Abstract
Background
Valacyclovir, an orally administered pro-drug of acyclovir, is utilized in the therapy of herpes simplex and herpes zoster infections. Little data regarding the pharmacokinetics, safety and tolerability are available for pediatric patients. This report describes acyclovir pharmacokinetics following valacyclovir administration in immunocompromised pediatric patients, compares pharmacokinetic parameters following oral valacyclovir and IV acyclovir, and provides a limited assessment of efficacy in the setting of active herpes zoster infection.
Procedure
A total of 37 immunocompromised children were enrolled on one of two studies. Pharmacokinetic data are available for 32 patients following valacyclovir (15 mg/kg) administration, 11 of whom also had pharmacokinetic sampling following IV acyclovir administration. Three patients received valacyclovir as treatment for herpes zoster infections.
Results
Mean (± SD) Cmax values for acyclovir following oral valacyclovir were 18.8 ± 7 uM with a total exposure of 4106 ± 1519 uM•min. The mean bioavailability of acyclovir from valacyclovir was 64%. Grade 1 nausea and emesis, which occurred in 5 patients was the only valacyclovir-related toxicity. Two of the three patients treated for herpes zoster had complete scabbing of lesions by day 9.
Conclusion
Valacyclovir (15 mg/kg) was well tolerated in pediatric patients and demonstrated excellent bioavailablity. Consideration should be given to the use of oral valacyclovir for the treatment of herpes zoster in clinically stable pediatric oncology patients.
Keywords: valacyclovir, acyclovir, pediatrics, pharmacokinetics
INTRODUCTION
Valacyclovir, the L-valyl ester of acyclovir, is approved by the United States Food and Drug Administration for the oral treatment of herpes zoster in immunocompetent adults.[1] Valacylovir is rapidly hydrolyzed to acyclovir in the liver and intestinal wall.[2, 3] Unlike oral acyclovir which has a bioavailability of only 10% to 20%, valacyclovir is more completely absorbed[4, 5] In adults, similar plasma acyclovir exposures may be obtained following oral administration of valacyclovir as compared to those achieved with doses of intravenous acyclovir.[5, 6] In adult normal volunteers, valacyclovir has the same safety profile as acyclovir, with the most common adverse events being headache and nausea.[4, 7]
Children at risk for disseminated varicella or herpes zoster infections are currently treated with intravenous acyclovir to prevent the complications of infection, and to accelerate healing. However, it has become an increasingly common practice to substitute valacyclovir for acyclovir, highlighting the need for pediatric data with regard to pharmacokinetics, safety and efficacy. Treatment with intravenous acyclovir requires either hospitalization, or extensive use of home health services, each with attendant expense and inconvenience. Identification of an oral drug with systemic exposures comparable to those following intravenous acyclovir may allow for the outpatient management of many of these children.
The primary aims of this study were to describe acyclovir pharmacokinetics following valacyclovir administration in immunocompromised pediatric patients and to compare pharmacokinetic parameters in a subset of patients following both oral valacyclovir and IV acyclovir. In addition, an assessment of efficacy in the setting of active HSV infection was performed in a small number of patients.
PATIENTS AND METHODS
Eligibility
This report includes data from two clinical protocols. Both studies included patients aged 2–18 years. Study A included patients with underlying malignancies who were receiving consolidation or maintenance chemotherapy. Study B included immunocompromised patients defined as patients who were receiving chemotherapy, had been treated with bone marrow transplantation or chemotherapy for an underlying malignancy or medical condition in the past 12 months, or had an underlying immunodeficiency syndrome. Stratum 1 of Study B enrolled patients with an acute herpes zoster infection (≤ 3 days of rash, limited to 3 or less dermatomes, and no evidence of dissemination). Stratum 2 enrolled bone marrow transplant patients who had positive HSV serology and were scheduled to receive acyclovir prophylaxis during the pre-transplant period. Complete eligibility criteria for both studies are included in Appendix I.
Drug Administration and Study Design
Valacyclovir was supplied by GlaxoSmithKline as 500 mg and 1 gm caplets. For patients unable to swallow caplets, or to administer an exact dose, a 50 mg/ml suspension with Ora-Plus (50:50 v/v) vehicle was extemporaneously prepared by the pharmacy. The suspension, which was sweetened with Syrpalta or Ora-Sweet was pharmacy stable for 21 days (≥ 90% potency by HPLC), and was previously demonstrated to have no statistically significant differences in pharmacokinetics as compared to valacylovir tablets.[8, 9]
Patients in Study A received a single 15 mg/kg valacyclovir dose (maximum dose 2 gm) in either the caplet or suspension form. Drug was administered at least one hour before or after oral intake of food. All patients had a follow up clinic visit to assess for possible adverse events at 7 ± 2 days following administration of study drug.
