Abstract
Purpose of review
It has been 10 years since pathological high-frequency oscillations (pHFOs) were described in the brain of epileptic animals and patients. This review summarizes progress in research on mechanisms of their generation and potential clinical applications over that period.
Recent findings
Initially, pHFOs were recorded with microelectrodes in the hippocampus of rodents and patients with mesial temporal lobe epilepsy (MTLE), but recently pHFOs have also been recorded with clinical depth and grid electrodes in multiple brain areas including the hippocampus and neocortex of patients with different types of epilepsy. One hypothesis is that pHFOs reflect fields of hypersynchronized action potentials (bursts of population spikes) within small discrete neuronal clusters responsible for seizure generation. Studies suggest that pHFOs can be used as a reliable biomarker for epileptogenesis, epileptogenicity, and the delineation of the epileptogenic region.
Summary
Recording of pHFOs with clinical electrodes provides a means for further investigation of their functional role in the epileptic brain and as a potential biomarker of epileptogenesis and epileptogenicity and for presurgical mapping.
Keywords: biomarkers, epilepsy, fast ripples, high-frequency oscillations
Introduction
Epilepsy is the most common serious neurological disorder, affecting people of all ages [1]. Ten percent of the world’s population have at least one epileptic seizure in a lifetime, and a third of these develop epilepsy. At any given time, 1% of the world’s population has active epilepsy. Despite a tremendous increase in the number of antiepileptic drugs made available over the past few decades, as many as 40% of people with epilepsy will fail to become seizure free with treatment. Future advances in diagnosis, treatment, and prevention will depend on improved understanding of the fundamental neuronal mechanisms underlying epilepsy disorders. Furthermore, pharmacotherapy in epilepsy continues to be trial-and-error because there are no reliable biomarkers that identify the presence or severity of epilepsy (epileptogenicity) or its development (epileptogenesis). A recently identified interictal electrophysiological epileptiform abnormality, termed pathological high-frequency oscillations (pHFOs), may reflect the primary neuronal disturbances responsible for epilepsy, providing opportunities to elucidate novel therapeutic and preventive targets and to develop effective biomarkers for clinical use.
Normal high-frequency oscillations
HFOs are local oscillatory field potentials that contain spectral power greater than 100 Hz and have a duration less than 1 s. Neuronal synchrony of unit firing increases during HFOs, facilitating synaptic transmission through local networks. Normal HFOs include ripple oscillations that were first described in CA1 of the nonprimate hippocampus [2–4], but are also found in CA3, subiculum, and entorhinal cortex [5, 6]. In rats, ripples contain spectral power between 100 and 200 Hz and occur most frequently during episodes of awake immobility and slow wave sleep (SWS). In CA1, and likely other hippocampal structures, ripples reflect summated fast inhibitory postsynaptic potentials (IPSPs) on the somata of pyramidal cells [7]. In-vivo studies using mice, bats, nonhuman primates, and humans have found ripples with spectral frequency and state-dependent characteristics similar to ripples in rats [8–11]. Ripples are believed to play an important role in information processing and consolidation of memory [12, 13].
In rodent and human neocortex, HFOs with spectral frequencies typically greater than 200 Hz may be evoked electrically or occur during sensory stimulation [14–18]. In cat neocortex, spontaneous ripple-frequency HFOs can be detected during natural sleep, and increase in amplitude during sleep-like states induced by ketamine anesthesia [19]. The mechanisms generating neocortical HFOs are not known, but evidence suggests that sensory-evoked HFOs may reflect population spikes from synchronously firing pyramidal cells and/or fast-spiking, possibly GABAergic, neurons [19–21].
Pathological high-frequency oscillations
In animal models of chronic limbic epilepsy, HFOs with spectral frequencies in the range of 250–600 Hz occur in dentate gyrus, CA1, and CA3 areas of hippocampus, subiculum, and entorhinal cortex, but only in rats that exhibit recurrent spontaneous seizures and not in those that have been subjected to an epileptogenic insult but do not exhibit epileptic seizures [22, 23]. These interictal HFOs, called fast ripples, are considered to be pathological based on results from several studies that showed interictal fast ripples are uniquely associated with sites of seizure onset [23, 24], fast ripples occur during the onset of some hippocampal seizures [25], and a greater number of fast ripples-generating sites correlate with a higher rate of seizures [26]. These results suggest that fast ripples can be a biomarker for the epileptogenic region, defined as the area of brain necessary and sufficient for the generation of spontaneous epileptic seizures, and also for assessing the degree of epileptogenicity. Many of these properties of fast ripples have been substantiated in patients with medically intractable mesial temporal lobe epilepsy (MTLE) [10, 22, 27–29, 30••, 31, 32].
