Abstract
The transport of germ cells across the seminiferous epithelium is composed of a series of cellular events during the epithelial cycle essential to the completion of spermatogenesis. Without the timely transport of spermatids during spermiogenesis, spermatozoa that are transformed from step 19 spermatids in the rat testis fail to reach the luminal edge of the apical compartment and enter the tubule lumen at spermiation, thereby entering the epididymis for further maturation. Step 19 spermatids and/or sperms that remain in the epithelium will be removed by the Sertoli cell via phagocytosis to form phagosomes and be degraded by lysosomes, leading to subfertility and/or infertility. However, the biology of spermatid transport, in particular the final events that lead to spermiation remain elusive. Based on recent data in the field, we critically evaluate the biology of spermiation herein by focusing on the actin binding proteins (ABPs) that regulate the organization of actin microfilaments at the Sertoli-spermatid interface, which is crucial for spermatid transport during this event. The hypothesis we put forth herein also highlights some specific areas of research that can be pursued by investigators in the years to come.
Keywords: Testis, spermatogenesis, ectoplasmic specialization, actin binding proteins, actin bundling proteins, branched actin polymerization inducing proteins, spermiogenesis, spermiation
2. Introduction
Spermatogenesis is a complex cellular process [1–4]. It is composed of several discrete cellular events that take place cyclically in the epithelium of the seminiferous tubule in mammalian testes, which include: (i) self-renewal of spermatogonia via mitosis, (ii) meiosis, (iii) spermiogenesis, and (iv) spermiation. In the seminiferous epithelium, spermatogonia that lie on the basement membrane in the basal compartment derived from spermatogonial stem cells located at the stem cell niche [5] undergo rapid expansion via mitosis after puberty, some of which differentiate into type A spermatogonia [6,7]. Some type A spermatogonia differentiate to type B, which are the germ cells that transform to preleptotene spermatocytes that are connected in “clones” by intercellular bridges (called known as tunneling nanotubes, TNT) to be transported across the blood-testis barrier (BTB) while differentiate into leptotene spermatocytes [8], so that spermatocytes undergo meiosis I and II to form haploid spermatids at the adluminal compartment behind the BTB (Figure 1). Spermatids (step 1) are then transformed into elongated spermatids (step 19) via spermiogenesis which are then lined up at the luminal edge of the tubule lumen. Thus, spermatozoa differentiated from step 19 spermatids are released into the tubular lumen at spermiation that takes place at late stage VIII of the epithelial cycle [9–13] (Figure 1).
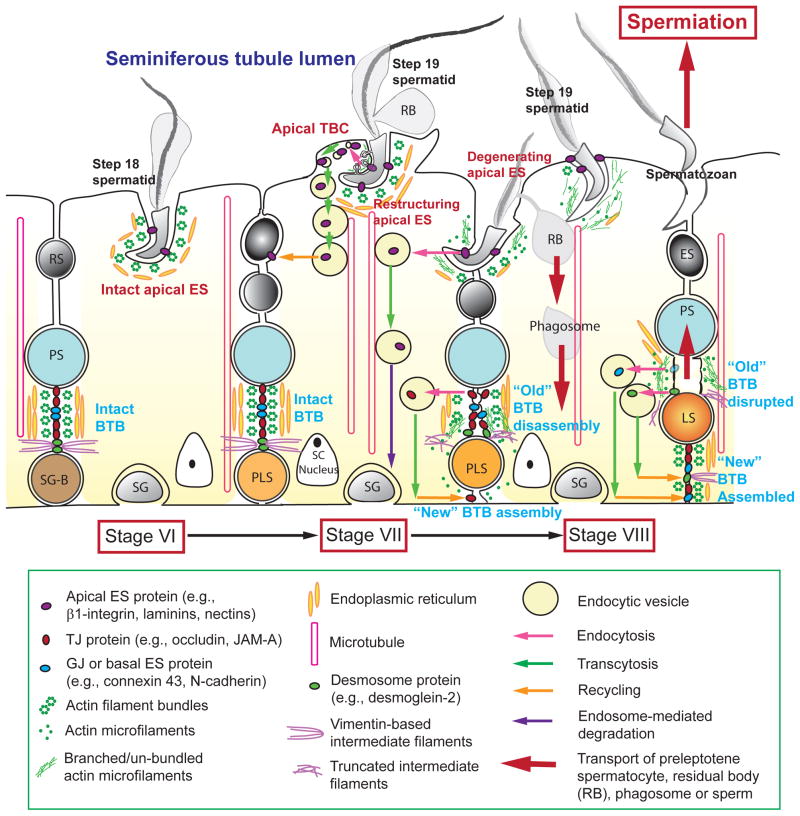
Figure 1. A schematic drawing that illustrates the cellular events of germ cell transport that occur in the seminiferous epithelium pertinent to the release of sperms at spermiation in the rat testis.
During the epithelial cycle, such as at stage VI, both the apical ES and the basal ES/BTB are intact in which actin microfilaments maintain the ES integrity via the formation of a bundled configuration. At stage VII, apical tubulobulbar complex (TBC) appears in the concave (ventral) side of the spermatid head, representing the site of apical ES that undergoes restructuring, in which endocytic vesicle-mediated endocytosis occurs, which is facilitated by the conversion of actin microfilaments from “bundled” to “un-bundled/branched” configuration. As such, “old” apical ES proteins, such as β1-integrin and nectin-2 or -3, can be transcytosed and recycled to assemble “new” apical ES. Similar events take place at the BTB in which “old” BTB above the preleptotene spermatocyte in transit at the BTB undergoes restructuring, and “old” BTB proteins, such as occludin and JAM-A, are transcytosed and recycled to assemble “new” BTB behind the preleptotene spermatocyte. Apical ES surrounding the head of step 19 spermatids continue to undergo degeneration until all the actin microfilaments are un-bundled and replaced with branched actin microfilaments and a mesh network of F-actin so that newly transformed spermatozoa are released into the tubule lumen at spermiation. Abbreviations used: SG-B, spermatogonium, type B; SG, spermatogonium; PS, pachytene spermatocyte; RS, round spermatid; SC, Sertoli cell; PLS, preleptotene spermatocyte; LS, leptotene spermatocyte; ES, elongating spermatid; RB, residual body.
As germ cells differentiate into more advanced stages during the epithelial cycle of spermatogenesis, they are also being transported by the Sertoli cell across the seminiferous epithelium from the basal to the adluminal (apical) compartment, so that spermatozoa transformed from step 19 spermatids at stage VIII of the epithelial cycle can be released to the tubule lumen at spermiation, entering the epididymis for further maturation (Figure 1). However, since germ cells per se, unlike fibroblasts, macrophages, neutrophils and other mammalian cells, are immotile cells, lacking the apparatus to elicit active locomotion, such as lamellipodia and filopodia found in other motile cells. Thus, germ cells that are analogous the “cargoes” must rely on actin microfilaments in the Sertoli cell that serve as the “vehicle” to be transported from the basal to the adluminal compartment along the microtubules that act as the “railroad track” [14–16] during the epithelial cycle of spermatogenesis. Studies have shown that the unique anchoring junction in the testis known as ectoplasmic specialization [ES, an actin microfilament-rich anchoring device using F-actin for attachment, also a testis-specific adherens junction (AJ)] serves as the “vehicle” and the microtubule acts as the “railroad track” to transport the cargo (germ cell) along the epithelium during the epithelial cycle [7,8,17–21]. ES is found at the Sertoli cell-cell interface and co-exists with actin-based tight junction (TJ) and gap junction (GJ) known as the basal ES. These junctions together with the intermediate filament-based desmosome constitute the blood-testis barrier (BTB) [7,22,23], one of the tightest blood-tissue barriers in the mammalian body [24–26], which also anatomically divides the seminiferous epithelium in the basal and the apical (adluminal) compartment. On the other hand, ES is also found in the apical compartment at the Sertoli-spermatid interface called the apical ES. Apical ES first appears in step 8 spermatids, however, once it assembles, unlike basal ES which coexists with TJ and GJ, it replaces both desmosome and GJ at the Sertoli-spermatid (step 1–7) interface [13,19,27], and it remains to be the only anchoring device until at late stage VIII when it undergoes complete degeneration to facilitate spermiation [17,21,28,29]. However, both apical and basal ES undergo restructuring at late stage VII to VIII so that proteins at the “old” ES can be endocytosed, transcytosed and recycled to assemble “new” apical and basal ES, in which giant endocytic vesicles are formed at both sites known as apical and basal tubulobulbar complex (TBC) [28,30–32] (Figure 1). In short, TBC is the ES that undergoes endocytic vesicle-mediated trafficking so that “old” ES proteins can be recycled for the assembly of “new” ES both in the basal (at the basal ES) and the apical (at the apical ES) compartment. Furthermore, TBC is also used to eliminate unwanted cytoplasmic debris in particular from the head region of late spermatids or Sertoli cells at the BTB [31,32]. In this context, it is of interest to note that many proteins at the desmosome and GJ that are found at the Sertoli-spermatid (step 1–7) interface (i.e., apical ES), and also many TJ- and GJ-proteins at the Sertoli cell-cell interface (i.e., basal ES), are being assimilated into the ES, even though ultrastructural features typical of TJ and GJ are not detected at the ES. Furthermore, proteins that are usually restricted to focal adhesion complex [FAC also known as focal contact, an actin-based anchoring junction at the cell-extracellular matrix (ECM) interface], such as focal adhesion kinase (FAK), c-Src (Rous sarcoma transforming virus or proto-oncogene tyrosine-protein kinase Src) and c-Yes (Yamaguchi sarcoma viral oncogene homolog1), are also found at the apical and/or basal ES, making the ES a hybrid anchoring junction, having the properties of AJ, GJ, TJ, desmosome and also FAC [33–35]. For instance, desmosome proteins desmoglein-2 and plakoglobin; GJ proteins connexin 43 and connexin 33; and TJ proteins JAM-C and CAR; are found at the apical and/or basal ES [36–39]
