Abstract
Deregulation of cell signaling is a vital part of cancer development. The mitogen activated protein kinase (MAPK) family is involved in regulating both cell growth and cell death. This family of kinases is negatively regulated by mitogen activated protein kinase phosphatases (MKPs). MKPs are dual specificity phosphatases that target threonine and tyrosine residues that appear in a TXY motif. There are eleven members of the MKP family. Expression of MKPs has been shown to be altered in many different types of cancer. Most of what is known centers on MKP-1, MKP-2 and MKP-3. This review will focus on their role in cancer development and progression.
Keywords: MKP-1, MKP-2, MKP-3, dual specificity phosphatases, MAPK signaling, cancer
Introduction
When it comes to cell signaling, it is important to investigate and understand both how the signaling pathway is turned on and how it is turned off. This is especially important in cancer, where aberrant cell signaling is a hallmark of the disease.1 One pathway that is commonly activated in cancer is the mitogen activated protein kinase (MAPK) pathway. While much research has been done on how turning on this pathway could affect the development and progression of cancer, scientists are just beginning to understand the ways in which shutting it down can also affect these processes. Mitogen activated protein kinase phosphatases (MKPs) dephosphorylate MAP kinases and attenuate their signaling.2 This review will focus on what is currently known about the role of MKP involvement in cancer.
MAP Kinase Signaling
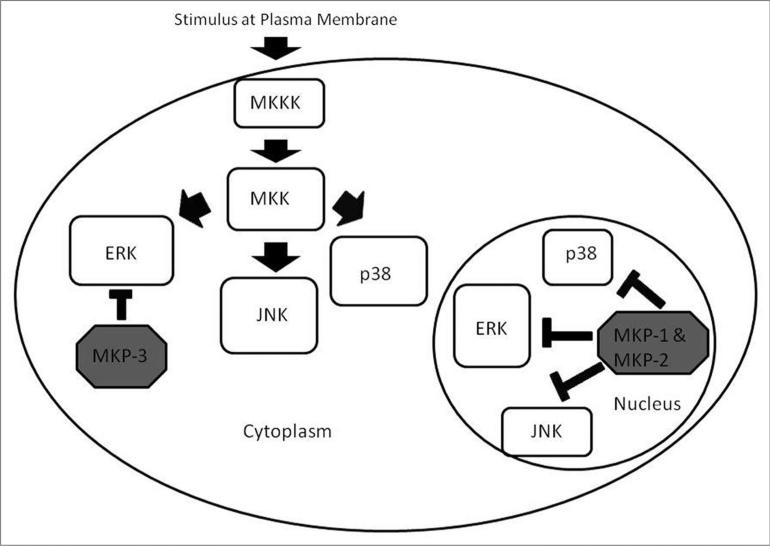
In order to fully appreciate the role MKPs play in MAP kinase signaling, it is helpful to understand the MAPK pathway itself. There are three branches of the MAPK cascade in mammalian cells: the extracellular signal regulated kinase (ERK), the c-Jun N-terminal kinase (JNK) and p38 MAP kinase. These three branches can be activated by varying cell stimuli, including growth factors, cytokines and cellular stress. ERK responds primarily to growth factors and thus promotes cell growth. JNK and p38 can have multiple effects, depending on the activating stimulus and context in which activation occurs.3-5 Response to cellular stress usually leads to apoptosis, while response to growth factors and cytokines usually promotes cell survival.3 MAP kinases receive stimuli from the cell surface through a signaling cascade. The general scheme is as follows: the stimulus is received at the plasma membrane, followed by activation of a MAP kinase kinase kinase (MKKK), activation of a MAP kinase Kinase (MKK) by a MKKK and finally activation of the MAPK by the MKK (Fig. 1).3 MAPKs are dually phosphorylated by the MKK on threonine and tyrosine residues in a TXY motif.2 All three of these kinases have multiple downstream targets, some of which include c-Jun and c-myc for ERK, c-Jun, Tau and IκBα for JNK and MAPKAP-K2 for p38 (reviewed in ref. 3). Due to their abilities to be activated by a variety of stimuli and to the variety of downstream targets, the three MAPK pathways are important players in the overall picture of cell signaling and must be tightly regulated.
