Abstract
OBJECTIVE
The purpose of this study was to evaluate initial experience with 18F-FDG PET/CT after pulmonary radiofrequency ablation of stage IA non–small cell lung cancer to determine whether treatment success or residual disease can be predicted with early postablation PET.
SUBJECTS AND METHODS
Thirty patients with medically inoperable stage IA non–small cell lung cancer (12 men, 18 women; median age, 76 years; range, 60–87 years) underwent outpatient CT-guided radiofrequency ablation over a 33-month period. Mean tumor size was 2.0 cm (range, 1.3–2.9 cm). PET/CT was performed within 60 days before radio-frequency ablation (RFA), within 4 days after RFA, and 6 months after RFA. Metabolic response was categorized as complete response or partial or no response at early post-RFA PET/CT and complete response, partial response, or progressive metabolic disease at 6-month post-RFA PET/CT and was compared with the 1-year clinical event rate (death, disease progression at contrast-enhanced CT, or repeat ablation).
RESULTS
Early PET/CT images, obtained within 4 days of RFA, were evaluable for 26 patients (23 at 6 months). Patients with a complete metabolic response at early PET/CT had a 1-year event rate of 43%, whereas those with partial or no response or disease progression had a 1-year event rate of 67% (p = 0.27). Patients with a complete metabolic response at 6-month PET/CT had a 1-year event rate of 0%. Those with a partial response and those with disease progression had an overall event rate of 75% (p = 0.001).
CONCLUSION
Early post-RFA PET/CT is not necessary and 6-month post-RFA PET/ CT findings correlate better with clinical outcome at 1 year.
Keywords: non–small cell lung cancer, PET/CT, radiofrequency ablation
Radiofrequency ablation (RFA) has become established as an effective alternative treatment of patients with early-stage, medically inoperable lung cancer [1–11]. RFA of stage I non– small cell lung cancer (NSCLC) has 78% 1-year and 27% 5-year survival rates compared with a 50% 1-year survival rate among patients undergoing observation alone [1]. Several studies have shown that RFA of lung tumors is safe and efficacious [1–11].
Contrast-enhanced CT and integrated PET/ CT with the glucose analogue 18F-FDG are the two principal imaging methods being used to evaluate treatment success after RFA for NSCLC. Appropriate imaging is needed to evaluate treatment response because early detection of residual tumor and recurrence can help determine which patients may benefit from repeat ablation or other therapies, such as stereotactic body radiotherapy. The optimal timing for follow-up imaging of patients after RFA has not been established, but some results indicate that early PET may be of benefit after RFA in the lung and liver. Okuma et al. [12] found that in rabbits the inflammatory changes visualized at PET after lung RFA were much less severe 1 day than 1–2 weeks after treatment. Liu et al. [13] found that PET/CT performed within 24 hours after RFA of liver metastasis can be effective in the detection of residual malignancy.
The American College of Surgeons Oncology Group study Z4033, a phase II trial of RFA in the care of patients with stage IA NSCLC judged to be medically inoperable, was designed to evaluate the safety and effectiveness of this treatment in a multicenter setting. In a substudy of this trial, the first cohort of patients (n = 30) underwent FDG PET/CT 1–4 days after RFA to determine whether treatment success or presence of residual disease could be predicted in comparison with clinical outcome at 1 year (death, disease progression on contrast-enhanced CT, or repeat ablation). We hypothesized that the early PET findings would not be confounded by the inflammatory changes known to develop after RFA and thus might be more reliable for detecting residual tumor.
