Abstract
In the present study we examined perceptual sensitivity to facial expressions of sadness among children at familial-risk for depression (N = 64) and low-risk peers (N = 40) between the ages 7 and 13(Mage = 9.51; SD = 2.27). Participants were presented with pictures of facial expressions that varied in emotional intensity from neutral to full-intensity sadness or anger (i.e., emotion recognition), or pictures of faces morphing from anger to sadness (emotion discrimination). After each picture was presented, children indicated whether the face showed a specific emotion (i.e., sadness, anger) or no emotion at all (neutral). In the emotion recognition task, boys (but not girls) at familial-risk for depression identified sadness at significantly lower levels of emotional intensity than did their low-risk peers. The high and low-risk groups did not differ with regard to identification of anger. In the emotion discrimination task, both groups displayed over-identification of sadness in ambiguous mixed faces but high-risk youth were less likely to show this labeling bias than their peers. Our findings are consistent with the hypothesis that enhanced perceptual sensitivity to subtle traces of sadness in facial expressions may be a potential mechanism of risk among boys at familial-risk for depression. This enhanced perceptual sensitivity does not appear to be due to biases in the labeling of ambiguous faces.
Offspring of depressed parents are at a greatly increased risk for mood disorders (for reviews, see Beardslee, Versage, & Giadstone, 1998; Goodman et al., 2011), with up to 50% of them developing major depression by the end of their adolescence (Weissman et al., 2005, 2006; Williamson, Birmaher, Axelson, Ryan, & Dahl, 2004). Consequently, efforts to identify potential mechanisms of risk have focused on examining how children at familial-risk for depression differ from their low-risk peers across multiple domains, such as genetics (e.g., Singh et al., 2011), parenting (e.g., Wilson & Durbin, 2010), and intra-individual factors (e.g., Silk, Shaw, Skuban, Oland, & Kovacs, 2006). Among these factors, the identification of atypical cognitive processes in high-risk children has received significant attention during the last decade. For example, studies have reported that high-risk children and adolescents are characterized by negatively biased attention to experimental stimuli (Joormann, Talbot, & Gotlib, 2007; Kujawa et al., 2011), memory recall (Mannie, Barnes, Bristow, Harmer, & Cowen, 2009), and attributions (Dearing & Gotlib, 2009). In the current study we examined another information processing domain, perceptual sensitivity to emotion cues in facial expressions, as a potential mechanism of risk that characterizes the offspring of depressed parents.
Research on information processing biases in psychopathology has often focused on examining attentional biases towards facial expressions of specific emotions, including biases in attention allocation/deployment, disengagement, and avoidance (see Cisler & Koster, 2010 for a recent review). One mechanism believed to contribute to some of these biases is enhanced feature detection capacity (Ohman, Flykt, & Esteves, 2001). That is, some attentional biases can occur, at least partially, as the result of an enhanced capacity to rapidly identify subtle emotional features at a perceptual, automatic and pre-awareness level (Öhman & Mineka, 2001), which in the case of facial expressions, depends upon the correct detection and decoding of facial features (Prkachin, 2003). In depression, such attentional biases appear to be specific to the identification of sad faces. For example, depressed adults display attentional biases toward dysphoric faces (Gotlib, Krasnoperova, Yue, & Joormann, 2004), and a tendency to label seemingly neutral expressions as sad (e.g., Gollan, Pane, McCloskey, & Coccaro, 2008; Gur et al., 1992; Surguladze et al., 2004). These biases are believed to maintain depressive symptoms by increasing depressed persons’ awareness of the negative affect of others (whether real or perceived), which then negatively impacts their interpersonal functioning (Gotlib et al., 2004). For example, higher perceived frequency of negative affect in others may help maintain the depressed person’s negative schemas about their social world. However, it is unclear whether these biases are due specifically to enhanced sensitivity to subtle traces of sadness because most previous adult studies have not examined perceptual sensitivity directly. Furthermore, it is also unknown whether such perceptual biases only maintain depression or are capable of inducing it. Thus, one important question is whether enhanced sensitivity to sadness is present in high-risk individuals before they become depressed.
There is some evidence that both at-risk and depressed youth display attentional bias toward sad faces (Hankin, Gibb, Abela, & Flory, 2010; Joormann et al., 2007; Kujawa et al., 2011). For example, Hankin et al. (2010) compared attentional biases to various facial expressions (e.g., sad, fearful) in depressed, anxious, comorbid and non-affected children using a modified version of the computer-based dot-probe paradigm (MacLeod, Mathews, & Tata, 1986). They found that depressed youth had difficulty disengaging from clearly identifiable sad faces while anxious youth had difficulty disengaging from fearful faces. However, evidence of perceptual sensitivity to emotion features in facial expressions is more scant. In fact, we are not aware of any study of perceptual sensitivity to facial expressions with depressed youth and the sole study of high-risk youth produced surprising results. Specifically, Joormann, Gilbert, and Gotlib (2010) examined perceptual sensitivity to sad faces among high-risk adolescent girls (mean age: 12.5 years; range: 9-14 years) who themselves had never been depressed but had mothers with multiple prior episodes of a depressive disorder. The authors found that high-risk girls displayed lower perceptual sensitivity to sad facial expressions, compared to their low-risk peers. That is, when viewing emotionally evocative facial expressions, high-risk girls needed more intense displays of sadness in order to correctly identify that affect.
Such under-sensitivity to sad cues among girls at high-risk for depression is contrary to expectations given the findings on attentional biases to facial expressions in similar pediatric populations (Hankin et al., 2010; Joormann et al., 2007; Kujawa et al., 2011) and facial identification biases in depressed adults (e.g., Gollan et al., 2008; Gur et al., 1992; Surguladze et al., 2004). Some methodological differences may account for their surprising finding. Joormann et al. (2010) utilized an innovative experimental protocol that involved watching video sequences of faces morphing from neutral to a target emotion. Participants were asked to respond as soon as they could identify the target emotion. Therefore perceptual sensitivity was assessed using a reaction time task, which raises the possibility that their findings may reflect group differences in reaction time to emotional stimuli instead of perceptual sensitivity, which has been observed in depressed adults (Gollan et al., 2008). In addition, the Joormann et al. (2010) sample included only females who were high-risk but had low levels of depression despite their relatively older age (Mage = 12.5). Although to our knowledge no study has examined sex differences in perceptual sensitivity in at risk youth, there is evidence of sex differences in factors that characterize youth at familial risk for depression (e.g., Silk et al., 2006). This raises the possibility that their findings were specific to older girls but not boys.