Figure 1.
Regulation of MAP kinase signaling by MKP-1, MKP-2 and MKP-3. Cell signals are received at the plasma membrane and transmitted through the MAP kinase signaling cascade. Each MAPK family member has its own specific MKKKs and MKKs. MKP dephosphorylation attenuates MAP Kinase activity. ERK, JNK and p38 are dephosphorylated by MKP-1 and MKP-2. MKP-3 dephosphorylates ERK. The other MKP family members also regulate MAPKs by dephosphorylation. MKP-1, MKP-2 and MKP-3 are shown here because their activity is the main focus of this review.
Map Kinase Phosphatases: Overview
The tight control needed by the MAPK pathways to effectively carry out their functions is achieved partially through the activity of MAP kinase phosphatases (MKPs), which are the endogenous negative regulators of MAPKs.2 MKPs belong to the dual-specificity phosphatase (DUSP) family and can dephosphorylate MAPKs through their recognition of the TXY motif present on the MAPK family members.2 The MKP family is made up of eleven members and can be grouped according to subcellular localization. MKP-1 (DUSP1), PAC-1 (DUSP2), MKP-2 (DUSP4) and hVH3 (DUSP5) can all be found in the nucleus. MKP-3 (DUSP6), MKP-4 (DUSP9) and MKP-X (DUSP7) can be found in the cytoplasm. MKP-5 (DUSP10), MKP-7 (DUSP16) and hVH5 (DUSP8) can be found in both the nucleus and cytoplasm.2 The final member of the MKP family, MK-STYX, is an inactive phosphatase.3 It is grouped with the other MKPs based on structural similarity. The structure of MKPs is highlighted by the presence of a C-terminal catalytic domain and an N-terminal domain that has two regions that are similar to the catalytic domain of cdc25.6,7 ERK, JNK and p38 are all dephosphorylated by MKPs. MKP-1 and MKP-2 are able to act on ERK, JNK and p38, which gives this signaling pathway an added layer of complexity.2 MKP-3, MKP-X and hVH3 dephosphorylate ERKs. MKP-5, MKP-7 and hVH5 dephosphorylate JNK and p38. MKP-4 and PAC-1 are able to dephosphorylate ERK and p38.2 The surface has barely been scratched for finding out how MKP activity relates to cancer, but progress is being made (Table 1). Most of what is known relates to MKP-1, the founding, and best characterized, member of the MKP family.
Table 1.
MKPs altered in cancer
| Phosphatase | Specificity | Subcellular location | Altered in | References |
|---|---|---|---|---|
| MKP-1 | ERK, JNK, p38 | nuclear | bladder, breast, colon, lung, ovarian, prostate | 2, 4, 5, 9-15 |
| MKP-2 | ERK, JNK, p38 | nuclear | breast, liver, ovarian, pancreatic | 9, 15-17 |
| MKP-3 | ERK | cytoplasmic | breast, ovarian, pancreatic | 9, 18-22 |
| MKP-4 | ERK, p38 | cytoplasmic | skin | 24 |
| MKP-7 | JNK, p38 | nuclear & cytoplasmic | leukemia | 26, 27 |
| PAC-1 | ERK, p38 | nuclear | ovarian | 23 |
MKP-1
Not much is known about the normal physiological functions of many of the MKP family members, but there is some evidence that MKP-1 may play a role in negatively regulating the immune response to bacterial lipopolysaccharide by increasing both proand anti-inflammatory cytokine production.8 MKP-1 expression has been shown to be altered in colon, prostate, bladder, ovarian, breast and non-small cell lung cancers.2,9 In colon, bladder and prostate cancer, MKP-1 is overexpressed in the early stages of disease, but expression seems to be lost as the disease progresses.9 Microarray analysis of tumor samples from nine patients participating in a Phase I/II clinical trial examining a treatment regimen consisting of bevacizumab and radiation therapy in colorectal cancer revealed that MKP-1 mRNA expression was consistently and significantly downregulated in all samples. The size of the decrease varied from patient to patient.10 MKP-1 expression has been linked to clinical outcome in ovarian cancer, where it has been correlated with shorter progression-free survival.9 Moderate to strong MKP-1 expression was seen in 