When examined by electron microscopy, ES is constituted by a tripartite ultrastructure composed of: (i) hexagonally arranged actin filament bundles that lie perpendicular to the Sertoli cell plasma membrane, which sandwiched in-between (ii) cisternae of endoplasmic reticulum and (iii) either the apposing Sertoli cell plasma membranes (basal ES) or the apposing Sertoli-spermatid plasma membranes (apical ES) [20,30] (Figure 1). Morphologically, apical and basal ES are indistinguishable even at the ultrastructural level under electron microscope except that the typical tripartite structure is found in both sides of adjacent Sertoli cells at the basal ES, but only in the Sertoli cell at the apical ES, not at the spermatid [20]. In fact, spermatids do not appear to contribute to the apical ES ultrastructurally even though they express many of the similar ES proteins found in Sertoli cells (e.g., nectin-2, afadin, N-cadherin, β-catenin) as well as unique proteins (e.g., nectin-3, laminin-α3, -β3, -γ3) not found in Sertoli cells [29,40]. Interestingly, constituent proteins at the apical vs. the basal ES are quite different [29,40]. Apical ES appears to mechanically grasp the head of spermatids which undergo rapid elongation and maturation via spermiogenesis, and to confer spermatid polarity so that the head of spermatids are pointing toward the basement membrane [20,41]. On the other hand, TBC consists of a cylindrical double-membrane core composed of the plasma membranes of two adjacent cells, cuffed by a network of actin microfilaments [8,30,42] (Figure 1). Basal TBC develops between adjacent Sertoli cells, which undergoes regressive changes during the epithelial cycle. Most membranous complexes arise during the early stages of the epithelial cycle at II–V and develop into large bulbous endings. At midcycle, namely stages VI–VII, most basal TBC display regressive changes and are eventually resorbed by Sertoli cell lysosomes. Basal TBC resorption is related to the impending breakdown of the “old” BTB above spermatocytes as these cells are being transported upward, crossing the BTB [30] (Figure 1). It is noted that basal TBC is not clustered at any specific and predictable location at the belt-like junctions, somewhat difficult to distinguish from elements of other junctional complexes [43].
Since both ES and TBC are actin-based ultrastructures, and the regulation of actin dynamics in unique testicular junctions are crucial to spermatogenesis, we focus on actin-binding and actin regulating proteins and their relationship with actin and other proteins at the ES in this review. We also focus our discussion herein on the spermatid transport across the seminiferous epithelium utilizing the apical ES since the biology of preleptotene spermatocyte transport at the basal ES/BTB has recently been reviewed [21,44,45].
3. Actin binding proteins (ABPs)
Actin is an essential component of the cytoskeleton, it is found in mammalian cells including Sertoli cells in the testis, existing in one of the two forms: globular, monomeric actin (G-actin), and filamentous polymeric actin (F-actin) [46–50]. G-actin is polymerized and assembled into double helices, forming F-actin. F-actin, also known as microfilament, has inherent polarity in which the rapidly growing end is the “barbed end” and the slow growing end the “pointed end” [51], which acts as the “vehicle” to transport the “cargo” which is the spermatid. Microfilaments can be cross-linked into higher order structures of meshes, bundles or composite bundled networks. These changes in the organization of the actin cytoskeleton thus confers plasticity to cells besides mechanical integrity, which also induces changes in the localization of the adhesion protein complexes at the TJ, such as occludin-ZO-1(zonula occludens-1) and claudin-ZO-1, apical ES such as integrin-laminin and nectin-afadin, which all use F-actin for their attachment [44]. Actin re-organization also enable cells to carry out various functions, including cell division, motility, contraction, phagocytosis and endocytic vesicle-mediated protein trafficing, besides conferring cell shape, polarity, adhesion and signal transduction [46,52]. Actin dynamics are tightly regulated by over 150 actin binding proteins (ABPs) that modulate localization, polymerization, cleavage, cross-linking, and organization of microfilaments, and they can be classified into two broad groups. One is proteins that regulate F-actin assembly and disassembly such as nucleation, barbed end capping, depolymerization, and monomer-binding. The other group is proteins that regulate higher-order F-actin structures, including F-actin bundling and F-actin cross-linking. Actin bundling proteins confer either parallel or antiparallel alignment of actin microfilaments and bundle F-actin into linear arrays. Some cross-linking proteins (e.g., filamin A) generate branched arrays of actin [46,51]. Since many proteins that are known to regulate cytoskeletal dynamics have not been studied in the testis, we limit our discussion herein on two functional groups of proteins that are intimately related to the organization of actin microfilaments at the ES pertinent to spermatogenesis. First, branched actin polymerization proteins that induce barbed end nucleation on existing microfilaments, effectively turning actin filaments from a “bundled” to “un-bundled/branched or mesh” configuration (Figures 2–4). Second, actin bundling proteins maintain ES integrity (Figures 2–4). Since an accompanying paper [53] focuses on the role of non-receptor protein tyrosine kinases on spermatid transport during the epithelial cycle, we limit our discussion herein on actin binding proteins (ABPs) and how the cross-talk of ABPs and protein tyrosine kinases, in particular FAK, are crucial to the biology of spermatid transport at spermiation.
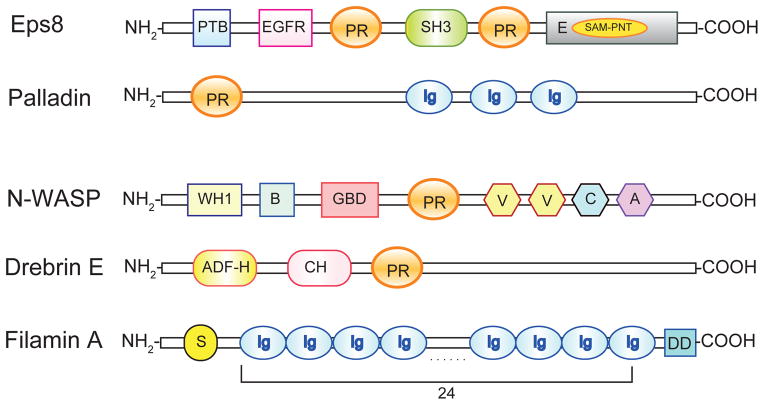
Figure 2. A schematic drawing that depicts the functional domains of the actin binding proteins (ABPs) that are known to regulate actin microfilaments at the ES in the mammalian testis.
The functional domains of actin bundling proteins Eps8 (also a barbed end capping protein) and palladin, as well as ABPs that are involved in creating branched microfilaments at the ES including N-WASP, drebrin E and filamin A. Abbreviations: PTB, phosphotyrosine binding domain; EGFR, epidermal growth factor receptor domain; PR, proline-rich region; SH3, Src homology 3 domain; E, effector region; SAM–PNT, Sterile α motif/Pointed domain; Ig, immunoglobulin-like domain; WH1, WASP homology domain; B, basic region; GBD, GTPase-binding domain; V, verprolin homology domain; C, cofilin homology domain; A, acidic region; ADF-H, actin depolymerizing factor homology domain; CH, charged/helical domain; S, spectrin-related domain; DD, dimerizing domain.
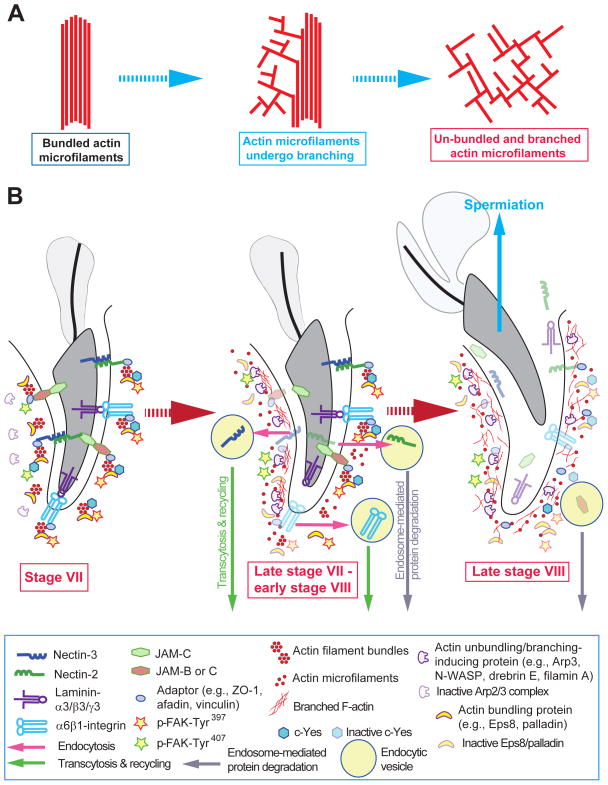
Figure 4. A hypothetical model depicting the likely events pertinent to the release of sperms at spermiation involving dynamic conversation of actin microfilaments from their bundled to un-bundled/branched configuration at the apical ES.