Therefore, in this study we compared youth (boys and girls aged 7 to 13) at high and low familial risk for depression in their ability to identify subtle features of facial expressions of sadness using a standard forced-choice feature detection paradigm (i.e., Pollak & Kistler, 2002) that more closely matches the procedures used with depressed adults (Gollan et al., 2008; Surguladze et al., 2004). We also examined perceptual sensitivity to anger as well as the ability to distinguish between anger and sadness in order to control for the specificity of the effect to sadness and the possibility of a sadness labeling bias, respectively. Based on findings with depressed adults, we hypothesized that our young, high-risk sample would show enhanced perceptual sensitivity to sad but not to angry cues in facial expressions. Specifically, we expected that high-risk children will identify sadness at lower levels of emotional intensity than low-risk peers (after controlling for the effects of age and depressive symptoms). Given the paucity of research examining sex differences in perceptual sensitivity to facial expressions in high-risk youth, our sex comparisons are exploratory.
Methods
Participants
This study included 104 youths between the ages of 7-13 (Mage = 9.51; SD = 2.27) who were at high or low-risk for depression and enrolled in a Program Project on risk factors for childhood-onset depression (COD). Of the children, 64 were at high-familial-risk for depression by virtue of having one biological parent with a documented history of childhood onset depression. The high-risk group included 45 families (17 families had more than one child in the study). The low-risk group included 36 families (3 families had more than one child in the study). The majority of both the proband and low-risk parents were mothers [n = 38 (84%) and n = 36 (100%), respectively]. The proband parents (Mage at time of current study = 30.80 years, age range: 23-37) were significantly younger at the time of this study than the low-risk parents (Mage = 34.16 years, age range: 26-44; t(72) = -3.07, p < .01). Proband parents were also less likely to be currently married than low-risk parents (33% vs. 57%, respectively; χ2 = 4.53, p = .03). Proband and low-risk parents did not significantly differ in educational history distribution with 84% of probands and 91% of low-risk parents having at least a high school diploma. College completion among both groups was relatively low, with only 3 (7%) of probands and 5 (16%) of low-risk parents having completed a 4-year degree (Fisher’s p = .28). The high and low-risk child samples did not differ significantly in sex (54% vs. 56% male), age (Mage = 9.52 vs. 9.39), and ethnic or racial backgrounds: the distributions were, respectively, 56% and 56% Caucasian, 16% and 33% African American, and 28% and 10% biracial or other.
All proband parents experienced the onset of their first episode of depression in childhood (CDEP). They were recruited by re-contacting individuals who had participated in past research studies as mood-disordered children, and through advertisements in the community, outpatient psychiatric clinics and related medical settings. The low-risk parents had no lifetime history of a major psychiatric disorder. Low-risk participants were recruited by re-contacting individuals who had participated in past research studies as psychologically-well children, using a geographically suitable Cole directory, and by advertising in a Women and Infants Center.
All parents were evaluated for this study via the Semi-Structured Clinical Interview for DSM-IV (SCID, First, Spitzer, Gibbon, & Williams, 1995). Specifically, the SCID was administered by trained professional clinicians to the parent and then separately to second informants (e.g., parent or partner) who provided information about the parent. Then pairs of psychiatrists independently reviewed these data, all accessible psychiatric and medical records, and provided a ‘best-estimate’ consensus diagnosis (Maziade et al., 1992) Most of the parent probands (>80%) had two or more episodes of depression since childhood. Informed consent or assent was obtained from all parents and children who participated in this study. All participants were compensated for participating. This study was approved by the Institutional Review Board of the University of Pittsburgh. For more details about the recruitment procedures, please see Forbes et al. (2006).
Assessment and Measures
Child symptoms and diagnostic status
Children’s depressive symptoms were quantified via the Children’s Depression Inventory (CDI; Kovacs & MHS Staff, 2003). The CDI, suitable for 7-17 year olds, contains 27 items, with a potential score range of 0 to 54, and has demonstrated adequate reliability and validity (coefficient alpha ranging from .71 to .89) (Kovacs & MHS Staff, 2003). In addition, all children were assessed for psychiatric disorders by trained masters-level clinicians using a semi-structured diagnostic interview; the Kiddie-Schedule for Affective Disorders and Schizophrenia – Present and Lifetime version (K-SADS-PL; Kaufman et al., 1997). Interviewers’ symptom ratings and diagnoses were then reviewed by two independent psychiatrists who provided final diagnoses using the ‘best-estimate’ consensus diagnosis (Maziade et al., 1992).
Facial expression identification
Children’s ability to recognize facial expressions of emotion was assessed via a morphed facial expressions recognition task developed by Pollak and Kistler (2002). Each child was tested in an experimental room by an experimenter while the parent was waiting in an adjacent room. The child was placed in a comfortable chair in front of a computer screen with designated computer keys for responses. The child was then presented with photographs of faces displaying different emotions (see below) and asked to indicate which emotion, if any, the face resembled most by choosing a left or right button on a response box; the buttons corresponded to words on the lower left or right corner of the picture (i.e., SAD vs. NOTHING., ANGER vs. NOTHING, or ANGER vs. SAD; see Figure 1).
Figure 1.

Selected Screen Shots of Trial Images for the sad identification condition. Faces of male actor and morphs between 10% and 90% of target emotion in each actor not shown. The target word was presented in equal frequency on the right or left of the face.
The stimuli of the original task (Pollak and Kistler 2002) consisted of morphed photographs of emotions that varied in emotional intensity on a continuum that started with one given emotion (e.g., sadness) which then segued into a neutral expression, and then changed into another emotion (e.g., anger). However, in order to more closely match the methods used in other studies of facial expression sensitivity in depressed adults (e.g., Surguladze et al., 2004), we used picture sequences that depicted neutral expressions morphing into sadness (i.e., Sad vs. Nothing) and neutral expressions morphing into anger (i.e., Anger vs. Nothing). This allowed us to examine perceptual sensitivity to ‘pure’ emotion features in the absence of other emotions. The Anger vs. Nothing condition was included to assess whether any observed enhanced perceptual sensitivity is specific to sadness or instead reflects enhanced sensitivity to all harsh emotions. In addition, we included a more standard condition in which facial expressions of anger morphed into sadness (i.e., Anger vs. Sadness), which allowed us to assess whether any observed group differences in perceptual sensitivity to sad cues are truly due to enhanced feature detection abilities as opposed to a tendency to mislabel ambiguous emotions as sadness.