57.6% of invasive primary ovarian tumors (n = 66).11 In a study involving a panel of ovarian cancer cell lines, MKP-1 expression was induced after treatment with cisplatin. Knockdown of MKP-1 in these cells with siRNA increased cisplatin induced cell-death.4 This indicates that MKP-1 may have a role in chemotherapy resistance. Potential involvement in chemotherapy resistance has also been seen in both lung and breast cancer. In lung cancer, overexpression of MKP-1 increased resistance to cisplatin.12 Overexpression of MKP-1 in breast cancer cells also protected them from apoptosis when treated with doxorubicin, mechlorethamine and paclitaxel.13 These results are important because it is thought that a variety of classes of chemotherapeutic agents carry out their apoptotic anticancer effect via the JNK pathway.5,13 Although MKP-1 is able to target ERK, JNK and p38, it has a much higher affinity for JNK and p38 in comparison to ERK.14 In a small clinical study, tumor samples were obtained from 14 breast cancer patients and examined expression of the three MAPKs. The study showed that ERK, JNK and p38 were all upregulated in malignant vs. non-malignant samples. The study also looked at JNK activity and found that it was 30% lower in malignant tissue than in normal tissue. Further investigation into the disparity between higher protein expression level and reduced activity level of JNK revealed that MKP-1, along with MKP-2 displayed increased expression in the malignant tissues.15 This suggests a possible mechanism for the decrease in JNK activity. Since a reduction in JNK activity may play a role in reducing the effectiveness of chemotherapy drugs, it is possible that down-regulating MKP-1 expression might be a novel way to combat chemotherapy resistance.
MKP-2
Considerably less is known about the contributions of MKP-2 to malignancy, but there is some evidence that it is involved in liver cancer and pancreatic cancer.16,17 In a study investigating hepatocarcinogenesis and hepatoma tissue, no expression of MKP-2 could be detected in normal tissue, but was present in three out of five primary hepatomas studied.16 MKP-2 mRNA levels were also elevated in ascites hepatoma cell lines compared to normal liver.16 The authors of this study suggest that MKP-2 expression might be used as a tumor marker in the liver.16 MKP-2 has also been linked to the suppression of ERK activity in pancreatic cancer cells harboring K-ras mutations.17 The expression of MKP-2 in pancreatic tumor cell lines correlated to MEK expression. When treated with a MEK inhibitor, PD98059, MKP-2 expression in BxPC-3 and Capan-1 cells was markedly decreased.17 MKP-2 has been shown to be co-expressed with MKP-1 in breast cancer15 and overexpressed in serous borderline tumors of the ovary.9
MKP-3
Similar to MKP-2, MKP-3 expression has been linked to Ras activity in pancreatic cancer.18,19 Unlike MKP-2, it is the loss of expression of MKP-3 that is associated with disease progression. A decrease in expression was observed in primary pancreatic tumor tissues classified as invasive carcinomas compared to in situ carcinomas.18 Another study revealed that many borderline lesions in which MKP-3 expression is missing contain K-ras mutations.19 A chemical carcinogenesis study using palytoxin found that treatment of H-ras expressing MCF10A cells resulted in a decrease in MKP-3 expression that corresponded to an increase in ERK expression.20 In breast cancer, changes in MKP-3 expression contributed to tamoxifen resistance in estrogen receptor alpha (ER-α) positive MCF-7 cells.21 Parental cells engineered for overexpression of MKP-3, in the presence of estrogen and treated with tamoxifen, showed a ten-fold increase in soft agar colony formation compared to control transfected cells. Colony formation was able to be blocked by ER-α antagonist ICI 182780 and MEK inhibitor PD98059.21 In addition to tamoxifen resistance, MKP-3 has also been implicated in cisplatin resistance in ovarian cancer. In both tumor tissue samples and ovarian cancer cell lines, MKP-3 protein expression was much lower than in normal samples and immortalized cell lines.22 Exogenous expression of MKP-3 in A2780cp cells, which are cisplatin resistant, increased their sensitivity to cisplatin treatment by up to 2.5-fold when compared to vector control cells.22