(A) As described in text, the final events that lead to the release of sperms at spermiation involve rapid conversion of actin microfilaments from their bundled to branched configuration, thereby unbundling the actin filaments at the apical ES, forming branched network. This, in turn, destabilizes the adhesion proteins, such as nectin-afadin, integrin-laminin, JAM-C-ZO-1, leading to the release of spermatozoa once they are transformed from step 19 spermatids in late stage VIII of the epithelial cycle (see Figure 1). (B) At early stage VII, step 19 spermatid remains anchored to the Sertoli cell in the seminiferous epithelium via cell adhesion proteins at the apical ES (see left panel), which is conferred by the action of c-Yes and p-FAK-Tyr397 and also p-FAK-Tyr407 to activate Eps8, palladin and/or α-actinin, so that these actin bundling proteins confer microfilaments the bundled configuration. However, in late stage VII (see mid panel), actin microfilaments at the concave (ventral) side of the spermatid head begin to undergo branched polymerization, induced by N-WASP/Arp2/3 complex facilitated by drebrin E, and possibly filamin A, due to a down-regulation of c-Yes and/or p-FAK-Tyr397 and –Tyr407. This site is also known as the apical tubulobulbar complex (TBC) so that “old” apical ES proteins can be recycled for the assembly of “new” apical ES for step 8 spermatids that appear in stage VIII of the cycle. While Eps8 and palladin are also expressed at the site, they are likely to be inactivated by p-FAK-Tyr407, losing their capability to induce bundling of actin microfilaments. Furthermore, these events of actin microfilament de-bundling rapidly spread over to the entire spermatid head at late stage VIII (see right panel), which in turn facilitates the release of sperms at spermiation.
4. Actin binding proteins (ABPs) that confer branched/meshed actin network at the ES in the mammalian testis
4.1. Arp2/3 (actin related protein 2/3) complex and N-WASP (neuronal Wiskott-Aldrich syndrome protein)
The Arp2/3 complex is a seven-subunit protein with of Arp2, Arp 3 and ARPC (Arp2/3 complex subunit) of 1 to 5. This 7-subunit Arp2/3 complex binds to the barbed end of an existing actin microfilament and induces branched actin nucleation at a 70° angle, thereby generating branched F-actin, effectively un-bundled an existing actin microfilament bundle [54–56]. This re-organization of F-actin thus leads to the formation of a dense meshwork of actin including lamellipodia leading edge in migrating cells [54,57]. However, the Arp2/3 complex is inactive until it is activated by cooperative interactions with ATP and nucleation-promoting factors [47,55,58]. Proteins from the WASP family, such as WASP, N-WASP, and the cortactin family are activators of Arp2/3 complex. Among these, N-WASP is considered to be a more potent activator of the Arp2/3 complex than cortactin [47,59,60]. All WASP family proteins share a common C-terminus responsible for nucleation promoting activity, which consists of the verprolin homology domain, cofilin homology domain, and acidic region (VCA domain) (Figure 2). The verprolin sequence binds actin monomers, whereas the acidic sequence binds to the Arp2/3 complex which induces changes in its conformation. N-WASP possesses two tandem verprolin motifs (Figure 2), leading to a more potent activity for Arp2/3 activation. WASPs also contain several other regulatory domains in the N-terminus including a WASP homology domain (WH1), a basic region, a GTPase-binding domain (GBD), and a proline-rich (PR) domain (Figure 2). These domains allow intrinsic regulation of its activity via auto-inhibition or mediated by external signals. N-WASP is auto-inhibited through interactions between the GBD and the VCA domain. This interaction prevents full length WASP from binding to and activating the Arp2/3 complex [60,61]. N-WASP, in turn, can be activated by Cdc42, and Src family kinases (e.g., c-Src), and also by WASP interacting SH3 protein (WISH) and the adaptor protein GRB2 via their SH3 (Src homology 3) domains [60]. N-WASP is also involved in clathrin-mediated endocytosis besides regulating endosome trafficking [60]. Arp3 is expressed in both Sertoli and germ cells in adult rat testes at a similar level; however, N-WASP is more abundant in germ cells, and cortactin is mostly found in Sertoli cells [62]. Spatiotemporal expression of Arp3 in the seminiferous epithelium is noted in the rat testis during the epithelial cycle: Arp3 is present at the BTB in various stages but barely detectable at stage VI–VII when the BTB remains “dormant” without restructuring, its expression is up-regulated considerably in stage VIII tubules when the BTB undergoes extensive restructuring [62]. Thus, the elevated expression of Arp3 at the BTB in stage VIII tubules facilitates the conversion of actin bundles to a mesh network to facilitate the transport of preleptotene spermatocytes at the site. Interestingly, the pattern of Arp3 expression at the apical ES at these stages is reciprocal of the BTB, being the highest at the concave (ventral) side of the head of elongating spermatids in stage VII tubules, and gradually diminishes to an undetected level at stage VIII prior to spermiation [62,63] (Figure 3). N-WASP is shown to co-localize with Arp3 at both the apical ES and BTB [62]. Cortactin and N-WASP are also detected at the concave side of elongated spermatid heads at stage VII during TBCs formation. Collectively, these findings suggest that activated Arp2/3 complex leads to actin nucleation and branching, assembling actin mesh network around protrusions of apical TBCs to facilitate protein endocytosis, transcytosis and recycling [62,64–66], with the likely involvement of N-WASP since it is known to participate in endocytic vesicle-mediated protein trafficking in other mammalian cells [60].
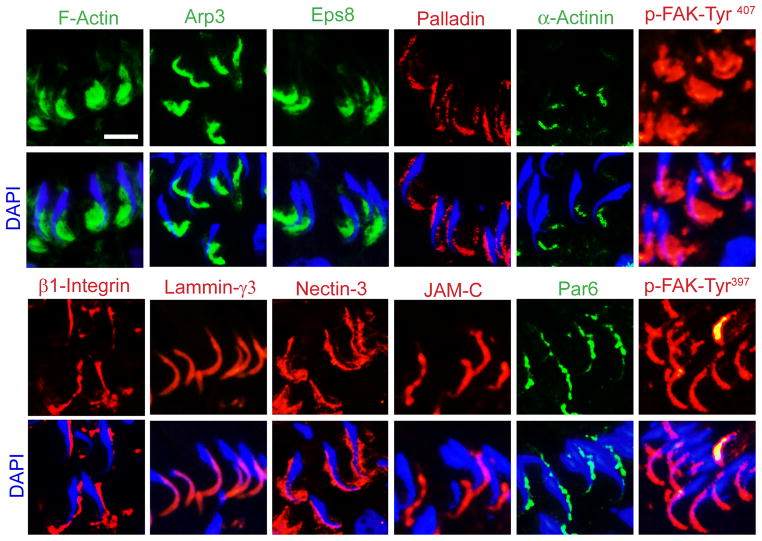
Figure 3. Spatiotemporal expression and localization of ABPs versus F-actin and selected apical ES proteins in the rat testis pertinent to the transport of spermatids for spermiation.
In this micrographs, the spatiotemporal localization of F-actin at the apical ES, surrounding the head of step 19 spermatids, in late stage VII tubules is compared to several ABPs, such as Arp3, Eps8, palladin, α-actinin, versus apical ES proteins, such as β1-integrin, laminin-γ3, nectin-3, JAM-C, and polarity protein Par6 as well as two ES regulatory proteins p-FAK-Tyr407 and p-FAY-Tyr397. These proteins are shown in either green or red fluorescence using corresponding specific antibodies as earlier reported [41,62,63,81,90,93,101]. See text for explanation. Scale bar, 10 μm, applies to all other micrographs in this panel.