Subjects and Methods
Patient Selection
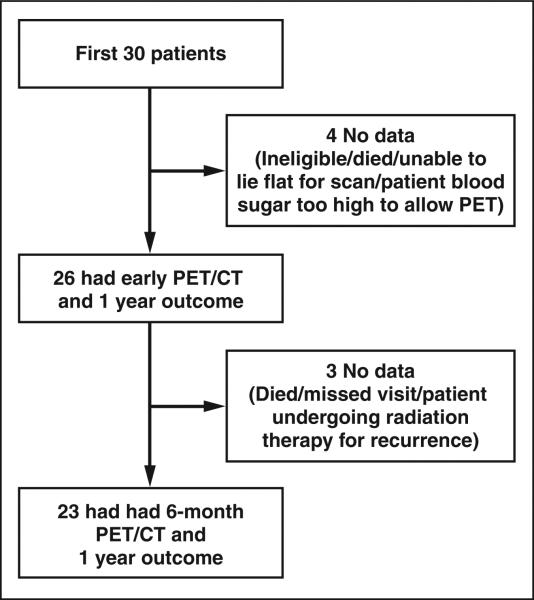
The American College of Surgeons Oncology Group study Z4033 was opened for accrual in December 2006. The eligibility criteria for this study are shown in Table 1. The patients in this sub-study were accrued from 11 participating institutions. All of the patients had biopsy-proved stage IA NSCLC. This study was HIPAA compliant and was approved by all participating hospital institutional review boards. All subjects gave written informed consent. The subjects for the PET substudy were the initial 30 patients who underwent percutaneous CT-guided RFA over the 33-month period through April 2009. The baseline patient characteristics are summarized in Table 2, and the Standards for Reporting of Diagnostic Accuracy diagram is shown in Figure 1.
TABLE 1.
Eligibility Criteria
| Patient Must Have | Major Criteria | Minor Criteria |
|---|---|---|
| Histologically confirmed clinical and imaging stage IA non–small cell lung cancer | Forced expiratory volume in 1 s, ≤ 50% predicted | Age ≥ 75 y |
| Evaluation by a thoracic surgeon and be deemed at high risk with respect to lung resection | Diffusion capacity of the lung for carbon dioxide, ≤ 50% predicted | Forced expiratory volume in 1 s, 51–60% predicted |
| FDG PET and CT of the chest and upper abdomen within 60 d before preregistration; pulmonary function testing within 120 d before registration | Diffusion capacity of the lung for carbon dioxide, 51–60% predicted | |
| Eastern Cooperative Oncology Group or Zubrod performance status 0, 1, or 2 | Pulmonary hypertension (pulmonary artery systolic pressure > 40 mm Hg) as estimated at echocardiography or right-heart catheterization | |
| At least one major criterion or two minor criteria | Poor left ventricular function (ejection fraction ≤ 40%) | |
| Resting or exercise Pao2 ≤ 55 mm Hg or oxygen saturation measured with pulse oximetry ≤ 88% | ||
| Pco2 > 45 mm Hg or Modified Medical Research Council Dyspnea Scale grade ≥ 3 | ||
TABLE 2.
Baseline Patient Characteristics
| Characteristic | Cohort Patients (n = 23) | All Patients (n = 30) | p a |
|---|---|---|---|
| Age (y) | 76 (60–87) | 78 (60–87) | 0.75 |
| Sex (no.) | 0.80b | ||
| Women | 13 (57) | 18 (60) | |
| Men | 10 (43) | 12 (40) | |
| Performance status | 0.78b | ||
| 0 | 3 (13) | 4 (13) | |
| 1 | 15 (65) | 17 (57) | |
| 2 | 5 (22) | 9 (30) | |
| Clinical stage (no.) | NAb | ||
| IA (T1N0M0) | 23 (100) | 30 (100) | |
| Histologic result | |||
| Squamous | 10 (43) | 12 (40) | 0.84b |
| Adenocarcinoma | 11 (48) | 14 (47) | |
| Other non–small cell carcinoma | 2 (9) | 3 (10) | |
| Poorly differentiated non–small cell carcinoma | 0 (0) | 1 (3) | |
| Size of nodule (cm) | 2 (1.3–2.9) | 2 (1.3–2.9) | 0.96 |
| Tumor standardized uptake value | 6.4 (0–14) | 6.4 (0–14) | 0.89 |
| Baseline forced expiratory volume in 1 s (mL) | 870 (430–2010) | 1040 (430–2010) | 0.57 |
| Baseline FEV1 (% of predicted) | 49 (13–102) | 48 (13–102) | 0.83 |
| Baseline forced vital capacity (mL) | 1840 (1090–2640) | 1840 (1090–2810) | 0.78 |
| Baseline diffusion capacity of the lung for carbon dioxide (% of predicted) | 41 (13–67) | 45 (13–67) | 0.74 |
Note—Values are median with range in parentheses or number with percentage in parentheses. NA = not applicable.
Wilcoxon rank sum test.
Chi-square test.
Fig. 1.
Standards for Reporting of Diagnostic Accuracy diagram shows numbers of patients in PET substudy cohort undergoing PET/CT 1–4 days and 6 months after RFA.