Other MKPs
PAC-1, MKP-4 and MKP-7 have also been shown to play a part in various cancer types. In a study of thirty-nine patient samples from serous ovarian tumors, high PAC-1 expression levels correlated with worse overall survival compared with those tumors with low PAC-1 levels.23 MKP-4 normally functions in placental development,24 but loss of expression has been connected to skin cancer development. Reintroduction of MKP-4 in malignant cells led to microtubule disruption and in vivo tumor suppression.25 Overexpression of MKP-7 in Rat-1 fibroblasts transformed with BCR-ABL showed a reduction in JNK activation and a decreased ability to transform both in vitro and in vivo.26 Downregulation of MKP-7 by miR-24, whose expression is induced by both AML-1 and AML1-ETO, has been linked to development of acute myeloid leukemia. Decreased MKP-7 led to increased phosphorylation of JNK and p38, which stimulated myeloid cell growth and inhibited differentiation.27
Implications for Treatment and Future Developments
There are many things still to learn about the roles of MKP-1, MKP-2 and MKP-3 in cancer. There are also many MKP family members about whose behavior little is known. Since growth signals are integral to cancer development and progression, understanding the regulatory methods that control them, or fail to control them properly, is critical to making therapies more effective. MKP-1 and MKP-3 have been linked to chemo-therapy resistance. MKP-2 expression could possibly be used as a biomarker in liver cancer. As more is learned about the role of MKPs in cancer development and progression, it could lead to the rational design of new therapies. Possibilities include small molecule inhibitors such as triptolide. Triptolide has been shown to potently inhibit MKP-1 in osteosarcoma cells and ovarian cancer.28,29 Additionally, a high-content assay has been developed to identify phosphatase inhibitors.30 This approach identified NSC 95397, which can inhibit MKP-1 and MKP-3 in vitro and restore paclitaxel sensitivity in breast cancer cells exposed to dexamethasone.31 A library of uracil quinolines that can inhibit MKP-1 has also been developed. These compounds could potentially be useful for investigating the role of MKP-1 in biological systems.32 Two other compounds with activity against MKP-1, PSI2106 and MDF2085, were identified from a pyrrole carboxamide library and are undergoing further investigation.33 Targeting MKPs that are aberrantly expressed might provide a more tumor specific therapeutic addition to traditional chemo-therapies and treatments currently being used in the clinic.
Acknowledgements
Apologies are extended to colleagues whose work could not be included due to space limitations. This work was supported by NIH grant R01 CA100073 to G.S. Wu.
Abbreviations
- MAPK
mitogen activated protein kinase
- MKP
mitogen activated protein kinase phosphatase
- ERK
extracellular signal regulated kinase
- JNK
c-jun N-terminal kinase
- MKKK
MAP kinase kinase kinase
- MKK
MAP kinase kinase
- DUSP
dual specificity phosphatase
- ER-α
estrogen receptor alpha
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Wu GS. Role of mitogen-activated protein kinase phosphatases (MKPs) in cancer. Cancer Metastasis Rev. 2007;26:579–85. doi: 10.1007/s10555-007-9079-6. [DOI] [PubMed] [Google Scholar]