4.2. Drebrin E (Developmentally regulated brain protein E)
Drebrin was first identified in avians as neuronal drebrin A (adult) [67]. Thus, drebrin A is the neuron-specific adult isoform, whereas drebrin E is expressed in the embryonic brain and in a wide range of non-neuronal cell types including the testis [51,68]. Drebrin is an actin-binding protein, involving in the formation of actin bundles, recruitment of proteins (e.g., chemokine receptor CXCR4) via changes in actin polymerization, building of dendritic spines and stabilization of gap junctions, actin remodeling via its interaction with Ras GTPases, and formation of lamellipodia and filopodia [67]. Drebrin is a member of the ADF-H (actin depolymerizing factor homology) domain family of actin-binding proteins. The ADF-H domain is a structurally conserved protein motif, with the ability to bind to both G- and F-actin (Figure 2) [51]. In addition to the N-terminal ADF-H domain, mammalian drebrin has second actin-binding domain, charged/helical (CH) actin binding motif (Figure 2). This motif is mainly responsible for F-actin interactions, because the ADF-H domain only binds F-actin weakly. The charged/helical domain seems to play a major role in determining the correct subcellular localization and activity of drebrin. Drebrin bind actin filaments in a 1:5 stoichiometry ratio, and the binding of drebrin to F-actin leads to structural and mechanical changes in the microfilament, such as increased stiffness and changes in the helical twist [51]. Drebrin stabilizes F-actin, modifies its structure, and modifies the interactions of other actin-binding proteins to microfilaments. Drebrin is found to regulate actin dynamics via the ability to compete with the binding of other actin regulatory proteins, such as α-actinin, fascin and tropomyosin to F-actin, to maintain the proper levels of other actin regulatory proteins in specific cellular domains in response to changes in environment, growth and development, pathological conditions and toxicants [51,67]. Drebrin also has PR domain near its C-terminus, serving as binding site for multiple proteins [51]. In the seminiferous epithelium of adult rat testes, drebrin E displays a restrictive stage-specific localization at the apical ES, as well as at the basal ES during the epithelial cycle [68]. Drebrin E is highly expressed at the basal ES at stage V–VI, but it diminishes considerably by stage VII–VIII and it remained almost non-detectable until stage IV [68]. At the apical ES, drebrin E is first detected at stage V, surrounding the entire head of elongating spermatids, but by stage VI its localization is “shifted” to localize most intensely and almost exclusively to the concave (ventral) side of the spermatid head [68]. Drebrin E structurally interacts with Arp3, and co-localized with Arp3 in stage VII tubules [68], these findings illustrate that drebrin E recruits the Arp2/3 complex to the apical ES. Drebrin E may serve as a platform to recruit necessary actin regulatory proteins to apical ES to affect F-actin filament bundles to confer apical ES dynamics to support spermatid transport during spermiogenesis.
4.3. Filamin A
Filamin A is a non-muscle ABP, an actin cross-linker and a scaffolding protein, which is known to maintain and regulate cytoskeleton structure and function in many epithelia including the seminiferous epithelium [69,70]. Filamin A is a dimeric protein composed of two identical polypeptide chains of 280 kDa each, the two chains self-associate non-covalently at the C-terminus via the dimerizing domain (DD) (Figure 2) to form a V-shaped functional protein. Because each monomer of filamin A contains a spectrin-related actin-binding domain at its N-terminus, each V-shaped dimeric filamin A binds to two F-actin, which favors perpendicular (i.e. at 90°) branching of F-actin [70,71]. Each N-terminal actin-binding domain followed by 24 Ig repeats, which contribute significantly to the actin binding strength of filamin A [72]. Similar Ig domain has been reported in a small number of vertebrate intracellular proteins, including myomesin, titin, and palladin [73]. Filamin A also anchors various transmembrane proteins to the actin cytoskeleton and provides a scaffold for a wide range of cytoplasmic and nuclear signaling proteins [69]. β1-Integrin and vimentin are known binding ligands of filamin A involved in cell adhesion [74,75]. Integrin tails connect to the actin cytoskeleton by binding directly to filamin A [76]. In the rat testis, filamin A is expressed exclusively by Sertoli cells cultured in vitro, distributed in cytoplasm in filamentous forms consistent with its function as an actin cross-linker [77]. In the testis, filamin A is predominantly (and also exclusively) expressed at the BTB near the basement membrane at the time the BTB begins to assemble at about 15–17 dpp (day postpartum), and its expression is considerably diminished during testicular maturation [77]. By 20 dpp, the expression of filamin A and its co-localization with F-actin in the epithelium begins to decline, and very weak staining of filamin A is detected in the testis by 90 dpp. Knockdown of filamin A in the testis by RNAi in vivo disrupts postnatal BTB assembly, which is mediated via a disorganization of the F-actin filament network and mis-localization of TJ and basal ES proteins at the BTB [77]. On 21 and 25 dpp after filamin A silencing, junctional adhesion molecules-A (JAM-A) and ZO-1 fail to be recruited to the BTB, localized diffusely at the BTB, thereby failing to recruit the BTB-associated proteins to the site to assemble a functional BTB. These findings thus illustrate that filamin A, besides serving as an organizer of actin-based cytoskeleton and cell structure, is involved in regulating cell adhesion function by recruiting adhesion protein complexes (e.g. JAM-A-ZO-1 and N-cadherin-β-catenin) to the BTB microenvironment for its assembly [77].
5. Actin binding proteins (ABPs) that confer actin filament bundles at the ES in the mammalian testis
5.1. Eps8 (epidermal growth factor receptor pathway substrate 8)
Eps8 was originally identified as a substrate of the epidermal growth factor receptor (EGFR), it belongs to a protein family that links growth factor stimulation to actin dynamics [78]. Eps8 is also a product of oncogene and an emerging target of anticancer therapy [79]. Eps8 is an important regulator of F-actin organization, which induces actin barbed end capping, actin bundling, and modulation of Rac GTPase-mediated actin remodeling [80,81]. Structurally, Eps8 contains several functional domains: a phosphotyrosine binding (PTB) domain, an EGFR binding region, two proline-rich (PR) sequences, a Src homology 3 (SH3) domain and a Sterile α motif/Pointed domain (SAM–PNT domain) overlapped by a C-terminal ‘effector’ region (Figure 2). PR sequences can interact with IRSp53 (insulin receptor tyrosine kinase substrate p53). The SH3 domain also interacts with Abi-1 (Abelson interacting protein-1, a scaffold protein), RN-tre and IRSp53. When Eps8 binds to Abi-1, accompanied by Sos-1 (son of sevenless 1, a Rac-specific guanine nucleotide-exchange factor) and PI3K (phosphatidylinositide 3-kinase), it activates Rac-mediated actin remodeling [82,83]. However, when Eps8 binds to RN-tre via the same SH3 domain, it inhibits Rab5-mediated receptor endocytosis. The core barbed end capping activity of Eps8 is encoded within the highly conserved C-terminal effecter region. The integrin cytoplasmic domain can also interact with Eps8 C-terminal effecter region, such as in colon cancer cells, it is likely that Eps8 plays a role in integrin signaling [81]. Eps8 is also associated with the late endosomal/lysosomal compartment. Eps8 also co-immunoprecipitates with Hsc70 and lysosomal associated membrane protein-2 (LAMP-2), which are key elements of the chaperone-mediated autophagy degradative pathway [80].
In adult rat testes, Eps8 is expressed in Sertoli and germ cells along with its functional partners, Abi-1, IRSp53, and Sos1, and is localized most prominently at the edges of spreading Sertoli cells cultured in vitro [81]. In the seminiferous epithelium, Eps8 is highly expressed at the F-actin-rich basal ES at the BTB and the apical ES at stage V–VI of the cycle. At stage VII, it shifted to the concave (ventral) side of elongated spermatids heads [81] (Figure 3). Eps8 is considerably diminished both at the basal ES and apical ES in stage VIII tubules, coinciding with BTB restructuring and apical ES disassembly at stage VIII [63,81]. This spatiotemporal expression of Eps8 in seminiferous epithelium during the epithelial cycle illustrates that Eps8, at stages V to early VII, may work as an actin barbed end capping protein and bundling protein, participate in the formation and maintenance of the characteristic actin filament bundles at the ES, which together with the Arp2/3 complex may facilitate the transport of spermatids across the epithelium. However, at late VII to VIII, it is involved in regulating endocytic vesicle-mediated protein trafficking via endocytosis and recycling of apical and basal ES in the seminiferous epithelium. This postulate is supported by findings via the knockdown of Eps8 by RNAi in Sertoli cells cultured in vitro with an established TJ-permeability barrier that mimics the BTB in vivo [81]. For instance, silencing of Eps8 leads to F-actin disorganization in Sertoli cells, such as stress fiber truncation, disorganization, and overgrowth [81], since actin microfilaments can no longer be capping and bundled, leading to barbed end nucleation possibly induced by the Arp2/3 complex. Eps8 knockdown also causes TJ protein mis-localization in which occludin and ZO-1 diffuse away from the cell-cell interface and internalized with occludin appears to form discrete cytoplasmic aggregates in the cell cytosol [81]. More importantly, Eps8 knockdown in vivo leads to germ cell loss from the epithelium and also BTB disruption [81], illustrating that Eps8 is a crucial regulator of actin dynamics by conferring germ cell adhesion and BTB integrity [81].