In Study B, patients in stratum 1 received valacyclovir for 5 to 10 days at a dose of 45 mg/kg/day (maximum dose 6 gm/day) divided TID. Complete study follow up information for this stratum is presented in Appendix A. Patients in stratum 2 received a single dose of valacyclovir as outlined in Study A. Eight hours later, a 60 minute intravenous infusion of acyclovir prophylaxis was initiated as prescribed by the patient’s bone marrow transplant physician. Adverse events for both studies were graded according to version 1 of the NCI Common Toxicity Criteria.
Pharmacokinetic Studies
Blood samples for assessment of acyclovir concentrations were collected in heparinized tubes. Samples were obtained prior to valacyclovir administration, and at 0.5, 1, 1.5, 2, 4, 6, and 8 hours following dose administration. Patients in Study B stratum 2 also had samples obtained at the listed time points following the completion of their initial dose of IV acyclovir. Samples were centrifuged at 1000×g for 15 minutes and the plasma was placed in a polypropylene tube. Urine was collected from the time of dosing to hour 8 in patients having urine collections for other reasons. Plasma and urine samples were stored at −80 °C until analysis. Plasma and urine samples were assayed for acyclovir using a modification of a previously described reverse phase high-performance liquid chromatography assay.[10] Additional assay information is presented in Appendix I.
Pharmacokinetic Data Analysis
Pharmacokinetic data were analyzed using compartmental and noncompartmental methods. Modeling was performed in ADAPT II (Biomedical Simulations Resource, Los Angeles, CA) using maximum likelihood estimation. Both one and two compartment models were fitted to the data and Akaike’s Information Criterion was used to determine the best fit. The model for acyclovir after valacyclovir administration included first order absorption with an absorption lag time (Tau). In these models, kel is the rate constant for elimination, and VC is the central volume of distribution. The apparent clearance (CL) was determined from the equation CL=Kel*VC, and half-life for each phase was determined from the model rate constants.
For noncompartmental analysis, the area under the concentration time curve (AUC) was calculated using the linear trapezoidal rule, and the residual area from the last quantifiable concentration to infinity was calculated using the approximation AUCtlast-∞= Ctlast/kel n.c., where kel n.c. is the apparent terminal elimination rate constant determined by log-linear regression of the terminal log-linear segment of the plasma concentration versus time curve. The apparent Cmax and Tmax values were estimated by visual inspection of the measured plasma drug concentration-time data for each subject. To avoid underestimation, the reported AUC and Cmax of acyclovir following i.v. administration of acyclovir were calculated using an estimated end of infusion concentration which resulted in a increase of 18% and 30% in the mean AUC and Cmax values respectively. Renal CL was derived by dividing the amount of drug collected in the urine over an 8 hour period by the plasma AUC8h. Bioavailability (F) of acyclovir after oral administration of valacyclovir was calculated as F= (AUCoral × doseiv)/(AUCiv × doseoral) utilizing molar doses for the calculation. For comparison, the acyclovir AUC and Cmax obtained following IV acyclovir administration were normalized to the molar equivalent (10.2 mg/kg) of the 15 mg/kg valacyclovir dose utilized in the study.
Statistics
A Mann-Whitney Rank Sum Test was used to evaluate demographic differences between the two study groups. The Wilcoxon Signed Rank Test was utilized for comparison of data in patients receiving both intravenous acyclovir and oral valacyclovir. Evaluation of drug exposure between different age populations One-Way Analysis of Variance was done and multiple comparisons were made using the Tukey-Kramer Method. A p-value of < 0.05 was required for statistical significance. SigmaStat 3.5 and S-Plus Version 6.2 for Windows were utilized for statistical analysis.
RESULTS
Patients
Thirty-seven patients were enrolled in these trials. Data from 5 patients were excluded from the analyses due to limited sampling (n=2) or storage issues (n=3). Data following valacyclovir administration are therefore reported for 32 patients; 16 patients from each study. Data are available for 11 patients following IV acyclovir administration in Study B. Data following urine collection and analysis was available for 21 patients. Characteristics of patients included in the pharmacokinetic analysis are outlined in Table I. There was no significant difference between the median ages or gender distribution of patients enrolled on either study.