In the intrahippocampal kainate model of MTLE, fast ripples and ripple-frequency HFOs were recorded in the epileptogenic dentate gyrus, an area where normal ripples do not occur in normal rats, within days to weeks after kainate injection, but only in those animals that later exhibited spontaneous seizures. Furthermore, shorter latencies to the first appearance of fast ripples or ripple-frequency HFOs in dentate gyrus correlated with shorter latencies to first spontaneous seizure [33]. These findings not only indicate that pHFOs predict seizures after an epileptogenic insult and could be a biomarker of epileptogenesis but also that pHFOs can occur with the same oscillatory frequency as normal ripples. Several important differences between pHFOs and normal ripples in rat hippocampus have been identified [34••]. Normal ripples reflect summated IPSPs and the spatial distribution of their neuronal generators is diffuse, whereas pHFOs are believed to reflect synchronized firing of abnormally bursting principal cells (i.e. burst of population spikes) localized to small discrete neuronal clusters embedded within tissue that does not generate pHFOs [35, 36].
The size and location of pHFO-generating neuronal clusters in dentate gyrus becomes stable over time [26], but local application of an inhibitory antagonist causes these clusters to increase in size [35]. This has led to the hypothesis that pHFO-generating neuronal clusters represent the basic underlying mechanism of these forms of epilepsy and that spontaneous seizures arise when reduction in tonic inhibitory influences results in their expansion, coalescence, and synchronization. This view is supported by the observation that the density of pHFO-generating clusters in dentate gyrus correlates with seizure frequency [26] and by the demonstration that the power of these oscillations increases during the transition to hypersynchronous seizures [25].
Neocortical HFOs occur during high-voltage spike and wave discharges in rats that could represent a model of absence epilepsy [37]. These events may reflect IPSPs in pyramidal cells, similar to mechanisms generating normal hippocampal ripples. Neocortical ripple-frequency HFOs have been recorded during spontaneous and electrically evoked electrographic seizures in anesthetized cats [38]. In this study, fast-spiking (presumably GABAergic) neurons did not fire at fixed latencies in relation to the neocortical HFOs, suggesting IPSPs may not be involved in synchronizing the neuronal discharge during some HFOs in neocortex. It is not clear from this study, however, whether these neocortical HFOs, and the proposed mechanism generating them, are pathological because the animals did not have chronic seizures. More research is needed to better understand the spatiotemporal properties and mechanisms supporting normal and pathological HFOs associated with neocortical epilepsy.
High-frequency oscillations in patients with epilepsy
The first human studies on interictal HFOs were recorded from microelectrodes positioned in hippocampus and entorhinal cortex of patients with MTLE [8, 22]. Human ripples in MTL structures that are presumably normal share several important characteristics with normal ripples found in the nonprimate hippocampus, including spectral frequency and bilateral occurrence [8, 28], broad area of tissue supporting generation and synchrony of neuronal discharges [27], highest occurrence during SWS and lowest duringREMsleep [10], and preferred discharge latencies of putative interneurons and pyramidal cells associated with the generation of ripples [39•]. Microelectrode data, and evidence that the occurrence of ripples correlates with correct recall of items after memory consolidation tasks [40•], suggest some ripples in human MTL reflect normal endogenous activity.
Properties of fast ripples recorded in patients resemble fast ripples in epileptic rats with respect to the following: spectral frequency, duration and association with the seizure-onset zone [22, 28], local generation in cell lamina that support evoked population spike discharge and abnormal synchrony of burst firing [27], and rates of occurrence that are highest during SWS and remain elevated during REM sleep [10]. In addition, with respect to the pathological substrate, particularly in patients with MTLE and hippocampal sclerosis, higher rates of fast ripples in hippocampus correlate with severity of local atrophy [41••], whereas higher rates of fast ripples and lower rates of ripples correlate with lower neuron densities in Ammon’s horn and dentate gyrus [42]. These data suggest that morphological abnormalities associated with hippocampal sclerosis may promote the generation of fast ripples and alter networks supporting ripples. It has not been possible, however, to distinguish normal from pathological ripple-frequency HFOs in human recordings.