5.2. Palladin
Palladin is an actin binding protein closely co-localized with α-actinin in stress fibers and focal adhesion complex (FAC) in mammalian cells [84,85]. It is widely expressed in vertebrate cells, besides stress fibers and FAC, palladin is also found in cell junctions, podosomes, lamellipodia and other actin-based subcellular structures [84,86]. In rodents and humans, palladin is expressed as several isoforms of 200-, 140-, 90-kDa [86]. All isoforms of palladin contain at least one PR region near its N-terminus, and three immunoglobulin (Ig)-like domains near its C-terminus (Figure 2). PR region serves as the docking site for proteins containing the SH3 domain, such as Src and Eps8, or Ena-Vasp homology domain 1 (EVH1)-containing proteins, such as vasodilatator-stimulated phosphoprotein (VASP)/WASP protein family, this thus targets Ena/VASP/WASP proteins to actin microfilament to modify its organization. Palladin is physically associated with Src SH3 domain which in turn activates Src via tyrosine phosphorylation in vivo [87]. The Ig domains in palladin can bind to actin and some actin binding proteins. The Ig domain of palladin contains actin binding sites, illustrating palladin can associate with F-actin directly to induce bundling/cross-linking of actin filaments [73,88]. Palladin modulates the morphology and viscoelastic response of actin network in a concentration-dependent manner. Increasing palladin concentrations cause structural transitions in actin network from a weakly cross-linked phase to a strongly bundled phase in which branched bundles span the entire network [52]. The Ig domain also binds directly to other actin binding proteins, such as ezrin (component of the cortical actin cytoskeleton) and α-actinin [84–86,89]. α-Actinin is also an actin cross-linking protein, and also a scaffold that recruits other signaling molecules to the cytoskeleton. Binding of palladin to α-actinin may offer more actin binding sites in a smaller volume, enabling the formation of tighter actin bundles, and facilitating bundle interconnectivity [52]. The ability of palladin to connect other molecules suggests its role as a cytoskeletal scaffold that brings other proteins with different functional modalities into higher-order molecular complexes [87]. Palladin, a 90–95 kDa polypeptide, is expressed by Sertoli and germ cells in the rat testis, co-localizing with actin filaments with a typical fibrillar punctuate staining in Sertoli cells in vitro [90]. Palladin is mostly found at the apical and the basal ES/BTB in the seminiferous epithelium, displaying a restrictive stage specific pattern. In stage IV–VII tubules, palladin is expressed at the apical ES, covering both the convex and the concave side of the head of spermatids, co-localizing with F-actin (Figure 3); however, from stage VII to stage VIII, palladin expression shifted and concentrated to the tip of spermatid heads and it is considerably diminished to a level almost not detectable at late stage VIII [90]. These findings thus suggest that palladin is used to maintain the actin filament bundles at the apical ES via changes in their localization during the epithelial cycle. Palladin is also found to be highly expressed at the basal ES/BTB at stage I–V but diminished at stage VII–VIII when the BTB undergoes restructuring [63,90]. A knockdown of palladin in Sertoli cells in vitro by RNAi disrupts the TJ function, which is associated with a disorganization of actin filaments [90]. This, in turn, impedes protein distribution at the TJ [90]. Its knockdown in vivo also perturbs F-actin organization that lead to a loss of spermatid polarity and adhesion, causing defects in spermatid transport and spermiation [90]. Palladin structurally interacts with Eps8 and Arp3, and co-localized with these proteins at the basal ES and at the concave side of spermatid heads in stage VII tubules [90]. Interaction of palladin and Eps8 may be mediated via the PR domain in palladin and the SH3 domain of Eps8. It has been reported that full length Eps8 is auto-inhibited and does not cap barbed ends of microfilaments, while the binding of Abi-1 alters the conformation of Eps8 and releases its barbed-end actin capping activity. The binding of palladin to Eps8 may be involved in a similar mechanism that modulates Eps8 barbed-end capping activity [91]. Palladin may interact with Arp3 through its binding with N-WASP. Thus, the precise interactions between palladin, Arp3 and Eps8 can induce rapid remodeling of the network of actin filaments at the ES to facilitate transport of spermatids across the seminiferous epithelium and of preleptotene spermatocytes across the BTB.
6. ABPs and spermatid transport during spermatogenesis
6.1. Introduction
As discussed above and shown in Figure 2, these ABPs are either actin bundling (e.g., Eps8, palladin) proteins, branched actin polymerization (N-WASP, drebrin) or branched actin-inducing (e.g., filamin A) proteins which bind to F-actin via different actin binding domains. For instance, filamin A has a spectrin-related actin-binding domain [71], drebrin E uses its ADF-H domain and charged/helical motif for actin binding [51], whereas palladin binds F-actin using the Ig domain [73] (Figure 2). Beside actin-binding, these ABPs also possess domains that allow them to interact with other proteins by recruiting additional functional proteins to the site to elicit remodeling of the actin cytoskeleton during the epithelial cycle. For example, Eps8, N-WASP, palladin all contain PR regions, whereas Eps8 and some other ABPs (e.g. cortactin) have SH3 domain [46]. Src family kinases are possess SH3 domains, and it is known that interactions of PR regions and SH3 domains are essential for signal transductions, such as between palladin and non-receptor kinases that regulate cell proliferation, differentiation, migration and transformation [92]. There are studies in the literature reporting that Eps8, Arp3 and palladin can be regulated by kinases and phosphatases that modulate their phosphorylation status, which, in turn, affect their intrinsic activities [87,91,93]. For instance, Eps8 can be tyrosine phosphorylated by a variety of protein tyrosine kinases [91]. Eps8 overexpression promotes the activity of FAK and c-Src, whereas Src can enhance tyrosine phosphorylation and protein synthesis of Eps8 which, in turn, up-regulate the expression and activity of FAK [79]. FAK has been shown to influence Arp 2/3 complex-mediated actin nucleation, modulating actin cytoskeleton organization at the basal ES of the Sertoli cell BTB through two likely mechanisms [93]. These include blockade of N-WASP from entering the nucleus following FAK-mediated phosphorylation, as well as the direct interaction of FAK 4.1/ezrin/radixin/moesin domain with Arp3, which enhances Arp2/3 activity [93]. In short, there are functional cross-talks between actin bundling proteins (e.g., Eps8, palladin), actin barbed end nucleation proteins (e.g., the Arp2/3 complex) and branched actin-inducing proteins (e.g., filamin A), which are likely mediated by non-receptor protein kinases as summarized in Section 6.2.
6.2. A current concept on cross-talks between non-receptor protein kinases (e.g., FAK) and ABPs that regulate spermatid transport during the epithelial cycle of spermatogenesis
As noted in the sections above, actin filament bundles at the apical ES surrounding the head of spermatids during spermiogenesis confer both adhesion and polarity to developing spermatids. However, these actin filament bundles (Figure 1) must be re-organized by alterning their configuration from “bundled”, “branched” and “un-bundled” as illstrated in Figure 4A via spatiotemporal expression of bundling-inducing proteins (e.g., Eps8, palladin) and branched actin-inducing proteins (e.g., Arp3, drebrin E and filamin A), thereby unbundling actin microfilaments at the apical ES to facilitate the transport of spermatids across the seminiferous epithelium. Based on the available data thus far, it is likely that the two phosphorylated forms of FAK, p-FAK-Tyr407 and p-FAK-Tyr397, and Src family kinases in particular c-Yes and c-Src, are playing crucial role in these events. This postulate is supported by the following observations. First, p-FAK-Tyr397 [94,95] and c-Yes [96] are highly and restrictively expressed at the convex (dorsal) side of spermatid heads which persists from stage VII–VIII until the degeneration of apical ES at late stage VIII when spermation takes place (Figure 3). p-FAK-Tyr397 also structurally interacts with c-Src, β1-integrin and vinculin at the apical ES [94] whereas c-Yes with Eps8 but not Arp3 or drebrin E [97]. Second, p-FAK-Tyr407 is predominantly expressed at the concave (ventral) side, but some can be detected at the convex (dorsal) side, of spermatid heads at stage VII–VIII (Figure 3) [93], and p-FAK-Tyr407 structurally associated with N-WASP and Arp3 [93]. More important, it was shown that p-FAK-Tyr407 modulates actin polymerization rate via its action on the N-WASP/Arp2/3 complex since overexpression of the FAK Y407E phoshomimetic mutant facilitated the interaction between N-WASP and Arp3 and significantly induced actin polymerization rate at the ES [93]. Third, a knockdown of c-Yes also induces actin polymerization rate at the ES [97]. Collectively, these findings thus illustrate that Eps8, palladin and Arp3, which are highly expressed at the concave side of spermatid heads at stage VII (Figure 3), can be selectively turned “on-or-off” by p-FAK-Tyr407, depending on the need in the microenvironment to accommodate spermatid transport during the epithelial cycle. Thus, their coexistence at the same site, namely the concave side of spermatid heads, do not necessarily cause a conflict of differential activity to induce changes in the organization of actin microfilaments. Instead, this represents a simple but efficient system to induce re-organization of actin filament bundles in response to the need of spermatid transport during the epithelial cycle. For instance, these protein kinases can selectively activate (or inactivate) either Eps8, palladin, Arp3, filamin A or drebrin E, so that only actin bundling or branched actin polymerization activity can be induced at the site. This possibility is supported by reports that palladin is a phosphoprotein that can be activated via phosphorylation on its Ser/Thr and/or Tyr residues. When Akt1 (also known as PKB, and it is a component of the apical ES in the rat testis [98]) phosphorylates palladin at Ser507, this in turn induces palladin to exert its activity to confer cross-linking of actin microfilaments into bundles [99]. On the other hand, palladin is also involved in subcellular targeting of c-Src [87], which can affect endocytic vesicle-mediated protein trafficking [100], such as those that take place at the apical TBC in stage VII–VIII of the epithelial cycle [28]. While the precise role of p-FAK-Tyr407 or -Tyr397 on the activation of palladin remains to be explored, p-FAK-Tyr397 structurally interacts with c-Src, thus, c-Src can be recruited to the apical ES to activate palladin to confer actin bundling.