TABLE I.
Patient Characteristics
| M:F | 17:15 |
| Age years median (range) | 9 (3–18) |
| Weight kg median (range) | 31 (13–103) |
| BSA m2 median (range) | 1.07 (0.6–2.33) |
| Diagnosis | |
| Leukemia | 19 |
| Ewings Sarcoma | 3 |
| Germ Cell Tumor | 3 |
| MDS | 2 |
| Medulloblastoma | 1 |
| NHL | 1 |
| Rhabodomyosarcoma | 1 |
| Rhabdoid | 1 |
| VAHS | 1 |
NHL= Non-Hodgkin’s Lymphoma, MDS= myelodysplastic syndrome, VAHS= viral associated hemophagocytic syndrome.
Pharmacokinetics
Valacyclovir Administration
A one-compartment model best described the plasma concentration vs. time curve of acyclovir following valacyclovir administration in 22 patients, and a 2-compartment model best described the data for the remaining 10 patients. The mean valacyclovir dose administered in these 32 patients was 14.97 mg/kg. Pharmacokinetic data are summarized in Table II. No statistically significant difference in these parameters existed between the patients in Study A as compared to Study B.
Table II.
Summary of Pharmacokinetic Parameters for Acyclovir Following Oral Valacyclovir Administration (15 mg/kg)
| Parameter | Mean ± SD | Median (range) | |
|---|---|---|---|
| Cmax | (uM) | 18.8 ± 7 | 17.1 (7.9 – 40.3) |
| Tmax | (min) | 128.7 ± 57 | 120 (60 – 250) |
| AUC∞ | (uM•min) | 4106 ± 1519 | 3934 (1672 – 8510) |
| CL | (ml/min/kg) | 11.4 ± 4.4 | 10.7 (5.2 – 23.4) |
| t ½ | (min) | 87.5 ± 29 | 80 (41.3 – 173) |
| VC | (L/kg) | 1.34 ± 0.65 | 1.23 (.33 – 3.3) |
| Tau | (min) | 23 ± 12.4 | 25.8 (1.2 – 70) |
| Renal CL | (ml/min/kg) | 3.03 ± 2.2 | 2.31 (0.35 – 8.21) |
VC= volume of distribution; CL=kel•VC
Table III compares pharmacokinetic parameters for three age groups of patients receiving valacyclovir. There was an increased exposure and decreased clearance with age. There was a statistically significant difference across the means (p=0.03 for AUC; p=0.0004 for clearance). There were statistically significant differences in exposure and clearance (p=0.02 for AUC; p=0.0003 for clearance) when the youngest (Group 1) was compared to the oldest (Group 3) patients. There was also a statistically significant difference in clearance between Group 1 and Group 2 (p=0.003). None of the other pairwise comparisons were statistically significant.