The number of HFO studies in presurgical patients has steadily risen with higher sampling rates and greater bandwidth that are now available on many clinical electrophysiology systems recording from standard clinical depth and subdural grid electrodes. These studies have extended the characterization of pHFOs in MTL structures and neocortex. Although they often have not attempted to distinguish between fast ripples and ripple-frequency HFOs, they have confirmed a strong association with the epileptogenic region, and when this distinction was made, fast ripples had a tighter correlation. Recent patient studies found higher rates of occurrences of HFOs within nonlesioned cortical seizure-onset areas compared with rates in lesioned cortical seizure-onset areas that included nodular heterotopias [43••]. These latter data indicate that abnormal HFOs are not limited to a specific type of epilepsy and may be a fundamental property of epileptogenicity common to many types of epilepsy, but also that in some types of epilepsy, electrophysiological disturbances may be remote to anatomical abnormalities.
HFOs in MTL and extratemporal structures occur more often during non-REM(NREM) sleep than during waking and REM sleep [44, 45] and the duration of HFOs is longest during SWS than during other sleep–wake states, but overall, properties of HFOs including significant differences in rates of occurrence of HFOs with respect to the seizure-onset area are consistent across sleep–wake states. These latter findings are consistent with previous studies that used recordings from NREM episodes to characterize spatial patterns of interictal HFOs in relation to seizure-onset areas [28, 30••, 31, 46, 47] and rates of interictal HFOs with respect to seizure frequency [48].
Direct brain electroencephalograph (EEG) recording of interictal spikes (IIS) can be useful for delineating the epileptogenic region, but the spatial extent of IIS is usually more widespread than the epileptogenic region and can even be contralateral to it. Recording with clinical electrodes provides an opportunity to evaluate whether pHFOs more reliably delineate the boundaries of the epileptogenic region than IISs do. Recent work has shown significant overlap in the spatial and temporal patterns of IIS and HFOs, with a greater proportion of IIS containing HFOs in seizure-onset areas compared with those outside these areas [31, 44, 49, 50]. Approximately, 40–50% of HFOs, however, occur independently of IIS. The occurrence of IIS with HFOs and HFOs alone are more sensitive in predicting the seizure-onset area than the occurrence of IIS alone [30••]. Furthermore, using surgical outcome to verify the boundaries of the epileptogenic region, patients with good surgical outcome have a significantly larger proportion ofHFO-generating sites removed than patients with poor surgical outcome, whereas the same relationship is less reliable for IIS or seizure-onset sites [51, 52]. These studies suggest that pHFOs not only delineate the epileptogenic region better than IIS, but also better than EEG identification of the site of ictal onset.
Electroencephalograph interictal spikes and high-frequency oscillations
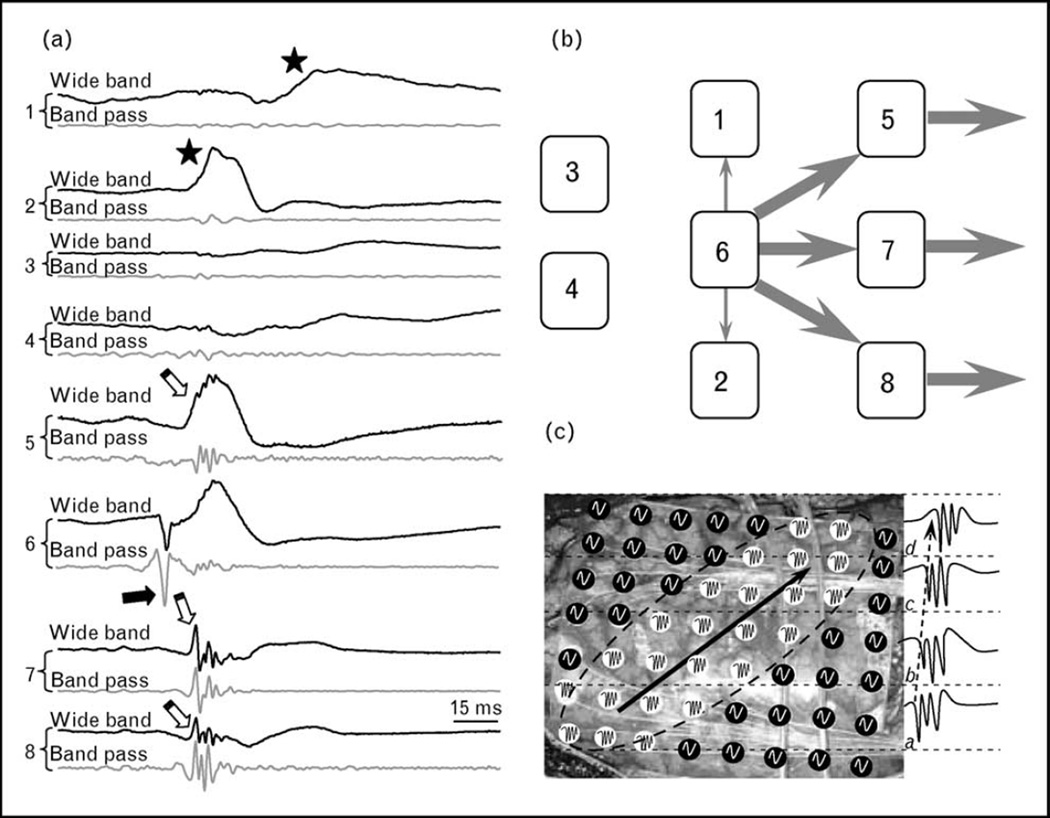