Based on the available data summarized herein, we now provide a model shown in Figure 4 to depict the likely events of spermatid transport pertinent to spermiation during spermatogenesis. As noted in Figure 4A, B, actin filament bundles at the apical ES is maintained by the concerted efforts of actin bundling proteins Eps8, palladin and others (e.g., α-actinin), in which p-FAK-Tyr397, p-FAK-Tyr407 and c-Yes maintain the proper phosphorylation status of these proteins so that these proteins are activated and they can exert their actin bundling (or barbed end capping) activity to confer actin microfilaments in a bundled configuration, such as at stage VII of the epithelial cycle (Figure 4A, B). These bundled actin microfilaments also support the proper localization of apical ES adhesion proteins, such as β1-integrin, laminin-γ3 chain, nectin-3, and JAM-C, at the apical ES since these proteins are using F-actin for their attachment to confer apical ES function (Figure 3). On this note, it is worthy to mention that at early to mid-stage VII, Eps8 is expressed also at the convex side, besides the concave side, of spermatid heads (see accompanied paper of Wan et al. in this issue); and by late stage VII, Eps8 and α-actinin that confer actin microfilament bundles are limited to the concave side of the spermatid head as shown in Figure 3. However, palladin remains abundantly expressed at the convex side of spermatid heads to confer actin microfilament bundles at late stage VII (Figure 3), illustrating the spatiotemporal expression of these ABPs that confer actin bundling across the spermatid head at the apical ES provides a unique mechanism to induce actin microfilament remodeling in the microenvironment. On the other hand, the Arp2/3 complex that is found at the concave side of spermatid heads remains inactivated, likely induced by p-FAK-Tyr407 and/or c-Yes which are also expressed at the same site (Figure 4B vs. Figure 3). At late stage VII of the cycle, when restructuring of the apical ES takes place at the concave side of the spermatid head that leads to the formation of the apical TBC, representing giant endocytic vesicles, is induced by the activation of the N-WASP/Arp2/3 complex via the action of p-FAK-Tyr407 and/or c-Yes (Figure 4B). Eps8, palladin, and α-actinin, however, are inactivated, even though they are expressed at the site, possibly via phosphorylation induced by these two p-FAK-Tyr407 and c-Yes (Figure 4A, B). The inactivation of Eps8 and pallaind (and also other actin bundling proteins, such as α-actinin) gradually spreads to the convex side of the spermatid head, which is likely induced by p-FAK-Tyr397 since it is found abundantly at this site (Figure 3), and bundles of actin microfilaments are replaced with the un-bundled and branched F-actin, destabilizing the adhesion protein complexes at the site, thereby facilitating the release of sperms at spermiation (Figure 4A, B). Similar events likely take place at the microenvironment at the Sertoli-spermatid interface for the transport of spermatid across the seminiferous epithelium. This model also highlights some areas of research that deserve attention in future studies, such as the nature of protein phosphorylation that activate and/or inactivate these ABPs.
7. Conclusing remarks and future persepctives
Herein, we provide an update on the likely involvement of ABPs and nonreceptor protein kinases on the transport of spermatids across the seminiferous epithelium during the epithelial cycle of spermatogenesis, in particular during the final steps of spermiation. We also provide a hypothetical model based on findings in the field as depicted in Figure 4. We envision that the activity ABPs that confer actin microfilaments their bundled or un-bundled/branched configuration at the apical ES are regulated by p-FAK-Tyr407, p-FAK-Tyr397, c-Yes and/or c-Src. There are many open questions remain to be answered. For instance, what molecule(s) are responsible for the timely activation of these ABPs? Are these molecules released from spermatids and sent to the Sertoli cells via hemi-channels of gap junctions that induce phosphorylation of the specific class of ABPs to confer the configuration of actin microfilaments at the site? Besides, actin cytoskeleton, the involvement of tubulin-based cytoskeleton at the apical ES remains to be carefully evaluated.
Highlights.
Remodeling of actin microfilaments at the ectoplasmic specialization (ES) is crucial to confer spermatid transport
ES remodeling is regulated by the antagonistic effects of actin bundling and branched actin polymerization-inducing proteins
Spermiation is an F-actin remodeling cellular event
Footnotes
This work was supported in part by grants from the National Institutes of Health (NICHD, U54 HD029990 Project 5 to C.Y.C.; and R01 HD056034 to C.Y.C.)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Kretser DM, Kerr JB. The cytology of the testis. In: Knobil E, Neill JB, Ewing LL, Greenwald GS, Markert CL, et al., editors. The Physiology of Reproduction. Vol. 1. New York: Raven Press; 1988. pp. 837–932. [Google Scholar]
- 2.Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 1363–1434. [Google Scholar]
- 3.Schlatt S, Ehmcke J. Regulation of spermatogenesis: an evolutionary biologist’s perspective. Sem Cell Dev Biol. 2014 doi: 10.1016/j.semcdb.2014.03.007. (in press) [DOI] [PubMed] [Google Scholar]
- 4.Cheng CY, Mruk DD. The biology of spermatogenesis: the past, present and future. Philos Trans R Soc Lond B Biol Sci. 2010;365:1459–1463. doi: 10.1098/rstb.2010.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Rooij DG. The spermatogonial stem cell niche. Microsc Res Tech. 2009;72:580–585. doi: 10.1002/jemt.20699. [DOI] [PubMed] [Google Scholar]
- 6.Schlatt S, Ehmcke J. Regulation of spermatogenesis: An evolutionary biologist’s perspective. Sem Cell Dev Biol. 2014 doi: 10.1016/j.semcdb.2014.03.007. (in press) 101016/jsemcdb201403007. [DOI] [PubMed] [Google Scholar]
- 7.Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell LD. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. Am J Anat. 1977;148:313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- 9.Russell LD, Bartke A, Goh JC. Postnatal development of the Sertoli cell barrier, tubular lumen, and cytoskeleton of Sertoli and myoid cells in the rat, and their relationship to tubular fluid secretion and flow. Am J Anat. 1989;184:179–189. doi: 10.1002/aja.1001840302. [DOI] [PubMed] [Google Scholar]
- 10.Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- 11.Parvinen M. Regulation of the seminiferous epithelium. Endocr Rev. 1982;3:404–417. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- 12.Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: Role in contraceptive development. Pharmacol Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- 14.Tang EI, Mruk DD, Cheng CY. MAP/microtubule affinity-regulating kinases, microtubule dynamics, and spermatogenesis. J Endocrinol. 2013;217:R13–R23. doi: 10.1530/JOE-12-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee NPY, Cheng CY. Ectoplasmic specialization, a testis-specific cell-cell actin-based adherens junction type: is this a potential target for male contraceptive development. Human Reprod Update. 2004;10:349–369. doi: 10.1093/humupd/dmh026. [DOI] [PubMed] [Google Scholar]
- 16.Guttman JA, Kimel GH, Vogl AW. Dynein and plus-end microtubule-dependent motors are associated with specialized Sertoli cell junction plaques (ectoplasmic specializations) J Cell Sci. 2000;113:2167–2176. doi: 10.1242/jcs.113.12.2167. [DOI] [PubMed] [Google Scholar]
- 17.Vogl AW, Vaid KS, Guttman JA. The Sertoli cell cytoskeleton. Adv Exp Med Biol. 2008;636:186–211. doi: 10.1007/978-0-387-09597-4_11. [DOI] [PubMed] [Google Scholar]
- 18.Vogl AW. Distribution and function of organized concentrations of actin filaments in mammalian spermatogenic cells and Sertoli cells. Int Rev Cytol. 1989;119:1–56. doi: 10.1016/s0074-7696(08)60648-8. [DOI] [PubMed] [Google Scholar]
- 19.Russell LD, Peterson RN. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol. 1985;94:177–211. doi: 10.1016/s0074-7696(08)60397-6. [DOI] [PubMed] [Google Scholar]
- 20.Russell LD. Observations on rat Sertoli ectoplasmic (‘junctional’) specializations in their association with germ cells of the rat testis. Tissue Cell. 1977;9:475–498. doi: 10.1016/0040-8166(77)90007-6. [DOI] [PubMed] [Google Scholar]
- 21.Cheng CY, Mruk DD. An intracellular trafficking pathway in the seminiferous epithelium regulating spermatogenesis: a biochemical and molecular perspective. Crit Rev Biochem Mol Biol. 2009;44:245–263. doi: 10.1080/10409230903061207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelletier RM. The blood-testis barrier: the junctional permeability, the proteins and the lipids. Prog Histochem Cytochem. 2011;46:49–127. doi: 10.1016/j.proghi.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Franca LR, Auharek SA, Hess RA, Dufour JM, Hinton BT. Blood-tissue barriers: Morphofunctional and immunological aspects of the blood-testis and blood-epididymal barriers. Adv Exp Med Biol. 2012;763:237–259. [PubMed] [Google Scholar]
