects the prostate cancer cell line LNCaP from TRAIL-induced apoptosis. J. Biol. Chem. 2001;276:10767–10774. doi: 10.1074/jbc.M005196200. [DOI] [PubMed] [Google Scholar]
- 19.Kandasamy K, Srivastava RK. Role of the phosphatidylinositol 3′-kinase/PTEN/Akt kinase pathway in tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in non-small cell lung cancer cells. Cancer Res. 2002;62:4929–4937. [PubMed] [Google Scholar]
- 20.Kwong J, Lee JY, Wong KK, Zhou X, Wong DT, Lo KW, Welch WR, Berkowitz RS, Mok SC. Candidate tumor-suppressor gene DLEC1 is frequently downregulated by promoter hypermethylation and histone hypoacetylation in human epithelial ovarian cancer. Neoplasia. 2006;8:268–278. doi: 10.1593/neo.05502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Zhou JY, Zhang L, Wu GS. Involvement of MKP-1 and Bcl-2 in acquired cisplatin resistance in ovarian cancer cells. Cell Cycle. 2009;8:3191–3198. doi: 10.4161/cc.8.19.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Zhou JY, Wu GS. ERK-dependent MKP-1-mediated cisplatin resistance in human ovarian cancer cells. Cancer Res. 2007;67:11933–11941. doi: 10.1158/0008-5472.CAN-07-5185. [DOI] [PubMed] [Google Scholar]
- 23.Lane D, Robert V, Grondin R, Rancourt C, Piche A. Malignant ascites protect against TRAIL-induced apoptosis by activating the PI3K/Akt pathway in human ovarian carcinoma cells. Int. J. Cancer. 2007;121:1227–1237. doi: 10.1002/ijc.22840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.