Therefore, the final task included 3 conditions presented in counter-balanced order. In the first condition, Sadness Identification, children were presented with pictures of sad faces varying in emotion intensity (from neutral to 100% sadness) and asked whether the face resembled sadness or nothing. The second condition, Anger Identification, children were presented with pictures of angry faces varying in emotion intensity (from neutral to 100% anger) and asked whether the face resembled anger or nothing. Finally, in the Sad vs. Anger Discrimination condition, faces mixing angry and sad features (morphing from 100% anger to 100% sadness) were presented randomly and the children had to decide whether the face resembled sadness or anger. The location of the target emotion word varied within stimuli presentation so that the correct target choice was presented with equal frequency but randomly on the left or right of the picture. Pictures were presented in blocks by condition (e.g., all anger identification pictures presented in the same block) but randomized within condition in regards to emotion intensity and location of words. The final stimulus set for sadness and anger identification included 22 pictures for each emotion, including 11 faces from one adult male and 11 faces from one adult female that were presented at 10% increments of intensity of the target emotion (e.g., 0% sad, 10% sad, […], 100% sad). The stimulus set for the sadness vs. anger discrimination condition also consisted of 22 pictures for each emotion. These pictures were presented at 10% increments of overlapping intensity ranging from 100% anger to 100% sadness. That is, each card displayed a morphing ratio of anger to sadness that varied as follow, 100%:0%, 90%:10%, 80%:20%, […], 0%:100%. We expected more response variability when presented with ambiguous faces (i.e., 40-60% emotion intensity) compared to “pure” faces (i.e., 0-30% or 70-100% emotion intensity). Thus, consistent with the original experimental design (Pollak & Kistler 2002), the images with 40, 50, and 60% cross-emotion morphing were presented 4 times for each actor and images with 0, 10, 20, 30, 70, 80, 90,100% cross-emotion morphing were presented twice for each actor. This oversampling of ambiguous faces facilitates the fitting of a reliable curve in this, more ambiguous, range of the morphing continua (see data analysis section). In total, participants completed 56 trials per condition: (3 emotion levels × 4 trials each) + (8 emotion levels × 2 trials each) × (2 actors) = 56 trials.
Data Analysis
Facial expression recognition index
Since the facial recognition task we used involves a two-option forced choice (e.g., sad vs. neutral), we created a response probability index that identifies the intensity of emotion that results in chance responses (see Pollak & Kistler, 2002). We call this index SP50 to refer to the average signal (emotion intensity) where the probability of identifying the target emotion equals chance (50%). Specifically, the probability of identifying the target emotion at a given signal strength, x, was defined by a logistic equation
where a is the inflection point (i.e., function midpoint), b is the slope, and c and d are the minimum and maximum emotion identification probabilities (0 <= c < d <= 1) of the curve. For each subject, nonlinear estimation is used to fit the above logistic curve using the data from multiple trials over a range of x. Once parameters a, b, c and d are estimated, the subject’s category boundary, SP50, may be computed by:
This index ranges from 0 to 10 and defines the boundary in intensity of the target emotion (e.g., from 0% to 100% sad) where the responses to the forced-choice task equal chance (i.e., P = .50). In the emotion identification conditions (i.e., Sad vs. Nothing and Anger vs. Nothing), lower SP50 scores denote that chance responses occur at lower intensity of the target emotion (i.e., sadness or anger), thus indicating higher perceptual sensitivity to subtle traces of the target emotion. For example, a SP50 score of 3 indicates that the target emotion is identified at greater rate than chance until the face contains only 30% of the target emotion, when chance responses are noted. In the Sad vs. Anger discrimination condition, anger was coded as the target emotion so that increasing scores indicate chance performance at increasing ratio of anger to sadness. Therefore, SP50 scores in this condition are interpreted based on their deviation from the expected midpoint of 5, which reflects faces containing 50% anger and 50% sadness. Scores above 5 suggest over identification of sadness given that chance responses would be noted at levels including a lower ratio of anger to sadness. For example, the SP50 score of 6 indicates that chance responses are observed when faces contain 60% anger and 40% sadness. Therefore, a child with an SP50 score of 6 would identify faces containing 50% anger and 50% sadness as sad more often than chance.
Hypothesis Testing
To test our hypotheses we used full-factorial ANOVA with a mixed random effects model framework (SAS PROC MIXED with ML estimation). Random effect models were used to account for families who had more than one child in the study. Our independent variables were risk status, sex, age and depressive symptoms and the two-way interactions between sex and risk status and between sex and CDI. The dependent outcome variable was SP50. Separate models were conducted for the three conditions (i.e., sad identification, anger identification, and sad vs. anger discrimination). To examine interaction effects, we compared models with decreasing levels of parsimony using a hierarchical framework, namely, Model 1: main effects and Model 2: main effects and two-way interactions. We examined changes in model fit using the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC) after the addition of the two-way interactions. Any decrease in the AIC and BIC in nested models of increasing complexity (more parameters) indicates a significant improvement in model fit (Bozdogan, 1987; Pan, 2001). We examined the nature of the interactions only when a model showed improved fit, based on at least one of these fit indices. Following recommendations to reduce Type I error, we replicated any significant findings without any covariates and reported such findings as needed (Simmons, Nelson, & Simonsohn, 2011).
Results
Characteristics of the Samples
Psychiatric diagnostic assessment revealed that 3 children in the high-risk group had already developed a depressive disorder and these children were excluded from all future analyses. Table 1 presents the means and standard deviations of key variables for the high- and low-risk groups of child participants by sex. The two groups did not differ in sex distribution, χ2 = .03 p >.10, age, t(92) = -.92, p > .10, or depression symptoms (CDI), t(92) = 0.27, p > .10. Likewise, there were no basic group differences in facial expression recognition of sadness, t(77) = 0.27, p >.10, or anger, t(90) = -1.72, p > .10. However, there were significant group differences in facial expression discrimination between sadness and anger. Specifically, high-risk children displayed significantly lower SP50 (M = 5.75, SD = .75) than their low-risk peers (M = 6.08, SD = .60), t(78) = -2.75, p = .03. Both groups tended to over identify sadness, but low-risk participants did so at even higher ratios of anger-to-sadness morphing (i.e., more anger than sadness features) than did their high-risk peers.