All of these patients underwent preablation PET/CT. Early PET/CT (1–4 days after RFA) was performed in 26 of 30 cases because of one death and three technical problems (one patient was deemed ineligible, one could not lie flat, and one had an elevated blood glucose concentration). A follow-up PET/CT study 6 months after RFA was scheduled for the 26 patients who underwent early PET/CT and was completed in 23 cases. The other three studies were not completed because of the death of one patient and technical problems (one patient missed the visit, one patient was undergoing radiation therapy for recurrent disease).
Response to treatment was based on the event rate 1 year after RFA. Events were defined as death, evidence of disease progression at contrast-enhanced CT, and repeat ablation. Early PET/CT and 6-month PET/CT results were compared with the event rate at 1 year.
Ablation Protocol
A radiofrequency generator (CC-1, Valleylab), perfusion pump, and cluster electrodes (Cool-tip, Valleylab) with variable lengths (10, 15, 20 cm) and fixed active-tip exposure (2.5 cm) were used according to the manufacturer's guidelines. CT guidance was used for placement of the radiofrequency electrode into the target tumor. CT images of the electrode placement within the target lesion were obtained before activation of the radiofrequency generator and were reviewed centrally along with the RFA treatment parameters by one of two principal investigators. Only patients with treatment deemed technically successful were included in the study.
For lesions smaller than 2 cm in diameter, central and distal positioning of the radiofrequency electrode was deemed adequate for the first ablation, and subsequent tandem ablation zones were developed during more proximal positioning. When possible, the target lesion was entered along its longitudinal axis to allow sequential overlapping tandem ablation during electrode withdrawal. For lesions larger than 2 cm in diameter, multiple overlapping ablation zones were developed to ensure adequate thermocoagulation of the target lesion. Each radiofrequency application lasted a maximum of 12 minutes at any one position with the maximum allowable current. The maximal intratumoral temperature was recorded from the device to ensure adequate thermocoagulation. An intratumoral temperature greater than 60°C was deemed adequate for a given electrode position.
FDG PET/CT Acquisition and Analysis
All studies were performed with integrated PET/CT scanners. Patients fasted for at least 4 hours before injection of FDG. Patients with a blood glucose concentration exceeding 200 mg/ dL were not included. PET/CT scans were acquired from the canthomeatal line to the proximal thigh 50–70 minutes after IV administration of FDG (0.14–0.21 mCi/kg). The attenuation-correction CT images were performed without IV contrast administration.
Qualitative and quantitative analysis of the early (1–4 days) and 6-month postablation PET/CT scans was performed at each participating site by a board-certified radiologist or nuclear physician with experience in PET/CT. For qualitative analysis, the images were assessed for central photo-penia and rim uptake (homogeneous vs nonhomogeneous) at the site of the index lesion. The quantitative analysis of the PET images entailed measurement of the maximum standardized up-take value (SUVmax) before and after ablation [14].
On the early postablation PET/CT scans, a complete metabolic response was defined as homogeneous peripheral rim activity and central photopenia at the ablation site. Partial or no metabolic response was defined as a discrete focus of peripherally increased uptake that was more intense than the rest of the peripheral activity, absence of expected central photopenia, or unchanged appearance of the tumor.
On the 6-month follow-up PET/CT scans, the patients’ conditions were categorized as complete metabolic response (no residual disease) based on the overall appearance of the ablation cavity (homogeneous activity at the ablation site without a discrete focus of increased uptake more intense than the rest of the peripheral activity); partial response if there was a discrete focus of peripherally increased uptake that was more intense than the rest of the peripheral activity; or progressive response if there was a focal interval increase in FDG avidity at the ablation site consistent with residual or recurrent malignancy.
For 16 of 23 patients, the preablation PET/CT and early and 6-month postablation PET/CT scans were available for central review. Images of the other seven patients were not available for central review because of various technical issues, such as corrupt files and incomplete or missing image series from the PET/CT examinations. The scans available for central review were evaluated for intensity of uptake at the ablation site relative to the intensity of mediastinal blood-pool activity on the early and 6-month postablation PET/CT scans, which was not specifically evaluated at the site readings. Central review was performed by a board-certified radiologist–nuclear physician with experience in PET and specific experience in post-RFA imaging.