- 24.Cheng CYE. Biology and Regulation of Blood-Tissue Barriers. Austin, TX: Landes Bioscience/Springer Science+Business Media, LLC; 2012. pp. 1–361. [Google Scholar]
- 25.Setchell BP. Blood-testis barrier, functional and transport proteins and spermatogenesis. Adv Exp Med Biol. 2008;636:212–233. doi: 10.1007/978-0-387-09597-4_12. [DOI] [PubMed] [Google Scholar]
- 26.Dym M, Fawcett DW. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970;3:308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- 27.Mruk DD, Cheng CY. Cell-cell interactions at the ectoplasmic specialization in the testis. Trends Endocrinol Metab. 2004;15:439–447. doi: 10.1016/j.tem.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Vogl AW, Young JS, Du M. New insights into roles of tubulobulbar complexes in sperm release and turnover of blood-testis barrier. Int Rev Cell Mol Biol. 2013;303:319–355. doi: 10.1016/B978-0-12-407697-6.00008-8. [DOI] [PubMed] [Google Scholar]
- 29.Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nature Rev Endocrinol. 2010;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Russell LD. Observations on the inter-relationships of Sertoli cells at the level of the blood-testis barrier: evidence for formation and resorption of Sertoli-Sertoli tubulobulbar complexes during the spermatogenic cycle of the rat. Am J Anat. 1979;155:259–279. doi: 10.1002/aja.1001550208. [DOI] [PubMed] [Google Scholar]
- 31.Russell LD. Spermatid-Sertoli tubulobulbar complexes as devices for elimination of cytoplasm from the head region in late spermatids of the rat. Anat Rec. 1979;194:233–246. doi: 10.1002/ar.1091940205. [DOI] [PubMed] [Google Scholar]
- 32.Russell LD. Further observations on tubulobulbar complexes formed by late spermatids and Sertoli cells in the rat testis. Anat Rec. 1979;194:213–232. doi: 10.1002/ar.1091940204. [DOI] [PubMed] [Google Scholar]
- 33.Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem Biophys Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vogl A, Pfeiffer D, Mulholland D, Kimel G, Guttman J. Unique and multifunctional adhesion junctions in the testis: ectoplasmic specializations. Arch Histol Cytol. 2000;63:1–15. doi: 10.1679/aohc.63.1. [DOI] [PubMed] [Google Scholar]
- 35.Yan HHN, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: a friend or a foe of spermatogenesis? BioEssays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mruk DD, Cheng CY. Desmosomes in the testis. Moving into an unchartered territory. Spermatogenesis. 2011;1:47–51. doi: 10.4161/spmg.1.1.15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc Natl Acad Sci USA. 2009;106:10213–10218. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang CQF, Mruk DD, Lee WM, Cheng CY. Coxsackie and adenovirus receptor (CAR) is a product of Sertoli and germ cells in rat testes which is localized at the Sertoli-Sertoli and Sertoli-germ cell interface. Exp Cell Res. 2007;313:1373–1392. doi: 10.1016/j.yexcr.2007.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gliki G, Ebnet K, Aurrand-Lions M, Imhof BA, Adams RH. Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature. 2004;431:320–324. doi: 10.1038/nature02877. [DOI] [PubMed] [Google Scholar]
- 40.Cheng CY, Mruk DD. Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol Rev. 2002;82:825–874. doi: 10.1152/physrev.00009.2002. [DOI] [PubMed] [Google Scholar]
- 41.Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proc Natl Acad Sci USA. 2008;105:9657–9662. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russell LD, Clermont Y. Anchoring device between Sertoli cells and late spermatids in rat seminiferous tubules. Anat Rec. 1976;185:259–278. doi: 10.1002/ar.1091850302. [DOI] [PubMed] [Google Scholar]
- 43.Du M, Young J, De Asis M, Cipollone J, Roskelley C, et al. A novel subcellular machine contributes to basal junction remodeling in the seminiferous epithelium. Biol Reprod. 2013;88:60. doi: 10.1095/biolreprod.112.104851. [DOI] [PubMed] [Google Scholar]
- 44.Su WH, Mruk DD, Cheng CY. Regulation of actin dynamics and protein trafficking during spermatogenesis - insights into a complex process. Crit Rev Biochem Mol Biol. 2013;48:153–172. doi: 10.3109/10409238.2012.758084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lie PPY, Cheng CY, Mruk DD. Signalling pathways regulating the blood-testis barrier. Int J Biochem Cell Biol. 2013;45:621–625. doi: 10.1016/j.biocel.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winder SJ, Ayscough KR. Actin-binding proteins. J Cell Sci. 2005;118:651–654. doi: 10.1242/jcs.01670. [DOI] [PubMed] [Google Scholar]
- 47.Lie PPY, Mruk DD, Lee WM, Cheng CY. Cytoskeletal dynamics and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1581–1592. doi: 10.1098/rstb.2009.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romet-Lemonne G, Jegou A. Mechanotransduction down to individual actin filaments. Eur J Cell Biol. 2013;92:333–338. doi: 10.1016/j.ejcb.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 49.Ip CKM, Wong AST. p70 S6 kinase and actin dynamics - a perspective. Spermatogenesis. 2012;2:44–52. doi: 10.4161/spmg.19413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kierszenbaum AL, Rivkin E, Tres LL. Molecular biology of sperm head shaping. Soc Reprod Fertil Suppl. 2007;65:33–43. [PubMed] [Google Scholar]
- 51.Poukkula M, Kremneva E, Seerlachius M, Lappalainen P. Actin-depolymerizating factor homology domain: a conserved fold performing diverse roles in cytoskeletal dynamics. Cytoskeleton. 2011;68:471–490. doi: 10.1002/cm.20530. [DOI] [PubMed] [Google Scholar]
- 52.Grooman B, Fujiwara I, Otey C, Upadhyaya A. Morphology and viscoelasticity of actin networks formed with the mutually interacting crosslinkers: palladin and alpha-actinin. PLoS One. 2012;7:e42773. doi: 10.1371/journal.pone.0042773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wan HT, Mruk DD, Tang EI, Xiao X, Cheng YH, et al. Role of non-receptor protein kinases in spermatid transport during spermatogenesis. Sem Cell Dev Biol. 2014 doi: 10.1016/j.semcdb.2014.04.013. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dos Remedios CG, Chhabra D, Kekic M, Dedova IV, Tsubakihara M, et al. Actin binding proteins: Regulation of cytoskeletal microfilaments. Physiol Rev. 2003;83:433–473. doi: 10.1152/physrev.00026.2002. [DOI] [PubMed] [Google Scholar]
- 55.Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem J. 2011;435:553–562. doi: 10.1042/BJ20102121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Suetsugu S. Activation of nucleation promoting factors for directional actin filament elongation: allosteric regulation and multimerization on the membrane. Semin Cell Dev Biol. 2013;24:267–271. doi: 10.1016/j.semcdb.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 57.Bisi S, Disanza A, Malinerno C, Frittoli E, Palamidessi A, et al. Membrane and actin dynamics interplay at lamellipodia leading edge. Curr Opin Cell Biol. 2013;25:565–573. doi: 10.1016/j.ceb.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Pfaendtner J, Volkmann N, Hanein D, Dalhaimer P, Pollard TD, et al. Key structural features of the actin filament Arp2/3 complex branch junction revealed by molecular simulation. J Mol Biol. 2012;416:148–161. doi: 10.1016/j.jmb.2011.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y, Aardema J, Corey SJ. Biochemical and functional significance of F-BAR domain proteins interaction with WASP/N-WASP. Sem Cell Dev Biol. 2013;24:280–286. doi: 10.1016/j.semcdb.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 60.Burianek LE, Soderling SH. Under lock and key: Spatiotemporal regulation of WASP family proteins coordinates separate dynamic cellular processes. Semin Cell Dev Biol. 2013;24:258–266. doi: 10.1016/j.semcdb.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamaguchi H, Miki H, suetsugu S, Ma L, Kirschner MW, et al. Two tandem verprolin homology domains are necessary for a strong activation of Arp2/3 complex-induced actin polymerization and induction of microspike formation by N-WASP. Proc Natl Acad Sci U S A. 2000;97:12631–12636. doi: 10.1073/pnas.190351397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc Natl Acad Sci USA. 2010;107:11411–11416. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wan HT, Mruk DD, Li SYT, Mok KW, Lee WM, et al. p-FAK-Tyr397 regulates spermatid adhesion in the rat testis via its effects on F-actin organization at the ectoplasmic specialization. Am J Physiol Endocrinol Metab. 2013;305:E687–E699. doi: 10.1152/ajpendo.00254.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Young JS, Guttman JA, Vaid KS, Vogl AW. Cortactin (CTTN), N-WASP (WASL), and clathrin (CLTC) are present at podosome-like tubulobulbar complexes in the rat testis. Biol Reprod. 2009;80:153–161. doi: 10.1095/biolreprod.108.070615. [DOI] [PubMed] [Google Scholar]