Table 1.
Means and standard deviations of key variables for offspring at high- and low-risk for depression.
| High Risk
|
Low Risk
|
Effect Size (d)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Males (N=30) |
Females (N=25) |
Total (N=55) |
Males (N=22) |
Females (N=17) |
Total (N=39) |
High vs. Low |
HRM vs LRM |
HRF vs. LRF |
|
|
|
|
|
|
|
|||||
| Age | 9.73(2.44) | 9.28(2.25) | 9.52(2.35) | 9.58(2.19) | 9.15(2.22) | 9.39(2.19) | 0.06 | 0.06 | 0.06 |
| CDI | 2.33(2.56) | 2.92(3.16) | 2.60(2.83) | 2.18(3.08) | 1.17(2.59) | 1.97(2.85) | 0.22 | 0.05 | 0.42 |
| SP50 Sadness | 4.89(.82) | 5.53(1.01) | 5.19(.96) | 5.73(1.11) | 5.08(.99) | 5.47(1.09) | -0.27 | -0.87* | 0.45 |
| SP50 Anger | 5.43(.67) | 5.35(.71) | 5.42(.70) | 5.71(1.05) | 5.14(.67) | 5.48(.96) | 0.02 | -0.33 | 0.30 |
| SP50 SvsA | 5.89(.86) | 5.62(.62) | 5.75(.75) | 6.37(.54) | 5.71(.45) | 6.08(.60) | -.49* | -.69* | -.13 |
| Sex (% male) | - | - | 54 | - | - | 56 | - | - | - |
CDI= Child Depression Inventory; HRM = High risk males; LRM = Low risk males; HRF = High risk females; LRF = Low risk females. SP50 SvsA = Sadness vs. Anger discrimination.
Mean difference p < .05.
Recognition of Sad Expressions
Sensitivity to pure traces of sadness in facial expressions was examined via a full factorial analysis of variance using mixed effects models: SP50 for sadness was the dependent variable and risk status, sex, age, and depressive symptoms (CDI) were the independent variables. The main effects model indicated that risk status (F1,69 = 0.60, p >.10), sex (F1,12 = 1.84, p > .10), age (F1,12 = 0.54, p > .10) and depressive symptoms (F1,12 = 0.04, p > .10) were not significant predictors of SP50 for Sadness. A model with the sex-by-risk status and sex-by-CDI interaction showed a significant improvement in fit (main effects model AIC 258.8 vs. two-level interaction model AIC 248.0) and the interaction of risk status by sex was significant (F1,11 = 7.62, p = 0.02) but the sex-by-CDI interaction was not significant (F1,11 = 0.22, p > .10). Post-hoc examination of least squares means revealed no group differences in SP50 for girls (Mean ±SE: 5.53 ±1.01 and 5.08 ±0.99 for high- vs. low-risk, respectively; t = 1.26, p > 0.10). In contrast, high-risk boys had significantly lower Sad vs. Neutral SP50 than control boys did (Mean ±SE: 4.89 ±0.82 vs. 5.73 ±1.11; t = -2.71, p = 0.02), indicating that boys at familial risk for depression reliably identified sadness at lower levels of intensity compared to their low-risk peers (See Figure 2 and 3). No other significant group differences were found between and within sex and risk status.
Figure 2.
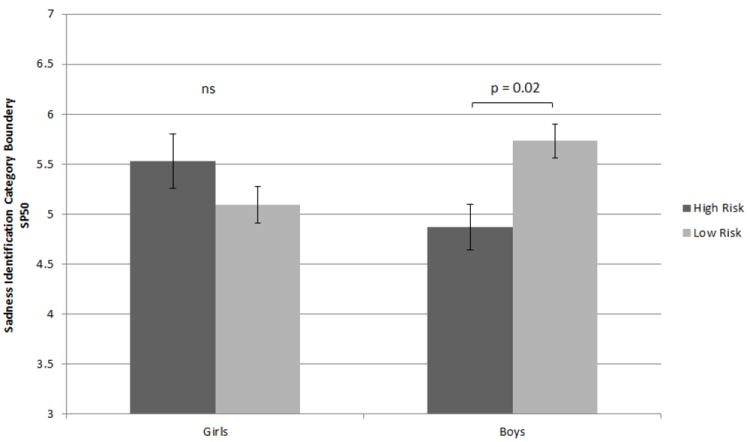
Mean difference in sadness identification category boundary among high- and low-risk boys and girls. Lower scores in SP50 represent higher perceptual sensitivity to subtle sad cues. Mean differences between high-risk boys and both low and high-risk girls were not significant. Error bars represent standard errors.
Figure 3.
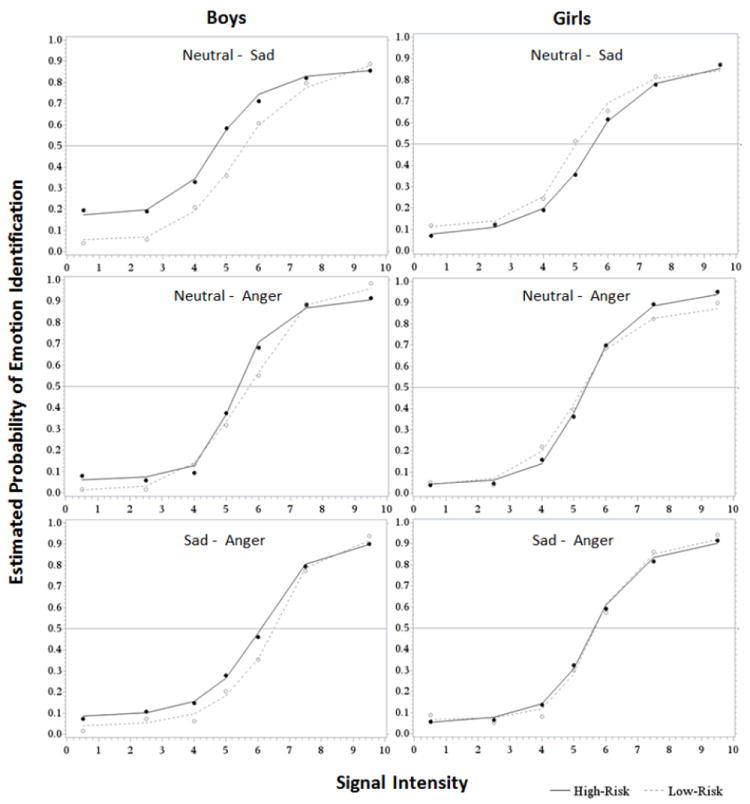
Results from emotion identification and discrimination task by sex and risk status. The Y axis refers to the probability that the target emotion would be identified relative the alternative choice. The target emotion is presented on the right for each choice pair. The X axis refers to the intensity of the target emotion relative to the alternative choice. For the emotion identification tasks (i.e., neutral vs. sad and neutral vs. anger) the X axis refers to the intensity of the motion. For the emotion discrimination task (i.e., sad vs. anger) the X axis refers to the intensity of anger relative to the intensity of sadness. For example, a signal strength of 9 = 90% anger and 10% sadness.