The mechanisms and functional significance associated with IIS containing pHFOs versus IIS without pHFOs are not known. If IIS reflect gigantic excitatory postsynaptic potentials [53], and pHFOs are bursts of population spikes [24, 35, 36], then IIS containing pHFOs may indicate that a majority of neurons in the recording area generate hypersynchronous action potentials that actively participate in the generation and propagation of epileptiform activity. Sites where IIS do not contain pHFOs may receive epileptiform input that is subthreshold for the generation of hypersynchronous discharges, which reduces the probability that epileptiform activity will be transmitted to postsynaptic targets. This concept is illustrated in Fig. 1. In part (a), eight simultaneously recorded EEG traces are presented. Four electrodes (number 5–8) record IIS that contain pHFOs; two other recording sites (1 and 2) record IIS that do not contain pHFOs, whereas the remaining two sites (3 and 4) record no epileptiform discharges. The earliest pHFO occurs under electrode 6 and reflects the local abnormal generation of hypersynchronous action potentials, which is basically the output of the network. One interpretation of these data is that the initial epileptiform signal is generated nearest to electrode 6, is then transmitted to electrodes 5, 7, and 8, and from these sites moves outside of the recorded network. Electrodes 1 and 2 also receive signal from electrode 6; however, it is subthreshold and the signal does not propagate outside of the network through these electrodes. Electrodes 3 and 4 do not receive input from areas near electrode 6 and do not participate in propagation of the epileptiform activity (Fig. 1b). Evidence to support this hypothesis will require careful analysis of the shape or morphology of IIS and spectral analysis for pHFO content to identify brain areas that are actively involved with the generation of epileptiform activity.
Figure 1. Functional difference between interictal spikes that do and do not contain pathological high-frequency oscillations.
(a) An example of an IIS simultaneously recorded from several electrode contacts in an epileptic rat. Recordings from microelectrodes 5–8 contain IIS with pHFOs, microelectrodes 1 and 2 contain EEG IIS without pHFOs and no IISs or pHFOs are present in microelectrodes 3 and 4. The pHFOs occurred first at microelectrode 6 and approximately 10 ms later at microelectrodes 5, 7, and 8. (b) A schematic of the role of each recoding sites in the propagation of epileptiform activity. After occurrence at recoding site 6, epileptiform activity propagates further through recording sites 5, 7, and 8 but not through recording sites 1 and 2 (see text for further explanation). (c) A hypothetical case of how mapping of IIS containing pHFOs could be used for determining which parts of neocortex participate in the propagation of epileptiform activity. Grid electrodes marked white record IISs containing pHFOs, whereas those marked black record IISs without pHFOs. pHFOs occur first in electrodes marked ‘A’, then progress through groups of electrodes marked ,‘B’, ‘C’, and ‘D’ (see examples of records on the right). In this case, the part of the neocortex outlined by the dashed ellipse actively participates in the generation and propagation of epileptiform activity from left to right, whereas other electrodes do not. IIS, interictal spike; pHFO, pathological high-frequency oscillation.
A potential application for this idea in the case of neocortical epilepsy is presented in Fig. 1c. The grid electrode contacts colored white denote sites that contain IIS with pHFOs and those colored black record IIS without pHFOs. Recordings indicate pHFOs occurred initially under the group of electrodes in an area labeled ‘a’ in Fig. 1c, and then are recorded consecutively at longer latencies near electrode sites labeled ‘b’, ‘c’, and ‘d’ (see examples of pHFOs on the right part of Fig. 1c). This pattern of pHFO activity could indicate to the neurosurgeon that the planned area of resection should include sites where pHFOs occurred, that is, area denoted by the ellipse in Fig. 1c.