Results
Twenty-six of the early post-RFA studies (81%) were performed within 48 hours after RFA (1 day after RFA for 11 patients, 2 days for 10 patients, 3 days for three patients, and 4 days for two patients). The pre-RFA median SUVmax was 6.1 (range, 1.0–14). The early post-RFA median SUVmax was 2.6 (range, 1.2–11.5). The 6-month post-RFA median SUVmax was 2.5 (range, 0.6–10.6).
Early post-RFA PET/CT showed complete metabolic response in 14 patients and partial or no metabolic response in 12 patients. The 1-year event rate was 43% (95% CI, 18–71%) among the patients with early complete metabolic response and 67% (95% CI, 35–90%) among the patients with early partial or no metabolic response. The difference in event rate was not statistically significant (p = 0.27, Fisher exact test) (Table 3).
TABLE 3.
Relation Between Early PET Tumor Response and Clinical Outcome at 1 Year
| Early PET Tumor Response | Clinical Outcome at 1 ya | |
|---|---|---|
| No Event | Event | |
| Complete response (n = 14) | 8 (57) | 6 (43) |
| Partial or no response (n = 12) | 4 (33) | 8 (67) |
Note—Values in parentheses are percentages. Fisher exact test, p = 0.27.
Event is death, progression, or repeat ablation.
Six-month post-RFA PET/CT showed seven patients had a complete metabolic response, nine had a partial response, and seven had progressive metabolic disease. The 1-year event rate was 0% (95% CI, 0–41%) among the patients with a complete metabolic response, 78% (95% CI, 40–97%) among the patients with a partial response, and 71% (95% CI, 29–96%) among the patients with progressive disease. These differences in event rates were statistically significant (p = 0.004, chi-square test). The overall event rate was 75% (95% CI, 48– 93%) among patients with a partial response and those with progression. This combined event rate was statistically significant in comparison with that among patients with a complete response (p = 0.001, Fisher exact test) (Table 4). At central review, it was observed that in 11 of 16 patients (69%) the early PET/ CT images showed uptake at the ablation site that was greater than the mediastinal blood-pool activity. This finding persisted on the 6-month PET/CT images in 9 of the 11 patients (82%).
TABLE 4.
Relation Between 6-Month PET Tumor Response and Clinical Outcome at 1 Year
| 6-Month PET Tumor Response | Clinical Outcome at 1 ya | |
|---|---|---|
| No Event | Event | |
| Complete response (n = 7) | 7 (100) | 0 (0) |
| Partial response (n = 9) | 2 (22) | 7 (78) |
| Progressive disease (n = 7) | 2 (29) | 5 (71) |
Note—Values in parentheses are percentages. Chi-square, p = 0.004; Fisher exact test, p = 0.001 (complete response vs partial response or progressive disease).
Event is death, progression, or repeat ablation.
Discussion
Imaging-guided thermal ablation with radio-frequency energy is becoming more commonly used to treat lung tumors in medically compromised patients. Treatment monitoring is an important part of the follow-up care of this group of patients.
In initial studies of RFA, contrast-enhanced CT was the primary means of follow-up imaging [7]. More recently, functional imaging with FDG PET is being used increasingly to assess treatment response in these patients. Akeboshi et al. [8] found that high FDG uptake by malignant tumors on PET scans before ablation was eliminated on post-RFA scans. Okuma et al. [9] used FDG PET for follow-up evaluation of patients with primary lung cancer and metastatic disease treated with RFA and found that FDG PET showed evidence predictive of regrowth of tumors earlier than did CT. Other studies have shown that complete disappearance of FDG uptake within a malignant pulmonary tumor after any treatment (surgery, radiotherapy, or chemotherapy) is a good prognostic indicator, and this principle is similarly applied to RFA [15, 16]. The limitations of PET as a tool for follow-up surveillance include low spatial resolution and the common presence of prominent circumferential FDG uptake on short-term follow-up images, reflecting the inflammatory reaction that surrounds an ablated tumor. A focus of FDG uptake along the ablation periphery that is more intense than the activity in the rest of the periphery may represent residual or recurrent tumor or a focally more intense inflammatory response. Differentiating these findings can be challenging at follow-up PET.