In order to minimize the possibility that one of the covariates was creating a suppression effect producing a Type I error, we replicated these models without including any covariates as recommended by Simmons et al., (2011). The model with the sex-by-risk status interaction showed improvement in fit over the main effects model (main effects model AIC 233.9 vs. two-level interaction model AIC 226.3) and the interaction of risk status-by-sex was still significant (F1,12 = 7.72, p = 0.01).
In addition, given that the group differences were observed only in boys, we examined whether the effects were due to higher levels of externalizing symptoms among the high-risk children as measured by the parent-reported Children Behavior Check List (CBCL; Achenbach & Rescorla, 2001). We first examined sex and group differences in externali zing symptoms. As expected, high-risk boys had significantly higher externalizing problems than control boys did (Mean ±SE: 19.28 ±1.90 vs. 7.75 ±1.86; t = -4.33, p < 0.01). In contrast, high- and low-risk girls differed only at trend level (Mean ±SE: 12.73 ±1.86 vs. 7.49 ±2.03; t = -1.88, p = 0.06). We then replicated the original models predicting SP50 for sadness using externalizing symptoms as an additional covariate. The model with the sex-by-risk status interaction showed improvement in fit over the main effects model (main effects model AIC 265.2 vs. two-level interaction model AIC 248.1) and the interaction of risk status by sex was still significant (F1,11 = 9.96, p < 0.01), suggesting that this effect was not due to sex differences in externalizing symptoms.
Recognition of Anger Expressions
Sensitivity to traces of anger in facial expression was also examined via a full factorial analysis is of variance using mixed effects: SP50 for anger was the dependent variable, and risk status, sex, age, and depressive symptoms (CDI) were the independent variables. The main effects model indicated that risk status (F1,77 = 0.4, p >.10), sex (F1,16 = 1.25, p > .10), age (F1,16 = 0.86, p > .10) and depressive symptoms (F1,16 = 0.92, p > .10) were not significant predictors of SP50 for Anger. A model with the sex-by-risk status and sex-by-CDI interaction did not improve the model fit (main effects model AIC 234.4 vs. two-level interaction model AIC 236.1). In addition, the interaction of risk status and sex was not significant (F1,15 = 2.25, p > 0.10). Likewise, the sex-by-CDI interaction was also not significant (F1,15= 0.06, p > 0.10).
Discrimination Between Sadness and Anger
Discrimination between traces of anger and sadness in facial expressions was examined via a full factorial analysis of variance using mixed effects: SP50 for anger vs. sadness was the dependent variable, and risk status, sex, age, and depressive symptoms (CDI) were the independent variables. The main effects model indicated that age (F1,16 = 0, p > .10) and depressive symptoms (F1,16 = 1.10, p > .10) were not significant predictors of SP50 for Anger vs. Sadness. However, both risk status (F1,76 = 3.85, p =.05) and sex (F1,16 = 16.87, p < .01) significantly predicted SP50 (See Figure 3). Specifically, high-risk children had significantly lower Anger vs. Sadness SP50 compared to low-risk children (Mean ±SE: 5.80 ±0.10 vs. 6.09 ±.09). The SP50 scores above 5 for both groups suggest that both tended to choose Sad in faces that included greater intensity of anger than sadness (i.e., sadness over-identification). However, the children at high-risk for depression had SP50 scores closer to 5 suggesting less sadness over-identification than their low-risk peers. In addition, girls had significantly lower Anger vs. Sadness SP50 than boys but both groups had scores above 5 (Mean ±SE: 5.69 ±0.10 vs. 6.20 ±.09). Thus, girls displayed less sadness over-identification than boys. A model with the sex-by-risk status interaction showed improvement in fit (main effects model AIC 202.6 vs. two-level interaction model AIC 201.7). However, the interaction of risk status and sex was not significant (F1,16 = 1.87, p > 0.10).
Discussion
In this study we examined whether enhanced perceptual sensitivity to sad cues was a potential mechanism of risk in the offspring of depressed parents. Contrary to our initial hypothesis, we did not find a main risk status effect on perceptual sensitivity when boys and girls were examined together. However, we found that sex of the child moderated the association between risk status and perceptual sensitivity. Specifically, high-risk boys displayed greater perceptual sensitivity to sad cues in facial expressions than did their low-risk same-sex peers. This effect was robust even after controlling for the role of age, depression symptoms, and externalizing problems. In contrast, we found no differences in perceptual sensitivity among high and low-risk girls. Therefore, our study indicates that enhanced perception of subtle traces of sadness may be a specific characteristic of boys at familial risk for depression. This is the first study to show that an information processing anomaly (i.e., enhanced facial expression recognition) that is believed to contribute to the attentional biases observed in depressed adults, may be present before the onset of major depression in high-risk boys.
Children of depressed parents have significantly greater risk of developing depression than their low-risk peers (Beardslee et al., 1998; Goodman et al., 2011), and thus the observed enhanced perceptual sensitivity for subtle expressions of sadness among high-risk boys may be one potential mechanism that explains such increased risk. Enhanced feature detection capacity may result in greater perception of actual negative affect in others and a more negative, albeit more realistic, picture of their social world, which could negatively impact their social interactions (Gotlib et al., 2004). For example, these children may be less likely to interact socially with peers when they notice subtle traces of sadness. This would not be surprising given that depression decreases parent-child interaction quality (Hammen, 2009) and thus these children may associate sadness in others with poor interpersonal experiences. In addition, these children may be more likely than their low-risk peers to identify sadness in their parents’ expressions and thus respond by experiencing negative affect themselves. However, as further discussed below, it is also possible that the observed sensitivity to sad expressions may not play an active role in development of depression but instead reflect specific and adaptive cognitive skills developed due to the greater exposure to sad faces in their home environment.