- 3.Boutros T, Chevet E, Metrakos P. Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death and cancer. Pharmacol Rev. 2008;60:261–310. doi: 10.1124/pr.107.00106. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Zhou JY, Wu GS. ERK-dependent MKP-1-mediated cisplatin resistance in human ovarian cancer cells. Cancer Res. 2007;67:11933–41. doi: 10.1158/0008-5472.CAN-07-5185. [DOI] [PubMed] [Google Scholar]
- 5.Zhou JY, Liu Y, Wu GS. The role of mitogen-activated protein kinase phosphatase-1 in oxidative damage-induced cell death. Cancer Res. 2006;66:4888–94. doi: 10.1158/0008-5472.CAN-05-4229. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson RJ, Keyse SM. Diverse physiological functions for dual-specificity MAP kinase phosphatases. J Cell Sci. 2006;119:4607–15. doi: 10.1242/jcs.03266. [DOI] [PubMed] [Google Scholar]
- 7.Keyse SM, Ginsburg M. Amino acid sequence similarity between CL100, a dual-specificity MAP kinase phosphatase and cdc25. Trends Biochem Sci. 1993;18:377–8. doi: 10.1016/0968-0004(93)90092-2. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases — regulating the immune response. Nat Rev Immunol. 2007;7:202–12. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- 9.Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008;27:253–61. doi: 10.1007/s10555-008-9123-1. [DOI] [PubMed] [Google Scholar]
- 10.Koukourakis MI, Giatromanolaki A, Sheldon H, Buffa FM, Kouklakis G, Ragoussis I, et al. Phase I/II trial of bevacizumab and radiotherapy for locally advanced inoperable colorectal cancer: vasculature-independent radiosensitizing effect of bevacizumab. Clin Cancer Res. 2009;15:7069–76. doi: 10.1158/1078-0432.CCR-09-0688. [DOI] [PubMed] [Google Scholar]
- 11.Denkert C, Schmitt WD, Berger S, Reles A, Pest S, Siegert A, et al. Expression of mitogen-activated protein kinase phosphatase-1 (MKP-1) in primary human ovarian carcinoma. Int J Cancer. 2002;102:507–13. doi: 10.1002/ijc.10746. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Xu J, Zhou JY, Liu Y, Wu GS. Mitogen-activated protein kinase phosphatase-1 is required for cisplatin resistance. Cancer Res. 2006;66:8870–7. doi: 10.1158/0008-5472.CAN-06-1280. [DOI] [PubMed] [Google Scholar]
- 13.Small GW, Shi YY, Higgins LS, Orlowski RZ. Mitogen-activated protein kinase phosphatase-1 is a mediator of breast cancer chemoresistance. Cancer Res. 2007;67:4459–66. doi: 10.1158/0008-5472.CAN-06-2644. [DOI] [PubMed] [Google Scholar]
- 14.Camps M, Nichols A, Arkinstall S. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 2000;14:6–16. [PubMed] [Google Scholar]
- 15.Wang HY, Cheng Z, Malbon CC. Overexpression of mitogen-activated protein kinase phosphatases MKP1, MKP2 in human breast cancer. Cancer Lett. 2003;191:229–37. doi: 10.1016/s0304-3835(02)00612-2. [DOI] [PubMed] [Google Scholar]
- 16.Yokoyama A, Karasaki H, Urushibara N, Nomoto K, Imai Y, Nakamura K, et al. The characteristic gene expressions of MAPK phosphatases 1 and 2 in hepatocarcinogenesis, rat ascites hepatoma cells, and regenerating rat liver. Biochem Biophys Res Commun. 1997;239:746–51. doi: 10.1006/bbrc.1997.7547. [DOI] [PubMed] [Google Scholar]
- 17.Yip-Schneider MT, Lin A, Marshall MS. Pancreatic tumor cells with mutant K-ras suppress ERK activity by MEK-dependent induction of MAP kinase phosphatase-2. Biochem Biophys Res Commun. 2001;280:992–7. doi: 10.1006/bbrc.2001.4243. [DOI] [PubMed] [Google Scholar]
- 18.Furukawa T, Sunamura M, Motoi F, Matsuno S, Horii A. Potential tumor suppressive pathway involving DUSP6/MKP-3 in pancreatic cancer. Am J Pathol. 2003;162:1807–15. doi: 10.1016/S0002-9440(10)64315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furukawa T, Fujisaki R, Yoshida Y, Kanai N, Sunamura M, Abe T, et al. Distinct progression pathways involving the dysfunction of DUSP6/MKP-3 in pancreatic intraepithelial neoplasia and intraductal papillarymucinous neoplasms of the pancreas. Mod Pathol. 2005;18:1034–42. doi: 10.1038/modpathol.3800383. [DOI] [PubMed] [Google Scholar]