Seizures and high-frequency oscillations
Reports of HFOs associated with seizure onsets were published more than 15 years ago [54, 55], but at that time, these data were obtained from a small number of patients and could not be repeated in other laboratories because electrophysiological recording equipment lacked the capacity to record wide bandwidth high-frequency EEG. Ictal HFOs have now been described in several animal and patient studies [29, 32, 52, 56–61] and their power can increase several minutes before seizure onset. Focal seizures that secondarily generalize are characterized by a relatively small area of pHFO generation at seizure onset that can move along the cortex and increase in size as the seizure progresses [62]. Although it has been suggested that alterations in the size of pHFO-generating neuronal clusters and increasing synchrony among them account for ictal transition to ictus [25, 35], more information about the fundamental neuronal mechanisms underlying this process is critical to understand the role of pHFOs in seizure genesis. For example, a preictal increase in HFO power that reflects bursts of population spikes may be an indication of enhanced synchronization of principal cell networks that reach threshold for propagation of ictal epileptiform activity to areas of the brain responsible for the manifestation of behavioral seizures. On the contrary, an increase in the power of preictal HFOs that reflect IPSPs may be an indication of interneuronal network activation to suppress propagation of epileptiform discharges and prevent seizure occurrence.
Normal versus pathological high-frequency oscillations
A critical issue that is currently limiting our understanding of the role HFOs play in epileptogenesis and epileptogenicity is our inability to reliably distinguish normal from pathological HFOs outside of the dentate gyrus. Addressing this problem will require identifying electrophysiological, anatomical, and pathological properties of the mechanisms and networks supporting the generation of different types of HFOs. Important new information will likely derive from advances in detection and power spectral analysis of wide bandwidthEEG[50, 63, 64], additional studies to understand the mechanisms and significance of IIS containing pHFOs and those without pHFOs, and analysis of simultaneous microelectrode and macroelectrode recordings using novel electrode contact sizes and configurations [46, 65]. Moreover, identifying clinical situations that are associated with increases or decreases in the occurrence of different types of HFOs, for example, sleep [66], medication withdrawal [67], or electrical stimulation [47], could provide further insights into the brain states and conditions that regulate their generation.
Conclusion
Data obtained from experimental animal models of MTLE and from patients with focal epilepsy due to a variety of limbic and neocortical lesions support the view that the spatial extent of pHFOs can reliably delineate the epileptogenic region, the area necessary and sufficient for spontaneous seizure generation. Evidence from animal studies also suggests that early occurrence of pHFOs predict the development of epilepsy after an epileptogenic insult, and features of pHFOs correlate with seizure frequency. These interictal oscillations, therefore, are among the very few potential candidate biomarkers of epileptogenesis and epileptogenicity. Remaining issues include the fact that it is not yet possible to distinguish normal HFOs from pHFOs in clinical recordings and so far spontaneous HFOs can only be detected with intracranial recordings.
Basic research on pHFOs indicate that these electrophysiological events reflect synchronized burst firing of principal cells within small discretely located neuronal clusters and that these clusters play an important role in seizure generation. pHFOs, therefore, could represent the primary epileptogenic abnormality in some forms of epilepsy. Elucidation of the fundamental neuronal mechanisms responsible for their development and their role in epileptogenesis, as well as ictogenesis, could provide insights into novel targets for treatment, prevention, and cure of epilepsy.
Acknowledgements
Support for the original research was provided by NS-02808 and NS-33310.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (p. 199).
- 1.World Health Organization. Atlas: epilepsy care in the world. Geneva: World Health Organization; 2005. International League Against Epilepsy. [Google Scholar]
- 2.Buzsaki G, Horvath Z, Urioste R, et al. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–1027. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- 3.O’Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- 4.Suzuki SS, Smith GK. Spontaneous EEG spikes in the normal hippocampus. II: Relations to synchronous burst discharges. Electroencephalogr Clin Neurophysiol. 1988;69:532–540. doi: 10.1016/0013-4694(88)90165-4. [DOI] [PubMed] [Google Scholar]