There have been few studies of the early posttreatment utility of FDG PET. Kang et al. [17] performed PET and CT 1 week after ablation and concluded that PET showed tumor destruction in 70% of cases whereas CT showed tumor destruction in 38% of cases. In our study, we sought to determine whether the findings at earlier PET/CT, 1–4 days after ablation, would be predictive of outcome at 1 year. The expected finding at early post-RFA PET/CT is fairly homogeneous peripheral rim activity with central photopenia, the latter representing completely ablated tissue containing no viable cells. The peripheral rim represents the margin between the ablation cavity and surrounding lung parenchyma that has sustained a partial RFA insult. This rim activity can be extremely intense, indicating a large accumulation of inflammatory cells (macrophages, histiocytes). A goal of performing early PET/CT was to allow detection of residual tumor before the onset of a marked inflammatory response. We found, however, that even PET/CT performed as early as 1 day after RFA often showed intense peripheral uptake greater than the mediastinal blood pool activity, a finding in 69% of our centrally reviewed cases. Our finding indicates that homogeneous rim activity is most compatible with post-RFA inflammatory changes, even if the rim activity is more intense than the mediastinal blood pool activity (Fig. 2).
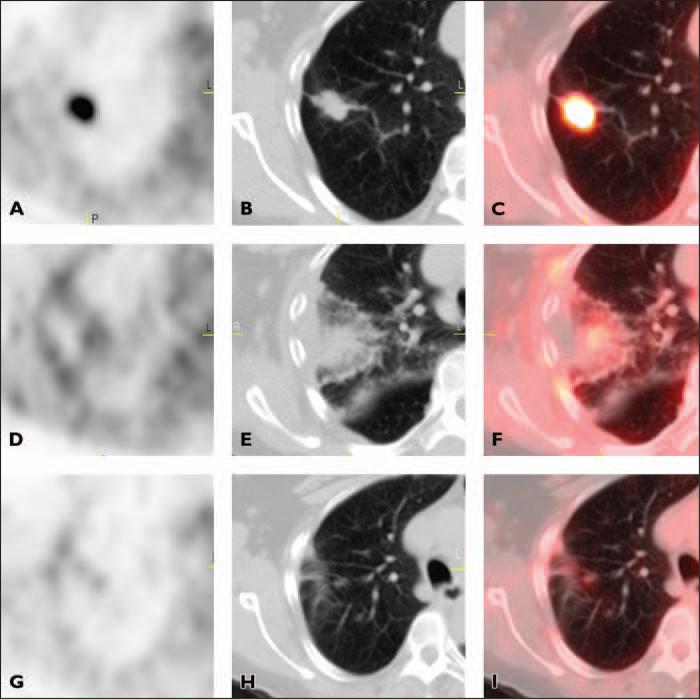
Fig. 2.
66-year-old woman with stage IA non–small cell lung cancer. A–C, Axial PET (A), CT (B), and fused PET/CT (C) images obtained before radiofrequency ablation (RFA) show area of intense FDG uptake corresponding to tumor in right upper lobe.
D–F, Axial PET (D), CT (E), and fused PET/CT (F) images obtained 3 days after RFA show homogeneous rim activity along periphery of ablation site with central photopenia (complete response). Rim activity is higher than background mediastinal activity.
G–I, Axial PET (G), CT (H), and fused PET/CT (I) images 6 months after RFA no longer show homogeneous rim activity. Mild linear FDG uptake at ablation site has intensity similar to mediastinal blood pool activity (complete response).
A discrete focus of increased FDG uptake along the periphery that was more intense than the rest of the rim activity was considered a partial response (Fig. 3). This focally more intense activity may represent residual malignancy or focally more intense inflammatory change. A pre-RFA PET/CT study helps with this differential diagnosis. Focal intense rim activity at the ablation site that is more intense than that of the tumor before RFA most likely represents focal intense inflammatory change. However, in patients in whom pre-RFA tumor uptake is mild, the post-RFA rim activity can mask the detection of residual tumor.
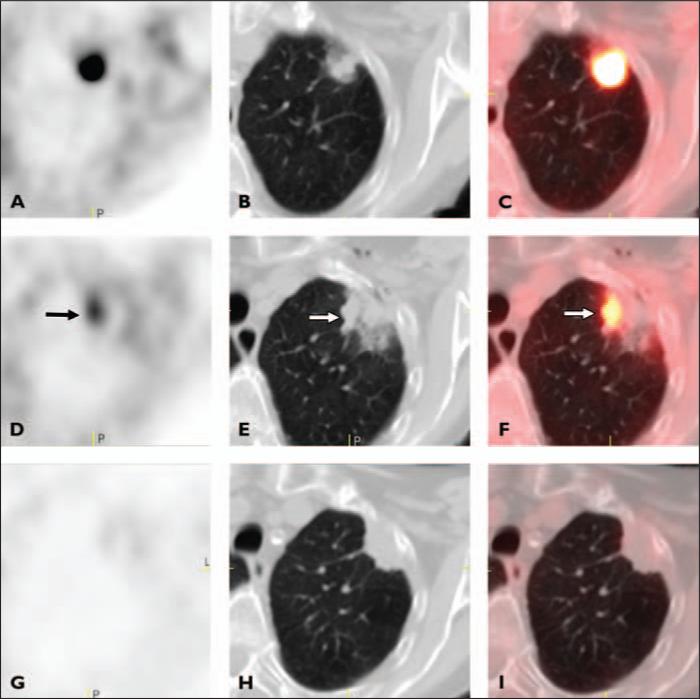
Fig. 3.