Surprisingly, we observed enhanced perceptual sensitivity among high-risk boys but not among high-risk girls. It is possible that our findings reflect true sex differences in risk factors for depression in this sample. Specifically, although adolescent girls display more cognitive vulnerabilities (e.g., maladaptive attributional styles) than boys (Hankin & Abramson, 2002), evidence for sex differences in pre-adolescence is more mixed. Some studies have observed slightly greater cognitive vulnerabilities, such as attributional style and hopelessness, in boys than girls (Abela, Brozina, & Haigh, 2002; Nolen-Hoeksema, Girgus, & Seligman, 1991). Given that about 80% of our sample was under 12 years of age, our findings may reflect one mechanism of risk that is unique to pre-adolescent boys. However, it is possible that our findings reflect underlying sex-specific processes that are unrelated to risk mechanisms. High-risk boys may have developed greater feature detection ability in response to differences in the way parents interact with high-risk boys and girls. That is, boys are more likely than girls to receive high rates of corporal punishment (Dietz, 2000; Gershoff, 2002) and depression in parents increases the risk of using harsh discipline (Eamon, 2001; Woodward & Fergusson, 2002). Thus, being able to identify subtle traces of sadness in depressed parents may be an adaptive skill for high-risk boys. Finally, although most of our proband parents were mothers (90%), most of the proband fathers in the sample had male offspring. Therefore, it is possible that the effect observed in high-risk boys was due to the impact of paternal depression. Although most research suggests that maternal depression is associated with greater risk than paternal depression (see Connell & Goodman, 2002; Hammen, 2009), some characteristics of depressed fathers could have impacted our results. For example, depressed fathers are up to four times more likely than non-depressed parents to use physical punishment (Davis, Davis, Freed, & Clark, 2011), and thus, as previously stated, boy’s ability to recognize depression may be especially adaptive.
In addition, our findings are not entirely consistent with the only previous examinations of attentional biases and perceptual sensitivity in youth at familial risk for depression (Joormann et al., 2010; Kujawa et al., 2011). For example, Kijawa et al. (2011) examined attentional biases in young offspring of depressed parents (Mage = 6) using a modified version of the dot-probe paradigm. They found that at-risk females, but not males, showed attentional bias towards sad faces when compared to their low-risk peers. Nonetheless, their experimental paradigm as executed (i.e., presenting faces at 1,500ms) is often interpreted as an attention disengagement task as opposed to attention allocation/deployment task (Gotlib et al., 2004), with the latter being more closely linked to feature detection capacity (see Cisler & Koster, 2010). Therefore, the Kujawa et al. (2011) study may be tapping into a different information processing domain that may not necessarily contradict our findings.
In contrast, Joormann et al. (2010) examined perceptual sensitivity to subtle traces of sadness in high-risk adolescent girls and found lower perceptual sensitivity to sadness in high-risk youth compared to their low-risk peers, which directly contradicts our findings. Joormann et al. (2010) preferentially sampled adolescent offspring (Mage = 12.5) who never had psychiatric disorders (including depression) despite their high-risk status; therefore, those cases may have included some resilient individuals. However, our study included a large number of younger pre-adolescent children (Mage = 9.5). Therefore, the low sensitivity to sad cues among high-risk girls in the Joormann et al. (2010) study may have served as a protective factor that enabled those offspring not to attend to, act upon, or be upset by sad cues in their surroundings. Finally, in the Joormann et al. (2010) study, participants watched a video of dynamically morphing faces and had to press a key as soon as they identified an emotion. Thus, perceptual sensitivity was defined by the speed of response: slower responses were interpreted as reflecting reduced perceptual sensitivity. This raises the possibility that the differences between high- and low-risk participants that Joormann et al. (2010) observed may have been impacted by differences in reaction time. In contrast, our estimation of perceptual sensitivity to sad cues was not confounded by, or dependent upon, the individual’s reaction time when watching dysphoric faces. We should note however, that although we did not find statistically significant differences in perceptual sensitivity between the high- and low-risk girls, the effect size of their mean differences was moderate (Cohen’s d = .45) and in the direction consistent with Joorman et al. (2010) findings.
Finally, the observed difference in sadness identification between high- and low-risk boys likely reflects true differences in feature detection capacity as opposed to differences in labeling biases. Specifically, if the observed difference in sadness identification was due to a tendency by high risk boys to label ambiguous faces as sad (i.e., labeling bias) we would expect to see such bias in the Sadness vs. Anger Discrimination condition, but this was not the case. In contrast, in the Sadness vs. Anger Discrimination condition, both high- and low-risk groups tended to label ambiguous faces that had greater ratio of anger to sad cues as sad, but this tendency was actually lower in high-risk children. This suggests that both groups displayed a sad over-identification bias of faces that had traces of both sadness and anger but the high-risk group did so at lower levels. It is likely that this sad over-identification finding reflects developmental differences in facial expression identification. Facial expression recognition abilities improve developmentally at different rates for different emotions. For example, there is evidence that children can recognize sadness at adult levels as much as four years before they can recognize anger at adult levels (Durand, Gallay, Seigneuric, Robichon, & Baudouin, 2007). Thus, the sad over-identification effect found in both groups may reflect age-related difficulty in the recognition of anger cues in mixed angry-sad faces. The question remains, however, as to why high-risk children displayed less difficulty with angry faces than their peers. Interestingly, Pollak et al., (2002) used this exact paradigm with abused and typically developing 9 year olds and found that non-abused controls significantly over-identified sadness in the Anger vs. Sad condition, which is in line with our findings. However, abused kids tended to over-identify anger instead (see Figure 2, page 9074) . This effect was interpreted as reflecting an adaptive ability to identify anger in abused kids. It is possible that it is also adaptive for our high-risk sample to discriminate between sadness and anger in their parents, which decreased the developmentally-appropriate difficulty in anger discrimination found in typically developing kids. All in all, our results from the emotion recognition condition point towards greater perceptual sensitivity to subtle sad features among high-risk boys and this effect was not likely due to a greater tendency to label ambiguous faces as sad.