- 65.Young JS, Guttman JA, Vaid KS, Vogl AW. Tubulobulbar complexes are intercellular podosome-like structures that internalize intact intercellular junctions during epithelial remodeling events in the rat testis. Biol Reprod. 2009;80:162–174. doi: 10.1095/biolreprod.108.070623. [DOI] [PubMed] [Google Scholar]
- 66.Young JS, Takai YK, KL, Vogl AW. Internalization of adhesion junction proteins and their association with recycling endosome marker proteins in rat seminiferous epithelium. Reproduction. 2012;143:347–357. doi: 10.1530/REP-11-0317. [DOI] [PubMed] [Google Scholar]
- 67.Cheng CY, Mruk DD. Actin binding proteins and spermatogenesis. Some unexpected findings. Spermatogenesis. 2011;1:99–104. doi: 10.4161/spmg.1.2.16913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li MWM, Xiao X, Mruk DD, Lam YL, Lee WM, et al. Actin binding protein drebrin E is involved in junction dynamics during spermatogenesis. Spermatogenesis. 2011;1:123–136. doi: 10.4161/spmg.1.2.16393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yue J, Huhn S, Shen Z. Complex roles of filamin-A mediated cytoskeleton network in cancer progression. Cell Biosci. 2013;3:7. doi: 10.1186/2045-3701-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Su WH, Mruk DD, Cheng CY. Filamin A: a regulator of blood-testis barrier assembly during post-natal development. Spermatogenesis. 2012;2:73–78. doi: 10.4161/spmg.20223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen H, Zhu X, Cong P, Sheetz MP, MNakamura F, et al. Differential mechanical stability filamin A rod segments. Biophys J. 2011;101:1231–1237. doi: 10.1016/j.bpj.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakamura F, Osborn TM, Hartemink CA, Hartwig JH, Stossel TP. Structural basis of filamin A functions. J Cell Biol. 2007;179:1011–1025. doi: 10.1083/jcb.200707073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beck MR, Dixon RD, Goicoechea SM, Murphy GS, Brungardt JG, et al. Structure and function of palladin’s actin binding domain. J Mol Biol. 2013;425:3325–3337. doi: 10.1016/j.jmb.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim H, Nakamura F, Lee W, Hong C, Perez-Sala D, et al. Regulation of cell adhesion to collagen via β1 integrins is dependent on interactions of filamin A with vimentin and protein kinase c epsilon. Exp Cell Res. 2010;316:1829–1844. doi: 10.1016/j.yexcr.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 75.Kim H, Nakamura F, Lee W, Shifrin Y, Arora P, et al. Filamin A is required for vimentin-mediated cell adhesion and spreading. Am J Physiol Cell Physiol. 2010;298:C221–C236. doi: 10.1152/ajpcell.00323.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ehrlicher AJ, Nakamura F, Hartwig JH, Weitz DA, Stossel TP. Mechanical strain in actin networks regulates FilGAP and integrin binding to filamin A. Nature. 2011;478:260–263. doi: 10.1038/nature10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Su WH, Mruk DD, Lie PPY, Lui WY, Cheng CY. Filamin A is a regulator of blood-testis barrier assembly during postnatal development in the rat testis. Endocrinology. 2012;153:5023–5035. doi: 10.1210/en.2012-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Di Fiore PP, Scita G. Eps8 in the midst of GTPases. Int J Biochem Cell Biol. 2002;34:1178–1183. doi: 10.1016/s1357-2725(02)00064-x. [DOI] [PubMed] [Google Scholar]
- 79.Li YH, Xue tY, He YZ, Du JW. Novel oncoprotein EPS8: a new target for anticancer theraphy. Future Oncol. 2013;9:1587–1594. doi: 10.2217/fon.13.104. [DOI] [PubMed] [Google Scholar]
- 80.Welsch T, Younsi A, Disanza A, Rodriguez JA, Cuervo AM, et al. Eps8 is recruited to lysosomes and subjected to chaperone-mediated autophagy in cancer cells. Exp Cell Res. 2010;316:1914–1924. doi: 10.1016/j.yexcr.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–2567. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Scita G, Tenca P, Frittoli E, Tocchetti A, Innocenti M, et al. Signaling from Ras to Rac and beyond: not just a matter of GEFs. EMBO J. 2000;19:2393–2398. doi: 10.1093/emboj/19.11.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Innocenti M, Tenca P, Frittoli E, Faretta M, Tocchetii A, et al. Mechanisms through which Sos-1 coordinates the activation of Ras and Rac. J Cell Biol. 2002;156:125–136. doi: 10.1083/jcb.200108035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Parast MM, Otey CA. Characterization of palladin, a novel protein localized to stress fibers and cell adhesions. J Cell Biol. 2000;150:643–656. doi: 10.1083/jcb.150.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Otey CA, Rachlin A, Moza M, Arneman D, Carpen O. The palladin/myotilin/myopalladin family of actin-associated scaffolds. Int Rev Cytol. 2005;246:31–58. doi: 10.1016/S0074-7696(05)46002-7. [DOI] [PubMed] [Google Scholar]
- 86.Goicoechea SM, Arneman D, Otey CA. The role of palladin in actin organization and cell motility. Eur J Cell Biol. 2008;87:517–525. doi: 10.1016/j.ejcb.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ronty M, Taivainen A, Heiska L, Otey C, Ehler E, et al. Palladin interacts with SH3 domains of SPIN90 and Src and is required for Src-induced cytoskeletal remodeling. Exp Cell Res. 2007;313:2575–2585. doi: 10.1016/j.yexcr.2007.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dixon RD, Arneman DK, Rachlin AS, Sundaresan NR, Costello MJ, et al. Palladin is an actin cross-linking protein that uses immunoglobulin-like domains to bind filamentous actin. J Biol Chem. 2008;283:6222–6231. doi: 10.1074/jbc.M707694200. [DOI] [PubMed] [Google Scholar]
- 89.Mykkanen OM, Gronholm M, Ronty M, Lalowski M, Salmikangas P, et al. Characterization of human palladin, a microfilament-associated protein. Mol Biol Cell. 2001;12:3060–3073. doi: 10.1091/mbc.12.10.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Qian X, Mruk DD, Wong EWP, Lie PPY, Cheng CY. Palladin is a regulator of actin filament bundles at the ectoplasmic specialization in the rat testis. Endocrinology. 2013;154:1907–1920. doi: 10.1210/en.2012-2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goicoechea SM, Arneman D, Disanza A, Garcia-Mata R, Scita G, et al. Palladin binds to Eps8 and enhances the formation of dorsal ruffles and podosomes in vascular smooth muscle cells. J Cell Sci. 2006;119:3316–3324. doi: 10.1242/jcs.03076. [DOI] [PubMed] [Google Scholar]
- 92.Asano E, Maeda M, Hasegawa H, Ito S, Hyodo T, et al. Role of palladin phosphorylation by extracellular signal-regulated kinase in cell migration. PLoS One. 2011;6:e29338. doi: 10.1371/journal.pone.0029338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lie PPY, Mruk DD, Mok KW, Su L, Lee WM, et al. Focal adhesion kinase-Tyr407 and -Tyr397 exhibit antagonistic effects on blood-testis barrier dynamics in the rat. Proc Natl Acad Sci USA. 2012;109:12562–12567. doi: 10.1073/pnas.1202316109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Siu MKY, Mruk DD, Lee WM, Cheng CY. Adhering junction dynamics in the testis are regulated by an interplay of β1-integrin and focal adhesion complex (FAC)-associated proteins. Endocrinology. 2003;144:2141–2163. doi: 10.1210/en.2002-221035. [DOI] [PubMed] [Google Scholar]
- 95.Beardsley A, Robertson DM, O’Donnell L. A complex containing α6β1-integrin and phosphorylated focal adhesion kinase between Sertoli cells and elongated spermatids during spermatid release from the seminiferous epithelium. J Endocrinol. 2006;190:759–770. doi: 10.1677/joe.1.06867. [DOI] [PubMed] [Google Scholar]
- 96.Xiao X, Mruk DD, Lee WM, Cheng CY. c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. Int J Biochem Cell Biol. 2011;43:651–665. doi: 10.1016/j.biocel.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xiao X, Mruk DD, Cheng CY. c-Yes regulates cell adhesion at the apical ectoplasmic specialization-blood-testis barrier axis via its effects on protein recruitment and distribution. Am J Physiol Endocrinol Metab. 2013;304:E145–E159. doi: 10.1152/ajpendo.00422.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J Biol Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- 99.Chin YR, Toker A. The actin-bundling protein palladin is an Akt1-specific substrate that regulates breast cancer cell migration. Mol Cell. 2010;38:333–344. doi: 10.1016/j.molcel.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xiao X, Mruk DD, Cheng FL, Cheng CY. c-Src and c-Yes are two unlikely partners of spermatogenesis and their roles in blood-testis barrier dynamics. Adv Exp Med Biol. 2012;763:295–317. doi: 10.1007/978-1-4614-4711-5_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yan HHN, Cheng CY. Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J Biol Chem. 2006;281:17286–17303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]