85-year-old woman with stage IA non–small cell lung cancer.
A–C, Axial PET (A), CT (B), and fused PET/CT (C) images obtained before radiofrequency ablation (RFA) show area of intense FDG uptake in left upper lobe.
D–F, Axial PET (D), CT (E), and fused PET/CT (F) images obtained 4 days after RFA show discrete focus of intense activity at medial margin of ablation site corresponding to opacity with irregular margins (arrow) (partial response).
G–I, Axial PET (G), CT (H), and fused PET/CT (I) images obtained 6 months after RFA images show minimal homogeneous rim activity with complete interval resolution of intense focus of increased activity in D–F (complete response).
The 6-month post-RFA PET/CT studies showed that patients with a complete or partial response had considerable variability in degree of diminished metabolic activity and qualitative appearance at the ablation site. Owing to the evolution of inflamma-tory changes, the ablation cavity often did not show peripheral homogeneous activity (Fig. 3). The central photopenia seen at early postablation PET/CT often is not apparent at 6-month postablation PET/CT if the peripheral activity decreases in intensity. Sing-nurkar et al. [18] also reported a variable appearance of postablation cavity on follow-up PET/CT scans obtained 1–4 months after RFA. An unfavorable response was characterized as focal uptake or as rim uptake with additional focal uptake in the same location as the original tumor. Continued PET/CT follow-up 1 year or more after treatment may be necessary to confirm recurrence. Detection of recurrent disease is critical because many patients may be candidates for repeat ablation or other therapies, such as stereotactic body radiotherapy.
The lack of correlation of the results of early PET/CT in comparison with clinical outcome 1 year after treatment indicates that early PET/CT is rarely of value. The optimal time for initial follow-up PET/CT after ablation has yet to be established. Singnurkar et al. [18] found a wide range of time postablation (1–4 months) when the initial follow-up PET/CT findings were predictive of local recurrence of malignancy. Deandreis et al. [19] performed postablation PET/CT 1 day, 1 month, and 3 months after RFA of 46 lung lesions (five lesions representing primary lung cancer, 41 lesions representing metastasis) and concluded that although the most appropriate postablation imaging time for PET/CT has yet to be assessed, performance of PET/CT 3 months after RFA may be a good interval for limiting false-positive results because of inflammatory uptake. The findings at PET/CT 1 day after RFA were not described in detail by Deandreis et al., therefore direct comparison of their early results and ours is not possible. It is not common for focally increased uptake at early PET/CT to be predictive of clinical evidence of disease recurrence during the first year after RFA. Figure 4 shows an uncommon case in which early PET/CT findings were predictive of the presence of biopsy-proved residual disease. The patient subsequently underwent repeat RFA of the area of recurrence.