Our study has a number of strengths and limitations. This is the first report of perceptual sensitivity to facial expressions of emotions among young boys and girls at familial risk for depression. Our results were obtained using a well-controlled, standard laboratory task that is not confounded by individual or group differences in reaction time, thus likely providing a more valid index of perceptual sensitivity. We also controlled for depressive symptoms and thus our findings are not due to group differences in depressive symptoms. However, our interpretation that greater perceptual sensitivity is a mechanism of risk is limited by the fact that we do not have information about eventual depression onset in these children. Thus, it is possible that such effect is just a characteristic of high-risk boys that does not play a role in the development of depression. For example, boys at familial-risk are exposed to more dysphoric affect in their parents than their low-risk peers (Forbes et al., 2008) and thus may become more skilled in identifying sadness. It is also possible that the observed effect reflects an early stage of the condition (i.e., prodrome) and thus be a result of depression as opposed to a risk factor. However, we did not find an association between perceptual sensitivity and depressive symptoms in this sample, which makes this explanation unlikely. In addition, our protocol did not include an evaluation of perceptual sensitivity to multiple other emotions (e.g., fear, happiness). We only studied sadness vs. anger because we wanted to examine specificity of perceptual biases for sadness, rather than all negative emotions. However, given the mounting interest in the role of positive emotions in depression, future studies should include happy faces in their emotional stimuli sets and examine perceptual sensitivity to happy faces in high-risk cohorts. Our study was cross-sectional and thus, we do not know whether the enhanced perceptual sensitivity observed in high-risk boys will predict future depression onset. Likewise, although we are interpreting our findings as reflective of perceptual sensitivity, it is possible that such sensitivity reflects attention allocation/deployment biases, which we did not directly test. Although this sounds like a chicken or the egg dilemma (i.e., are attention biases due to high perceptual sensitivity or vice-versa?), most current conceptualizations of biases view feature detection as a potential mechanism (as opposed to an outcome) of attentional biases (Cisler & Koster, 2010). Furthermore, our sample size was relatively small and it is possible that the study had insufficient power to identify subtle differences in some analyses. Finally, most parents of our high-risk children were mothers, which prevented us from examining the differential effects of maternal vs. paternal depression.
In conclusion, our findings indicate that young, male offspring of parents with a history of depression may have enhanced perceptual sensitivity to sad facial cues. This unusual skill may be a mechanism that contributes to the development of depression in male offspring of depressed parents. Future studies should examine if individual differences in sensitivity to sad cues among high-risk children predict the onset or severity of depressive disorders as they move into adolescence and young adulthood.
Key Points.
Children of depressed parents are a high risk for developing depression. Identifying differences between high and low risk children can help us understand potential mechanisms of risk transmission.
We examined perceptual sensitivity to sad cues in facial expressions among children at familial-risk for depression and low-risk peers.
We found that high-risk boys, but not girls, displayed enhanced perceptual sensitivity to sadness when compared to their low-risk peers, suggesting these biases may be present in boys before onset of major depression.
Oversensitivity to sad cues in facial expressions may be a mechanism of risk among male offspring of depressed parents.
Acknowledgments
This study was supported by NIMH Program Project grant PO1 MH056193 to Dr. Kovacs. Preparation of this article was partially supported by NIMH grant RO1 MH085722. The CDI is published by MHS Inc. from which Dr. Kovacs receives royalties.
Footnotes
Conflict of interest: No conflict of interest
References
- Abela JRZ, Brozina K, Haigh E. An examination of the response styles theory of depression in third- and seventh-grade children: A short-term longitudinal study. Journal of Abnormal Child Psychology. 2002;30(5):515–527. doi: 10.1023/a:1019873015594. [DOI] [PubMed] [Google Scholar]
- Achenbach T, Rescorla LA. Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Beardslee WR, Versage EM, Giadstone TRG. Children of affectively ill parents: A review of the past 10 years. Journal of the American Academy of Child & Adolescent Psychiatry. 1998;37(11):1134–1141. doi: 10.1097/00004583-199811000-00012. [DOI] [PubMed] [Google Scholar]
- Bozdogan H. Model selection and Akaike’s information criterion (AIC): The general theory and its analytical extensions. Psychometrika. 1987;52(3):345–370. [Google Scholar]
- Cisler JM, Koster EHW. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review. 2010;30(2):203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell AM, Goodman SH. The association between psychopathology in fathers versus mothers and children’s internalizing and externalizing behavior problems: A meta-analysis. Psychological Bulletin. 2002;128(5):746–773. doi: 10.1037/0033-2909.128.5.746. [DOI] [PubMed] [Google Scholar]
- Davis RN, Davis MM, Freed GL, Clark SJ. Fathers’ Depression Related to Positive and Negative Parenting Behaviors With 1-Year-Old Children. Pediatrics. 2011;127(4):612–618. doi: 10.1542/peds.2010-1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearing K, Gotlib I. Interpretation of Ambiguous Information in Girls at Risk for Depression. Journal of Abnormal Child Psychology. 2009;37(1):79–91. doi: 10.1007/s10802-008-9259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz TL. Disciplining children: characteristics associated with the use of corporal punishment. Child Abuse & Neglect. 2000;24(12):1529–1542. doi: 10.1016/s0145-2134(00)00213-1. [DOI] [PubMed] [Google Scholar]