- 5.Chrobak JJ, Buzsaki G. High-frequency oscillations in the output of the hippocampal–entorhinal axis of the freely behaving rat. J Neurosci. 1996;16:3056–3066. doi: 10.1523/JNEUROSCI.16-09-03056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Csicsvari J, Hirase H, Czurko A, et al. Fast network oscillations in the hippocampal ca1 region of the behaving rat. J Neurosci. 1999;19:1–4. doi: 10.1523/JNEUROSCI.19-16-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ylinen A, Bragin A, Nadasdy Z, et al. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bragin A, Engel J, Jr, Wilson CL, et al. High-frequency oscillations in human brain. Hippocampus. 1999;9:137–142. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Buhl DL, Harris KD, Hormuzdi SG, et al. Selective impairment of hippocampal gamma oscillations in connexin-36 knock-out mouse in vivo. J Neurosci. 2003;23:1013–1018. doi: 10.1523/JNEUROSCI.23-03-01013.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staba RJ, Wilson CL, Bragin A, et al. High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol. 2004;56:108–115. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- 11.Ulanovsky N, Moss CF. Hippocampal cellular and network activity in freely moving echolocating bats. Nat Neurosci. 2007;10:224–233. doi: 10.1038/nn1829. [DOI] [PubMed] [Google Scholar]
- 12.Buzsaki G. The hippocampo-neortical dialogue. Cereb Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- 13.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 14.Cracco RQ, Cracco JB. Somatosensory evoked potential in man: far field potentials. Electroencephalogr Clin Neurophysiol. 1976;41:460–466. doi: 10.1016/0013-4694(76)90057-2. [DOI] [PubMed] [Google Scholar]
- 15.Curio G, Mackert BM, Burghoff M, et al. Localization of evoked neuromagnetic 600 Hz activity in the cerebral somatosensory system. Electroencephalogr Clin Neurophysiol. 1994;91:483–487. doi: 10.1016/0013-4694(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 16.Eisen A, Roberts K, Low M, et al. Questions regarding the sequential neural generator theory of the somatosensory evoked potential raised by digital filtering. Electroencephalogr Clin Neurophysiol. 1984;59:388–395. doi: 10.1016/0168-5597(84)90040-6. [DOI] [PubMed] [Google Scholar]
- 17.Jones MS, Barth DS. Spatiotemporal organization of fast (>200 Hz) electrical oscillations in rat vibrissa/barrel cortex. J Neurophysiol. 1999;82:1599–1609. doi: 10.1152/jn.1999.82.3.1599. [DOI] [PubMed] [Google Scholar]
- 18.Nakano S, Hashimoto I. Comparison of somatosensory evoked high-frequency oscillations after posterior tibial and median nerve stimulation. Clin Neurophysiol. 1999;110:1948–1952. doi: 10.1016/s1388-2457(99)00145-5. [DOI] [PubMed] [Google Scholar]
- 19.Grenier F, Timofeev I, Steriade M. Focal synchronization of ripples (80– 200 Hz) in neocortex and their neuronal correlates. J Neurophysiol. 2001;86:1884–1898. doi: 10.1152/jn.2001.86.4.1884. [DOI] [PubMed] [Google Scholar]
- 20.Barth DS. Submillisecond synchronization of fast electrical oscillations in neocortex. J Neurosci. 2003;23:2502–2510. doi: 10.1523/JNEUROSCI.23-06-02502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones MS, MacDonald KD, Choi B, et al. Intracellular correlates of fast (>200 Hz) electrical oscillations in rat somatosensory cortex. J Neurophysiol. 2000;84:1505–1518. doi: 10.1152/jn.2000.84.3.1505. [DOI] [PubMed] [Google Scholar]
- 22.Bragin A, Engel J, Jr, Wilson CL, et al. Hippocampal and entorhinal cortex high-frequency oscillations (100–500Hz) in human epileptic brain and in kainic acid-treated rats with chronic seizures. Epilepsia. 1999;40:127–137. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 23.Bragin A, Engel J, Jr, Wilson CL, et al. Electrophysiologic analysis of a chronic seizure model after unilateral hippocampal ka injection. Epilepsia. 1999;40:1210–1221. doi: 10.1111/j.1528-1157.1999.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 24.Bragin A, Wilson CL, Engel J., Jr Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: a hypothesis. Epilepsia. 2000;41:S144–S152. doi: 10.1111/j.1528-1157.2000.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 25.Bragin A, Azizyan A, Almajano J, et al. Analysis of chronic seizure onsets after intrahippocampal kainic acid injection in freely moving rats. Epilepsia. 2005;46:1592–1598. doi: 10.1111/j.1528-1167.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 26.Bragin A, Wilson CL, Engel JJ. Spatial stability over time of brain areas generating fast ripples in the epileptic rat. Epilepsia. 2003;44:1233–1237. doi: 10.1046/j.1528-1157.2003.18503.x. [DOI] [PubMed] [Google Scholar]
- 27.Bragin A, Wilson CL, Staba RJ, et al. Interictal high frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol. 2002;52:407–415. doi: 10.1002/ana.10291. [DOI] [PubMed] [Google Scholar]
- 28.Staba RJ, Wilson CL, Bragin A, et al. Quantitative analysis of high frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–1752. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- 29.Khosravani H, Mehrotra N, Rigby M, et al. Spatial localization and time-dependant changes of electrographic high frequency oscillations in human temporal lobe epilepsy. Epilepsia. 2008;50:605–616. doi: 10.1111/j.1528-1167.2008.01761.x. [DOI] [PubMed] [Google Scholar]
- 30. Jacobs J, LeVan P, Chander R, et al. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–1907. doi: 10.1111/j.1528-1167.2008.01656.x. This study provides evidence that rates of HFOs identify seizure onset areas with greater sensitivity and specificity compared to rates of interictal spikes.