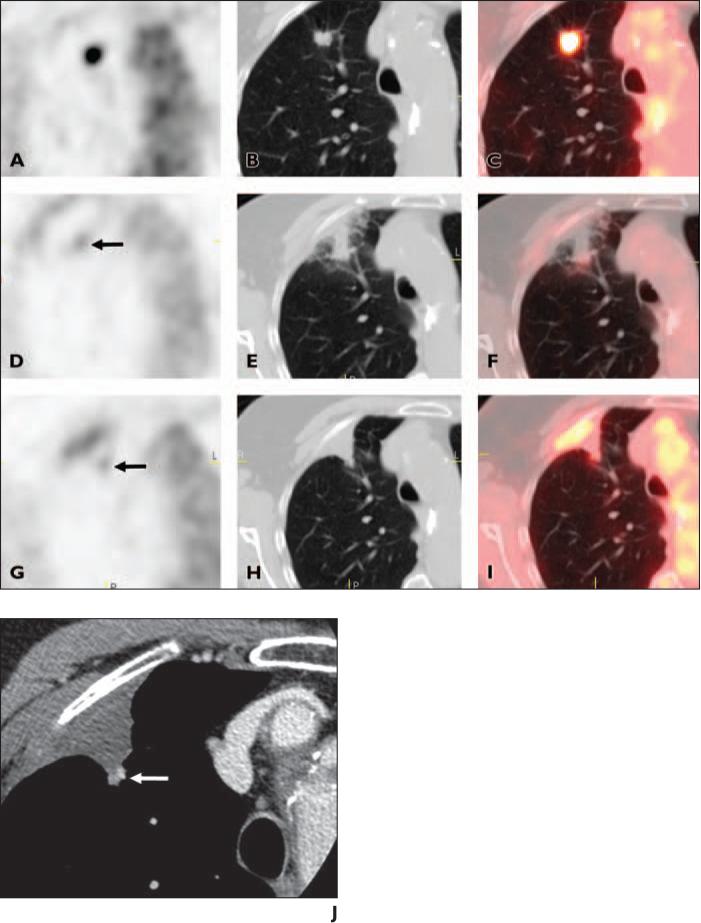
Fig. 4.
87-year-old woman with stage IA non–small cell lung cancer.
A–C, Axial PET (A), CT (B), and fused PET/CT (C) images show area of intense FDG uptake corresponding to tumor in right upper lobe.
D–F, Axial PET (D), CT (E), and fused PET/CT (F) images obtained 3 days after radiofrequency ablation (RFA) show more discrete focus of increased activity (arrow, D) at medial aspect of ablation site (partial response). Ablation site otherwise exhibits mild peripheral uptake and central photopenia.
G–I, Axial PET (G), CT (H), and fused PET/CT (I) images obtained 6 month after RFA show persistent and slightly more intense discrete focus of increased activity (arrow, G) at medial portion of ablation site corresponding to small nodular opacity at posterome-dial aspect of triangular ablation cavity (progressive disease).
J, Axial CT densitometry image obtained 6 months after RFA shows enhancement of nodular focus at medial aspect of ablation cavity (arrow) that corresponds to location of discrete focal activity in D–I.
One weakness of this study was that 4 of 30 patients did not undergo early PET/CT and 7 of 30 patients did not undergo 6-month PET/CT. This dropout rate is to be expected in a study of this kind because of the frailty of the population being studied.
Conclusion
Contrary to our expectations, the findings at early post-RFA PET/CT were confounded by posttreatment inflammatory changes in many patients and were not predictive of clinical outcome at 1 year. Therefore, early PET/CT does not appear to be useful in the care of patients who have undergone RFA. This knowledge helps in the appropriate use of PET/CT after RFA and results in economic savings because it obviates early postablation PET/CT. Although the optimal time for the initial follow-up PET/CT examination after ablation has yet to be established, the findings at 6-month post-RFA PET/CT did correlate with clinical outcome and thus may provide valuable information for patient care.
Acknowledgments
We thank the American College of Surgeons Oncology Group staff, in particular Heidi Nelson and David Ota, for their assistance in the development of this manuscript; the investigators and their site research teams; and the patients and their caregivers who participated in this study.
Supported by grant U10 CA 76001 from the U.S. National Cancer Institute to the American College of Surgeons Oncology Group.
Footnotes
Presented at the 2009 annual meeting of the Radiological Society of North American (ID 8011786).