- Durand K, Gallay M, Seigneuric A, Robichon F, Baudouin JY. The development of facial emotion recognition: The role of configural information. Journal of Experimental Child Psychology. 2007;97(1):14–27. doi: 10.1016/j.jecp.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Eamon MK. Antecedents and socioemotional consequences of physical punishment on children in two-parent families. Child Abuse & Neglect. 2001;25(6):787–802. doi: 10.1016/s0145-2134(01)00239-3. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID-I/P, Version 2.0) New York: Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- Forbes EE, Shaw DS, Fox NA, Cohn JF, Silk JS, Kovacs M. Maternal depression, child frontal asymmetry, and child affective behavior as factors in child behavior problems. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47(1):79–87. doi: 10.1111/j.1469-7610.2005.01442.x. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Shaw DS, Silk JS, Feng X, Cohn JF, Fox NA, Kovacs M. Children’s affect expression and frontal EEG asymmetry: transactional associations with mothers’ depressive symptoms. Journal of Abnormal Child Psychology. 2008;36(2):207–221. doi: 10.1007/s10802-007-9171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoff ET. Corporal punishment by parents and associated child behaviors and experiences: A meta-analytic and theoretical review. Psychological Bulletin. 2002;128(4):539–579. doi: 10.1037/0033-2909.128.4.539. [DOI] [PubMed] [Google Scholar]
- Gollan JK, Pane HT, McCloskey MS, Coccaro EF. Identifying differences in biased affective information processing in major depression. Psychiatry Research. 2008;159(1-2):18–24. doi: 10.1016/j.psychres.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SH, Rouse MH, Connell AM, Broth MR, Hall CM, Heyward D. Maternal depression and child psychopathology: A meta-analytic review. Clinical Child and Family Psychology Review. 2011;14(1):1–27. doi: 10.1007/s10567-010-0080-1. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. Journal of Abnormal Psychology. 2004;113(1):127–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Research. 1992;42(3):241–251. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- Hammen CL. Children of depressed parents. In: Gotlib IH, Hammen CL, editors. Handbook of depression. Vol. 2. New York, N.Y: The Guilford Press; 2009. pp. 275–297. [Google Scholar]
- Hankin BL, Abramson LY. Measuring Cognitive Vulnerability to Depression in Adolescence: Reliability, Validity, and Gender Differences. Journal of Clinical Child & Adolescent Psychology. 2002;31(4):491. doi: 10.1207/S15374424JCCP3104_8. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Gibb BE, Abela JRZ, Flory K. Selective attention to affective stimuli and clinical depression among youths: Role of anxiety and specificity of emotion. Journal of Abnormal Psychology. 2010;119(3):491–501. doi: 10.1037/a0019609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gilbert K, Gotlib IH. Emotion identification in girls at high risk for depression. Journal of Child Psychology and Psychiatry. 2010;51(5):575–582. doi: 10.1111/j.1469-7610.2009.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology. 2007;116(1):135–143. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kovacs M, Staff MHS. Children’s Depression Inventory (CDI): Technical Manual Update. North Tonawanda, NY: Multi-Health Systems, Inc; 2003. [Google Scholar]
- Kujawa A, Torpey D, Kim J, Hajcak G, Rose S, Gotlib I, Klein D. Attentional Biases for Emotional Faces in Young Children of Mothers with Chronic or Recurrent Depression. Journal of Abnormal Child Psychology. 2011;39(1):125–135. doi: 10.1007/s10802-010-9438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95(1):15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Mannie ZN, Barnes J, Bristow GC, Harmer CJ, Cowen PJ. Memory impairment in young women at increased risk of depression: Influence of cortisol and 5-HTT genotype. Psychological Medicine. 2009;39(05):757–762. doi: 10.1017/S0033291708004248. [DOI] [PubMed] [Google Scholar]
- Maziade M, Roy MA, Fournier JP, Cliche D, Merette C, Caron C, Garneau Y, Montgrain N, Shriqui C, Dion C. Reliability of best-estimate diagnosis in genetic linkage studies of major psychoses: results from the Quebec pedigree studies. American Journal of Psychiatry. 1992;149(12):1674–1686. doi: 10.1176/ajp.149.12.1674. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Girgus JS, Seligman MEP. Sex differences in depression and explanatory style in children. Journal of Youth and Adolescence. 1991;20(2):233–245. doi: 10.1007/BF01537610. [DOI] [PubMed] [Google Scholar]
- Ohman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. Journal of Experimental Psychology General. 2001;130(3):466–478. doi: 10.1037//0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: Toward an evolved module of fear and fear learning. Psychological Review. 2001;108(3):483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57(1):120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Kistler DJ. Early experience is associated with the development of categorical representations for facial expressions of emotion. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(13):9072–9076. doi: 10.1073/pnas.142165999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prkachin GC. The effects of orientation on detection and identification of facial expressions of emotion. British Journal of Psychology. 2003;94(1):45–62. doi: 10.1348/000712603762842093. [DOI] [PubMed] [Google Scholar]
- Silk JS, Shaw DS, Skuban EM, Oland AA, Kovacs M. Emotion regulation strategies in offspring of childhood-onset depressed mothers. Journal of Child Psychology and Psychiatry. 2006;47(1):69–78. doi: 10.1111/j.1469-7610.2005.01440.x. [DOI] [PubMed] [Google Scholar]
- Simmons JP, Nelson LD, Simonsohn U. False-positive psychology. Psychological Science. 2011;22(11):1359–1366. doi: 10.1177/0956797611417632. [DOI] [PubMed] [Google Scholar]
- Singh AL, D’Onofrio BM, Slutske WS, Turkheimer E, Emery RE, Harden KP, Heath AC, Madden PAF, Statham DJ, Martin NG. Parental depression and offspring psychopathology: a children of twins study. Psychological Medicine. 2011;41(7):1385–1395. doi: 10.1017/S0033291710002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze SA, Young AW, Senior C, Brébion G, Travis MJ, Phillips ML. Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology. 2004;18(2):212–218. doi: 10.1037/0894-4105.18.2.212. [DOI] [PubMed] [Google Scholar]
- Weissman M, Wickramaratne P, Nomura Y, Warner V, Pilowsky D, Verdeli H. Offspring of depressed parents: 20 years later. American Journal of Psychiatry. 2006;163(6):1001. doi: 10.1176/ajp.2006.163.6.1001. [DOI] [PubMed] [Google Scholar]
- Weissman M, Wickramaratne P, Nomura Y, Warner V, Verdeli H, Pilowsky DJ, Grillon C, Bruder G. Families at high and low risk for depression: A 3-Generation Study. Archives of General Psychiatry. 2005;62(1):29–36. doi: 10.1001/archpsyc.62.1.29. [DOI] [PubMed] [Google Scholar]
- Williamson DE, Birmaher B, Axelson DA, Ryan ND, Dahl RE. First episode of depression in children at low and high familial risk for depression. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43:291–297. doi: 10.1097/00004583-200403000-00010. [DOI] [PubMed] [Google Scholar]
- Wilson S, Durbin CE. Effects of paternal depression on fathers’ parenting behaviors: A meta-analytic review. Clinical Psychology Review. 2010;30(2):167–180. doi: 10.1016/j.cpr.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Woodward LJ, Fergusson DM. Parent, child, and contextual predictors of childhood physical punishment. Infant and Child Development. 2002;11(3):213–235. [Google Scholar]