- 31.Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130:2354–2366. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- 32.Jirsch JD, Urrestarazu E, LeVan P, et al. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–1608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- 33.Bragin A, Wilson CL, Almajano J, et al. High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–1023. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- 34. Engel J, Jr, Bragin A, Staba R, Mody I. High-frequency oscillations: what is normal and what is not? Epilepsia. 2009;50:598–604. doi: 10.1111/j.1528-1167.2008.01917.x. It is the first publication that critically reviewed normal and pathological HFOs.
- 35.Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. J Neurosci. 2002;22:2012–2021. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bragin A, Wilson CL, Engel J., Jr Voltage depth profiles of high-frequency oscillations after kainic acid-induced status epilepticus. Epilepsia. 2007;48:35–40. doi: 10.1111/j.1528-1167.2007.01287.x. [DOI] [PubMed] [Google Scholar]
- 37.Kandel A, Buzsaki G. Cellular-synaptic generation of sleep spindles, spike-and- wave discharges, and evoked thalamocortical responses in the neocortex of the rat. J Neurosci. 1997;17:6783–6797. doi: 10.1523/JNEUROSCI.17-17-06783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grenier F, Timofeev I, Steriade M. Neocortical very fast oscillations (ripples, 80–200 Hz) during seizures: intracellular correlates. J Neurophysiol. 2003;89:841–852. doi: 10.1152/jn.00420.2002. [DOI] [PubMed] [Google Scholar]
- 39. Le Van Quyen M, Bragin A, Staba R, et al. Cell type-specific firing during ripple oscillations in the hippocampal formation of humans. J Neurosci. 2008;28:6104–6110. doi: 10.1523/JNEUROSCI.0437-08.2008. This study identifies neuronal correlates of normal HFOs in humans.
- 40. Axmacher N, Elger CE, Fell J. Ripples in the medial temporal lobe are relevant for human memory consolidation. Brain. 2008;131:1806–1817. doi: 10.1093/brain/awn103. The first study that described a role of ripple oscillations in human cognitive processes.
- 41. Ogren JA, Wilson CL, Bragin A, et al. Three dimensional surface maps link local atrophy and fast ripples in human epileptic hippocampus. Ann Neurol. 2009;66:783–791. doi: 10.1002/ana.21703. This study found a strong association between hippocampal atrophy and areas generating pHFOs.
- 42.Staba RJ, Frighetto L, Behnke EJ, et al. Increased fast ripple-to-ripple ratios correlate with reduced hippocampal volumes and neuron loss in temporal lobe epilepsy patients. Epilepsia. 2007;48:2130–2138. doi: 10.1111/j.1528-1167.2007.01225.x. [DOI] [PubMed] [Google Scholar]
- 43. Jacobs J, LeVan P, Chatillon C-E, et al. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132:1022–1037. doi: 10.1093/brain/awn351. This publication provides evidence that HFOs represent a marker for seizure-onset areas independent of the underlying disorder.