Table III.
Age Comparision of Acyclovir Pharmacokinetic Parameters following Valacyclovir
| Age Mean (range) |
No. Subjects | AUC (uM•min) Mean ± SD |
CL (ml/min/kg) Mean ± SD |
|---|---|---|---|
| Group-1: 2– <6 yrs 3.75 (3–5) |
8 | 2925 ± 974 | 16.2 ± 4.5 |
| Group-2: 6 – <12 yrs 8.3 (6–11) |
12 | 4292 ± 1105 | 10.4 ± 2.9 |
| Group-3: 12–18 yrs 15 ( 12–18) |
12 | 4707 ± 1797 | 9.1 ± 3.1 |
Acyclovir Administration
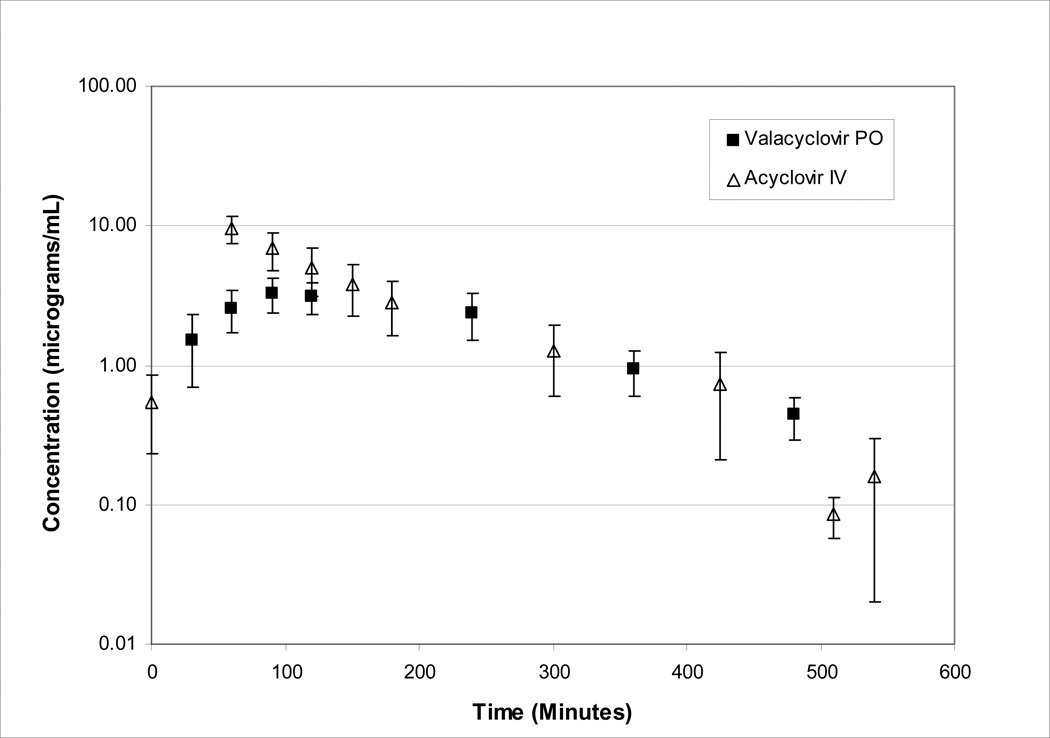
Eleven patients had pharmacokinetic sampling performed after both oral valacyclovir (mean dose 14.4 mg/kg) and IV acyclovir (mean dose 9.2 mg/kg) administration. The mean plasma acyclovir concentration-time profiles following administration of intravenous acyclovir and oral valacyclovir are shown in Figure 1.
Figure 1.
Plasma pharmacokinetics of acyclovir after intravenous administration of acyclovir (mean dose= 9.2 mg/kg) and oral administration of valacyclovir (mean dose 14.4 mg/kg). Data denote mean ± SD values representative of population studied (n=11).
For IV acyclovir, a one-compartment model best fit 6 patients, and a two-compartment model for 5 patients. The acyclovir clearance (mean ± SD) was 7.5 ± 2.9 ml/min/kg and the terminal half-life (t½) was 119.4 ± 62 minutes. One patient had a markedly prolonged t½ of 294.5 minutes. When this patient was excluded, the mean ± SD t½ was 101.8 ± 25 min.
Table IV compares the PK parameters of IV acyclovir to acyclovir after oral valacyclovir administration acyclovir in each of the 11 patients who received both drugs. The Tmax (mean ± SD) was shorter after IV acyclovir (29 ± 6.5 min) than after oral valacyclovir (131 ± 59 min). In addition, the peak acyclovir plasma concentrations (normalized for dose) were lower following oral valacyclovir (18 ± 4 uM) administration compared with IV acyclovir (48 ± 15 uM; p=0.001). The t½ following IV acyclovir administration was longer than following oral valacyclovir, however this was not statistically significant (p=0.067), and may be a reflection of longer post dose sampling following IV drug administration. For the eleven patients studied, the mean bioavailability was 64% ± 13.5% with a range from 40–87%. No association between concomitant medications and clearance was noted.
Table IV.