References
- 1.Dupuy DE, Zagoria RJ, Akerley W, et al. Percutaneous radiofrequency ablation of malignancies in the lung. AJR. 2000;174:57–59. doi: 10.2214/ajr.174.1.1740057. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg SN, Dupuy DE. Image-guided radiofrequency tumor ablation: challenges and opportunities. Part 1. J Vasc Interv Radiol. 2001;12:1021–1032. doi: 10.1016/s1051-0443(07)61587-5. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi NS, Dupuy DE. Image-guided radiofrequency ablation as a new treatment option for patients with lung cancer. Semin Roentgenol. 2005;40:171–181. doi: 10.1053/j.ro.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Fernando HC, De Hoyos A, Landreneau RJ, et al. Radiofrequency ablation for the treatment of non-small cell lung cancer in marginal surgical candidates. J Thorac Cardiovasc Surg. 2005;129:639–644. doi: 10.1016/j.jtcvs.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Steinke K, Glenn D, King J, et al. Percutaneous imaging-guided radiofrequency ablation in patients with colorectal pulmonary metastases: 1-year follow-up. Ann Surg Oncol. 2004;11:207–212. doi: 10.1245/aso.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Suh R, Reckamp K, Zeidler M, Cameron R. Radiofrequency ablation in lung cancer: promising results in safety and efficacy. Oncology. 2005;19(suppl 4):12–21. [PubMed] [Google Scholar]
- 7.Suh RD, Wallace AB, Sheehan RE, Heinze SB, Goldin JG. Unresectable pulmonary malignancies: CT-guided percutaneous radiofrequency ablation—preliminary results. Radiology. 2003;229:821–829. doi: 10.1148/radiol.2293021756. [DOI] [PubMed] [Google Scholar]
- 8.Akeboshi M, Yamakado K, Nakatsuka A, et al. Percutaneous radiofrequency ablation of lung neoplasms: initial therapeutic response. J Vasc Interv Radiol. 2004;15:463–470. doi: 10.1097/01.rvi.0000126812.12853.77. [DOI] [PubMed] [Google Scholar]
- 9.Okuma T, Okamura T, Matsuoka T, et al. Fluorine-18-fluorodeoxyglucose positron emission tomography for assessment of patients with unresectable recurrent or metastatic lung cancers after CT-guided radiofrequency ablation: preliminary results. Ann Nucl Med. 2006;20:115–121. doi: 10.1007/BF02985623. [DOI] [PubMed] [Google Scholar]
- 10.Simon CJ, Dupuy DE, DiPetrillo TA, et al. Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology. 2007;243:268–275. doi: 10.1148/radiol.2431060088. [DOI] [PubMed] [Google Scholar]
- 11.Dupuy DE, Goldberg SN. Image-guided radiofrequency tumor ablation: challenges and opportunities. Part 2. J Vasc Interv Radiol. 2001;12:1135–1148. doi: 10.1016/s1051-0443(07)61670-4. [DOI] [PubMed] [Google Scholar]
- 12.Okuma T, Matsuoka T, Okamura T, et al. 18F-FDG small-animal PET for monitoring the therapeutic effect of CT-guided radiofrequency ablation on implanted VX2 lung tumors in rabbits. J Nucl Med. 2006;47:1351–1358. [PubMed] [Google Scholar]
- 13.Liu ZY, Chang ZH, Lu ZM, et al. Early PET/CT after radiofrequency ablation in colorectal cancer liver metastases: is it useful? Chin Med J (Engl) 2010;123:1690–1694. [PubMed] [Google Scholar]
- 14.Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50(suppl 1):11S–20S. doi: 10.2967/jnumed.108.057182. [DOI] [PubMed] [Google Scholar]
- 15.Patz EF, Jr, Connolly J, Herndon J. Prognostic value of thoracic FDG PET imaging after treatment for non-small cell lung cancer. AJR. 2000;174:769–774. doi: 10.2214/ajr.174.3.1740769. [DOI] [PubMed] [Google Scholar]
- 16.Akhurst T, Downey RJ, Ginsberg MS, et al. An initial experience with FDG-PET in the imaging of residual disease after induction chemotherapy for lung cancer. Ann Thorac Surg. 2002;73:259–264. doi: 10.1016/s0003-4975(01)03257-x. [DOI] [PubMed] [Google Scholar]
- 17.Kang S, Luo R, Liao W, Wu H, Zhang X, Meng Y. Single group study to evaluate the feasibility and complications of radiofrequency ablation and usefulness of post treatment position emission tomography in lung tumours. World J Surg Oncol. 2004;2:30. doi: 10.1186/1477-7819-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singnurkar A, Solomon SB, Gönen M, et al. 18F-FDG PET/CT for the prediction and detection of local recurrence after radiofrequency ablation of malignant lung lesions. J Nucl Med. 2010;51:1833–1840. doi: 10.2967/jnumed.110.076778. [DOI] [PubMed] [Google Scholar]
- 19.Deandreis D, Leboulleux S, Dromain C, et al. Role of FDG PET/CT and chest CT in the follow-up of lung lesions treated with radiofrequency ablation. Radiology. 2011;258:270–276. doi: 10.1148/radiol.10092440. [DOI] [PubMed] [Google Scholar]