- 20.Warmka JK, Mauro LJ, Wattenberg EV. Mitogen-activated protein kinase phosphatase-3 is a tumor promoter target in initiated cells that express oncogenic Ras. J Biol Chem. 2004;279:33085–92. doi: 10.1074/jbc.M403120200. [DOI] [PubMed] [Google Scholar]
- 21.Cui Y, Parra I, Zhang M, Hilsenbeck SG, Tsimelzon A, Furukawa T, et al. Elevated expression of mitogen-activated protein kinase phosphatase 3 in breast tumors: a mechanism of tamoxifen resistance. Cancer Res. 2006;66:5950–9. doi: 10.1158/0008-5472.CAN-05-3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan DW, Liu VW, Tsao GS, Yao KM, Furukawa T, Chan KK, et al. Loss of MKP3 mediated by oxidative stress enhances tumorigenicity and chemoresistance of ovarian cancer cells. Carcinogenesis. 2008;29:1742–50. doi: 10.1093/carcin/bgn167. [DOI] [PubMed] [Google Scholar]
- 23.Givant-Horwitz V, Davidson B, Goderstad JM, Nesland JM, Trope CG, Reich R. The PAC-1 dual specificity phosphatase predicts poor outcome in serous ovarian carcinoma. Gynecol Oncol. 2004;93:517–23. doi: 10.1016/j.ygyno.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Christie GR, Williams DJ, Macisaac F, Dickinson RJ, Rosewell I, Keyse SM. The dual-specificity protein phosphatase DUSP9/MKP-4 is essential for placental function but is not required for normal embryonic development. Mol Cell Biol. 2005;25:8323–33. doi: 10.1128/MCB.25.18.8323-8333.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Lagowski J, Sundholm A, Sundberg A, Kulesz-Martin M. Microtubule disruption and tumor suppression by mitogen-activated protein kinase phosphatase 4. Cancer Res. 2007;67:10711–9. doi: 10.1158/0008-5472.CAN-07-1968. [DOI] [PubMed] [Google Scholar]
- 26.Hoornaert I, Marynen P, Goris J, Sciot R, Baens M. MAPK phosphatase DUSP16/MKP-7, a candidate tumor suppressor for chromosome region 12p12-13, reduces BCR-ABL-induced transformation. Oncogene. 2003;22:7728–36. doi: 10.1038/sj.onc.1207089. [DOI] [PubMed] [Google Scholar]
- 27.Zaidi SK, Dowdy CR, van Wijnen AJ, Lian JB, Raza A, Stein JL, et al. Altered Runx1 subnuclear targeting enhances myeloid cell proliferation and blocks differentiation by activating a miR-24/MKP-7/MAPK network. Cancer Res. 2009;69:8249–55. doi: 10.1158/0008-5472.CAN-09-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z, Zhou JY, Kanakapalli D, Buck S, Wu GS, Ravindranath Y. High level of mitogen-activated protein kinase phosphatase-1 expression is associated with cisplatin resistance in osteosarcoma. Pediatr Blood Cancer. 2008;51:754–9. doi: 10.1002/pbc.21727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Zhou JY, Zhang L, Wu GS. Involvement of MKP-1 and Bcl-2 in acquired cisplatin resistance in ovarian cancer cells. Cell Cycle. 2009;8:3191–8. doi: 10.4161/cc.8.19.9751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogt A, Lazo JS. Implementation of high-content assay for inhibitors of mitogen-activated protein kinase phosphatases. Methods. 2007;42:268–77. doi: 10.1016/j.ymeth.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vogt A, McDonald PR, Tamewitz A, Sikorski RP, Wipf P, Skoko JJ, et al. A cell-active inhibitor of mitogen-activated protein kinase phosphatases restores paclitaxel-induced apoptosis in dexamethasone-protected cancer cells. Mol Cancer Ther. 2008;7:330–40. doi: 10.1158/1535-7163.MCT-07-2165. [DOI] [PubMed] [Google Scholar]
- 32.Arnold DM, Foster C, Huryn DM, Lazo JS, Johnston PA, Wipf P. Synthesis and biological activity of a focused library of mitogen-activated protein kinase phosphatase inhibitors. Chem Biol Drug Des. 2007;69:23–30. doi: 10.1111/j.1747-0285.2007.00474.x. [DOI] [PubMed] [Google Scholar]
- 33.Lazo JS, Skoko JJ, Werner S, Mitasev B, Bakan A, Koizumi F, et al. Structurally unique inhibitors of human mitogen-activated protein kinase phosphatase-1 identified in a pyrrole carboxamide library. J Pharmacol Exp Ther. 2007;322:940–7. doi: 10.1124/jpet.107.122242. [DOI] [PubMed] [Google Scholar]