Comparison of Acyclovir Pharmacokinetic Parameters Following Oral Valacyclovir and IV Acyclovir Administration
| Valacyclovir (15 mg/kg) p.o. | Acyclovir i.v. | Normalized To IV Acyclovir Dose of 10.2 mg/kg |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Age (yrs) |
Cmax (uM) |
AUC∞ (uM•min) |
CL (ml/min/kg) |
T½ (min) |
Bioavailability % |
Dose (mg/kg) |
Cmax (uM) |
AUC∞ (uM•min) |
CL (ml/min/kg) |
T½ (min) |
Cmax (uM) |
AUC∞ (uM•min) |
| 1 | 18 | 23.7 | 7855 | 5.4 | 66 | 54 | 12.8 | 81.5 | 17970^ | 3.7 | 103 | 65 | 14287 |
| 2 | 16 | 17.1 | 4829 | 8.6 | 48.2 | 40 | 6.8 | 47.6 | 7976 | 4.0 | 98 | 72 | 12010 |
| 3 | 6 | 25.2 | 4017 | 11.7 | 73 | 87 | 15.5 | 53.8 | 7005 | 9.5 | 130 | 35 | 4601 |
| 4 | 11 | 16.9 | 5287 | 7.5 | 60 | 63 | 6.7 | 48.4 | 5842 | 5.8 | 58 | 73 | 8891 |
| 5 | 4 | 16.5 | 2653 | 17.5 | 78.1 | 80 | 11.4 | 37.7 | 3840 | 13.3 | 101 | 34 | 3421 |
| 6 | 8 | 22.4 | 2638 | 17.9 | 65.6 | 53 | 8.4 | 28.7 | 4019 | 8.7 | 78 | 35 | 4902 |
| 7 | 9 | 16.3 | 4723 | 8.9 | 113 | 72 | 7.6 | 31.2 | 4759 | 7.3 | 90 | 42 | 6392 |
| 8 | 18 | 15.6 | 3008 | 5.51 | 41.4 | 63 | 5.6 | 23.4 | 3490 | 6.5 | 294 | 42 | 6314 |
| 9 | 18 | 14.4 | 5089 | 7.5 | 121.6 | 60 | 5.7 | 22.1 | 4866 | 5.4 | 135 | 39 | 8665 |
| 10 | 5 | 10 | 2195 | 18.5 | 99.7 | 56 | 9.5 | 44.7 | 4012 | 11.1 | 134 | 48 | 4302 |
| 11 | 4 | 21.2 | 4937 | 8.6 | 122.8 | 74 | 10.7 | 46.6 | 7122 | 6.8 | 90 | 44 | 6766 |
| Mean ± SD | 10.6 ± 9 | 18 ± 4 | 4294 ± 1635 | 10.7 ± 5 | 80.8 ± 29 | 63.8 ± 13.5 | 9.2 ± 3 | 42.3 ± 17 | 6446 ± 4110 | 7.5 ± 2.9 | 119.4 ± 62 | 48 ± 15 | 7323 ± 3384 |
Safety and Efficacy
Among 37 patients receiving a single dose of valacyclovir, 5 patients had grade 1 emesis (n=4) and nausea (n=1) possibly, probably or definitely related to study drug. No other adverse events were attributed to valacyclovir. No patients displayed clinical or laboratory evidence of thrombotic thrombocytopenic purpura.
Three patients, 2 females and 1 male, with active herpes zoster were treated on stratum 1 of Study B. Patients were 9–11 years of age and had the following diagnosis: Ewings sarcoma, medulloblastoma, and acute lymphoblastic leukemia. One patient was removed from study on day 7 because of progression of zoster lesions. She was started on acyclovir 500 mg/m2/dose IV every 8 hours, and did not have any further progression. On acyclovir treatment day 5 her ANC rose to 760 and she was discharged home to complete a total 10 day course of acyclovir. Her acyclovir exposure following the initial dose of valacyclovir was similar to the group mean at 4,615 uM•min. The remaining 2 patients had complete scabbing of their lesions at day 9, at which time valacyclovir was discontinued. Complete clearing of the lesions was noted by the day 15 visit. Pharmacokinetic data were available for one patient, and acyclovir exposure after the initial valacyclovir dose was 4,288 uM•min, similar to the group mean.
DISCUSSION
Acyclovir is routinely used in the prophylaxis and treatment of HSV and VZV infections in immunocompromised pediatric patients. The poor bioavailability of oral acyclovir necessitates frequent dosing and results in inferior drug exposure compared with intravenous administration. In contrast, valacyclovir has excellent bioavailability and results in high systemic acyclovir exposures. However, few data in pediatric patients regarding the pharmacokinetics of this agent are available.
This study of pharmacokinetic parameters following oral valacyclovir administration in pediatric patients aids our understanding of the drug in this age group. The mean CL, t½, Cmax, and AUC values for acyclovir in this study were in the range previously reported in children receiving similar valacyclovir doses.[11][12] The mean ± SD t½ was shorter (1.45 ± 0.48 h) in children than in adults who received a comparable dose based on body weight (mean values between 2.6–3.3 hours).[5][12][4] There was a trend towards a greater mean exposure and decreased CL with increasing age (Table III).