- 44.Bagshaw AP, Jacobs J, LeVan P, et al. Effect of sleep stage on interictal high-frequency oscillations recorded from depth macroelectrodes in patients with focal epilepsy. Epilepsia. 2008;50:617–628. doi: 10.1111/j.1528-1167.2008.01784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clemens Z, Molle M, Eross L, et al. Temporal coupling of parahippocampal ripples, sleep spindles and slow oscillations in humans. Brain. 2007;130:2868–2878. doi: 10.1093/brain/awm146. [DOI] [PubMed] [Google Scholar]
- 46.Schevon CA, Trevelyan AJ, Schroeder CE, et al. Spatial characterization of interictal high frequency oscillations in epileptic neocortex. Brain. 2009;132:3047–3059. doi: 10.1093/brain/awp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacobs J, Zijlmans M, Zelmann R, et al. Value of electrical stimulation and high frequency oscillations (80–500 Hz) in identifying epileptogenic areas during intracranial EEG recordings. Epilepsia. 2009 doi: 10.1111/j.1528-1167.2009.02389.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zijlmans M, Jacobs J, Zelmann R, et al. High frequency oscillations and seizure frequency in patients with focal epilepsy. Epilepsy Res. 2009;85:287–292. doi: 10.1016/j.eplepsyres.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urrestarazu E, Jirsch JD, LeVan P, et al. High-frequency intracerebral EEG activity (100–500 Hz) following interictal spikes. Epilepsia. 2006;47:1465–1476. doi: 10.1111/j.1528-1167.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi K, Jacobs J, Gotman J. Detection of changes of high-frequency activity by statistical time-frequency analysis in epileptic spikes. Clin Neurophysiol. 2009;120:1070–1077. doi: 10.1016/j.clinph.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacobs J, Zijlmans M, Zelmann R, et al. High frequency EEG oscillations correlate with outcome of epilepsy surgery. Ann Neurol. doi: 10.1002/ana.21847. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ochi A, Otsubo H, Donner EJ, et al. Dynamic changes in ictal high frequency oscillations in neocortical epilepsy using multiple band frequency analysis. Epilepsia. 2007;48:286–296. doi: 10.1111/j.1528-1167.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- 53.de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol. 2001;63:541–567. doi: 10.1016/s0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- 54.Allen PJ, Fish DR, Smith SJM. Very high-frequency rhythmic activity during SEEG suppression in frontal lobe epilepsy. Electroencephalogr Clin Neurophysiol. 1992;82:155–159. doi: 10.1016/0013-4694(92)90160-j. [DOI] [PubMed] [Google Scholar]
- 55.Fisher RS, Webber WR, Lesser RP, et al. High-frequency EEG activity at the start of seizures. J Clin Neurophysiol. 1992;9:441–448. doi: 10.1097/00004691-199207010-00012. [DOI] [PubMed] [Google Scholar]
- 56.Traub RD, Whittington MA, Buhl EH, et al. A possible role for gap junctions in generation of very fast EEG oscillations preceding the onset of perhaps initiating, seizures. Epilepsia. 2001;42:153–170. doi: 10.1046/j.1528-1157.2001.26900.x. [DOI] [PubMed] [Google Scholar]
- 57.Worrell GA, Gardner AB, Stead SM, et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–937. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Worrell GA, Parish L, Cranstoun SD, et al. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127:1496–1506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
- 59.RamachandranNair R, Ochi A, Imai K, et al. Epileptic spasms in older pediatric patients: Meg and ictal high-frequency oscillations suggest focal-onset seizures in a subset of epileptic spasms. Epilepsy Res. 2008;78:216–224. doi: 10.1016/j.eplepsyres.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Akiyama T, Otsubo H, Ochi A, et al. Focal cortical high-frequency oscillations trigger epileptic spasms: confirmation by digital video subdural EEG. Clin Neurophysiol. 2005;116:2819–2825. doi: 10.1016/j.clinph.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 61.Jacobs J, Zelmann R, Jirsch J, et al. High frequency oscillations (80–500 Hz) in the preictal period in patients with focal seizures. Epilepsia. 2009;50:1780–1792. doi: 10.1111/j.1528-1167.2009.02067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akiyama T, Otsubo H, Ochi A, et al. Topographic movie of ictal high-frequency oscillations on the brain surface using subdural EEG in neocortical epilepsy. Epilepsia. 2006;47:1953–1957. doi: 10.1111/j.1528-1167.2006.00823.x. [DOI] [PubMed] [Google Scholar]
- 63.Crepon B, Navarro V, Hasboun D, et al. Mapping interictal oscillations greater than 200 Hz recorded with intracranial macroelectrodes in human epilepsy. Brain. 2010;133:33–45. doi: 10.1093/brain/awp277. [DOI] [PubMed] [Google Scholar]
- 64.Gardner AB, Worrell GA, Marsh E, et al. Human and automated detection of high-frequency oscillations in clinical intracranial EEG recordings. Clin Neurophysiol. 2007;118:1134–1143. doi: 10.1016/j.clinph.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Gompel JJ, Stead SM, Giannini C, et al. Phase I trial: safety and feasibility of intracranial electroencephalography using hybrid subdural electrodes containing macro- and microelectrode arrays. J Neurosurg. 2008;25:E23–E29. doi: 10.3171/FOC/2008/25/9/E23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zelmann R, Zijlmans M, Jacobs J, et al. Improving the identification of high frequency oscillations. Clin Neurophysiol. 2009;120:1457–1464. doi: 10.1016/j.clinph.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zijlmans MM, Jacobs JM, Zelmann RM, et al. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009;72:979–986. doi: 10.1212/01.wnl.0000344402.20334.81. [DOI] [PMC free article] [PubMed] [Google Scholar]