The mean acyclovir AUCs following oral valacyclovir (15 mg/kg; 320–640 mg/m2) were nearly three fold higher; 5.5 ± 2.4 vs. 15.4 ± 5.7 ug-hr/ml respectively compared to children 3 weeks to 7 years of age (n=18) dosed with 600 mg/m2 of oral acyclovir.[13] The mean bioavailability of valacyclovir in this population was 64%, exceeding the mean value of 43% previously reported in pediatric patients,[11] but in a range reported in adults.[14] The differences in bioavailability in the pediatric studies may reflect the different doses utilized, with decreased acyclovir bioavailability associated with the higher doses per body weight.
The Cmax values following valacyclovir in our study were in the same range as those previously reported in children receiving the same acyclovir dose per body surface area.[15][12] The mean Cmax (4.3 ± 1.6 ug/ml) and concentration at 8 hours following valacylovir (0.4 ± 0.25 ug/ml) fall within reported ranges for concentrations of acyclovir inhibiting HSV-1 and VZV growth in culture by 50% (0.02–13.5 and 0.12–10.8 ug/ml respectively).[16–20] Data related to Tmax and Cmax values were obtained from noncompartmental analysis. Given the initial sampling time point of 30 minutes post infusion, our Tmax estimate is likely prolonged in patients receiving IV acyclovir. In our study, one patient had a markedly prolonged terminal elimination half-life following i.v. acyclovir. When this patient was excluded, the mean ± SD t½ (1.69 ± 0.41 h) was shorter compared to previously reported values in adults (mean range 2.3–2.44 h),[5][14][6] but was similar to that reported in immunocompromised children (1.8 ± 0.38 h).[11] The estimated total clearance in the study population was similar to that previously reported in children in the same age range (413 ± 110 vs. 335 ± 109 ml/min/1.73m2).[15]
Valacylovir was well tolerated and only associated with grade 1 nausea and emesis following both single or prolonged drug administration. These events occurred primarily in patients receiving extemporaneously prepared suspension and were likely related to the formulation. Two of the three patients receiving valacyclovir for active herpes zoster infections had scabbing of all lesions by day 9, indicating that valacyclovir may be an acceptable substitute for IV acyclovir in this patient population. However, this study was not powered to provide efficacy data and further studies are required to make definitive recommendations.
In conclusion, valacylovir was well tolerated and showed favorable pharmacokinetics in pediatric patients at the dose studied. In this study, valacyclovir has significantly greater bioavailability (64%) as compared to that reported with oral acyclovir (10–20%), and therapeutic responses were noted in the small subset of patients treated with herpes zoster. These data support the recommendation that further consideration should be given to the use of valacyclovir for the treatment of herpes zoster in clinically stable pediatric oncology patients or in the setting of HSV prophylaxis in pediatric patients undergoing bone marrow transplantation.
Acknowledgments
Supported in part by:
UO1 HD37242-04S1, Network of Pediatric Pharmacology Research Units, NICHD M01-RR00188, General Clinical Research Center, NCRR
GlaxoSmithKline
REFERENCES
- 1.Shinkai I, Ohta Y. New drugs--reports of new drugs recently approved by the FDA. Valacyclovir. Bioorganic & medicinal chemistry. 1996;4(1):1–2. doi: 10.1016/0968-0896(96)00041-7. [DOI] [PubMed] [Google Scholar]
- 2.Burnette TC, de Miranda P. Metabolic disposition of the acyclovir prodrug valaciclovir in the rat. Drug metabolism and disposition: the biological fate of chemicals. 1994;22(1):60–64. [PubMed] [Google Scholar]
- 3.de Miranda P, Burnette TC. Metabolic fate and pharmacokinetics of the acyclovir prodrug valaciclovir in cynomolgus monkeys. Drug metabolism and disposition: the biological fate of chemicals. 1994;22(1):55–59. [PubMed] [Google Scholar]
- 4.Weller S, Blum MR, Doucette M, et al. Pharmacokinetics of the acyclovir pro-drug valaciclovir after escalating single- and multiple-dose administration to normal volunteers. Clin Pharmacol Ther. 1993;54(6):595–605. doi: 10.1038/clpt.1993.196. [DOI] [PubMed] [Google Scholar]
- 5.Soul-Lawton J, Seaber E, On N, et al. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob Agents Chemother. 1995;39(12):2759–2764. doi: 10.1128/aac.39.12.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoglund M, Ljungman P, Weller S. Comparable aciclovir exposures produced by oral valaciclovir and intravenous aciclovir in immunocompromised cancer patients. The Journal of antimicrobial chemotherapy. 2001;47(6):855–861. doi: 10.1093/jac/47.6.855. [DOI] [PubMed] [Google Scholar]
- 7.Fife KH, Barbarash RA, Rudolph T, et al. Valaciclovir versus acyclovir in the treatment of first-episode genital herpes infection. Results of an international, multicenter, double-blind, randomized clinical trial. The Valaciclovir International Herpes Simplex Virus Study Group. Sexually transmitted diseases. 1997;24(8):481–486. doi: 10.1097/00007435-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Fish DN, Vidaurri VA, Deeter RG. Stability of valacyclovir hydrochloride in extemporaneously prepared oral liquids. Am J Health Syst Pharm. 1999;56(19):1957–1960. doi: 10.1093/ajhp/56.19.1957. [DOI] [PubMed] [Google Scholar]
- 9.Simon DNF MW, Deeter RG. Pharmacokinetics and safety of valaciclovir in children with Epstein-Barr virus illness. Drugs in R&D. 2002;3(6):365–373. doi: 10.2165/00126839-200203060-00001. [DOI] [PubMed] [Google Scholar]
- 10.Page T, Sherwood C, Connor JD, et al. Simple reversed-phase high-performance liquid chromatography quantitation of ganciclovir in human serum and urine. Journal of chromatography. 1996;675(2):342–346. doi: 10.1016/0378-4347(95)00381-9. [DOI] [PubMed] [Google Scholar]
- 11.Eksborg S, Pal N, Kalin M, et al. Pharmacokinetics of acyclovir in immunocompromized children with leukopenia and mucositis after chemotherapy: can intravenous acyclovir be substituted by oral valacyclovir? Med Pediatr Oncol. 2002;38(4):240–246. doi: 10.1002/mpo.1317. [DOI] [PubMed] [Google Scholar]
- 12.Nadal D, Leverger G, Sokal EM, et al. An investigation of the steady-state pharmacokinetics of oral valacyclovir in immunocompromised children. J Infect Dis. 2002;186(Suppl 1):S123–S130. doi: 10.1086/342968. [DOI] [PubMed] [Google Scholar]
- 13.Sullender WM, Arvin AM, Diaz PS, et al. Pharmacokinetics of acyclovir suspension in infants and children. Antimicrob Agents Chemother. 1987;31(11):1722–1726. doi: 10.1128/aac.31.11.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steingrimsdottir H, Gruber A, Palm C, et al. Bioavailability of aciclovir after oral administration of aciclovir and its prodrug valaciclovir to patients with leukopenia after chemotherapy. Antimicrob Agents Chemother. 2000;44(1):207–209. doi: 10.1128/aac.44.1.207-209.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blum MR, Liao SH, de Miranda P. Overview of acyclovir pharmacokinetic disposition in adults and children. Am J Med. 1982;73(1A):186–192. doi: 10.1016/0002-9343(82)90088-2. [DOI] [PubMed] [Google Scholar]
- 16.Andrei G, Sienaert R, McGuigan C, et al. Susceptibilities of several clinical varicella-zoster virus (VZV) isolates and drug-resistant VZV strains to bicyclic furano pyrimidine nucleosides. Antimicrob Agents Chemother. 2005;49(3):1081–1086. doi: 10.1128/AAC.49.3.1081-1086.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andrei G, Snoeck R, Reymen D, et al. Comparative activity of selected antiviral compounds against clinical isolates of varicella-zoster virus. Eur J Clin Microbiol Infect Dis. 1995;14(4):318–329. doi: 10.1007/BF02116525. [DOI] [PubMed] [Google Scholar]
- 18.Biron KK, Elion GB. In vitro susceptibility of varicella-zoster virus to acyclovir. Antimicrob Agents Chemother. 1980;18(3):443–447. doi: 10.1128/aac.18.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.GlaxoSmithKline. Product Information. Research Triangle Park (NC): 2007. Valtrex (valacyclovir hydrochloride) caplets. [Google Scholar]
- 20.Machida H. Comparison of susceptibilities of varicella-zoster virus and herpes simplex viruses to nucleoside analogs. Antimicrob Agents Chemother. 1986;29(3):524–526. doi: 10.1128/aac.29.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]