Abstract
Background
Adult survivors of childhood Central Nervous System (CNS) tumors may be at risk for pulmonary dysfunction. This study enumerates the incidence of pulmonary dysfunction and explores associations between craniospinal irradiation (CSI) and pulmonary dysfunction among survivors of childhood CNS tumors.
Methods
Participants included Childhood Cancer Survivor Study (CCSS) cohort members treated for CNS malignancies when < age 21, who survived five+ years, and sibling comparisons. Medical records were abstracted and participants completed questionnaires that asked about the nature and timing of pulmonary dysfunction. Incidence rates were calculated, and Poisson regression, adjusted for chemotherapy exposures, was used to evaluate associations between CSI and pulmonary dysfunction.
Results
Survivor participants (N=1,653) were 54.7% male, median age at diagnosis 7.6 (range 0–21), and median time from cohort entry 18.5 (range 3.3–33.9) years. The incidence of pulmonary dysfunction (per 1,000 person years) was 9.1 (95% CI 7.8–10.6) for emphysema/obliterative bronchiolitis and >3.0 for asthma, chronic cough and need for extra oxygen. Rates of fibrosis (RR 2.0, 95% CI 1.0–3.9), chest wall abnormalities (RR 19.0, 95% CI 4.2–85.7), chronic cough (RR 1.6, 95% CI 1.2–2.1) and need for supplemental oxygen (RR 2.5, 95% CI 1.9–3.3) were higher among survivors than among siblings. Survivors treated with CSI were 10.4 (95% CI 7.6–14.4) times more likely than those not exposed to report chest wall deformity.
Conclusion
Adult survivors of CNS malignancy have high rates of pulmonary dysfunction 5+ years after diagnosis. Survivors treated with CSI should be monitored for pulmonary disease to permit early interventions.
Keywords: childhood survivors, CNS malignancy, pulmonary late effect, craniospinal radiation
Introduction
Recent estimates from the Surveillance, Epidemiology and End Results program suggest that in the modern era, 70% of children diagnosed with Central Nervous System (CNS) malignancies will become 5-year survivors.[1] Nevertheless, curative treatment has not come without cost. Documented complications of anti-cancer therapy in this population include neurocognitive, neurologic, and neuroendocrine sequelae, as well as secondary neoplasms.[2–5] In addition, because many CNS tumor survivors were treated with craniospinal irradiation (CSI) and/or chemotherapy, their lungs were exposed to potential toxins, placing them at risk for pulmonary damage.
The lung is one of the most radiation-sensitive organs in the body. In addition to the direct effects on lung tissue,[6] spinal field radiation may also affect spinal growth,[7] and is associated with scoliosis and chest wall deformity.[8,9] Reduced lung volume and limited chest wall mobility may compound direct injury to the lung and contribute to the pathogenesis of long-term pulmonary dysfunction.[10] Two studies have suggested an association between CSI and late-onset pulmonary dysfunction among childhood CNS tumor survivors.[11,12] However, because of small sample sizes and heterogeneous radiation doses, neither investigation was able to evaluate the association between radiation dose and pulmonary dysfunction.
Children with CNS tumors often receive chemotherapy in addition to radiation. Some agents, for example bleomycin, carmustine (BCNU), lomustine (CCNU), busulfan, and cyclophosphamide, are also associated with pulmonary injury.[13] In a reported series of 17 children who survived after treatment with CSI and BCNU for malignant CNS tumors, nine died later from pulmonary fibrosis.[14–16] Among the eight patients still alive 25 years after diagnosis, seven had radiologic evidence of upper zone pulmonary fibrosis.[14]
The Childhood Cancer Survivor Study (CCSS) provides an opportunity to confirm, in a well- defined cohort, the associations between CSI and long-term pulmonary complications among survivors of childhood onset CNS tumors. Therefore, the aims of this study were to enumerate the incidence of pulmonary dysfunction, and explore potential associations between CSI and pulmonary dysfunction among survivors of childhood CNS tumors.
Methods
Participants
The CCSS is a retrospective cohort study designed to evaluate the impact of childhood cancer and its treatment on long-term function and health.[17,18] Participants were diagnosed and treated at one of 26 collaborating institutions in North America between 1970 and 1986 when 21 years of age or younger. Institutional review boards of participating centers reviewed and approved the CCSS protocol. Cohort entry was limited to individuals who survived for at least five years after original diagnosis. Participants have completed multiple questionnaires since their original enrollment (available at http://ccss.stjude.org). Institutional Board Approval was obtained for Human Subjects Research at each collaborating center. The study population for this analysis included 1,653 childhood onset CNS tumor survivors who consented to medical record abstraction and a sibling comparison group (N=4,023).
Questionnaire and medical record abstraction
The primary outcomes of interest were pulmonary conditions reported by participants on the baseline, year 2000, and year 2007 surveys. Conditions were categorized into seven groups: (1) pulmonary fibrosis; (2) emphysema/obliterative bronchiolitis; (3) interstitial pneumonia/pleurisy; (4) abnormal chest wall (kyphosis, scoliosis, acquired deformity of the chest or ribs); (5) chronic cough/shortness of breath; (6) asthma; and (7) need for extra oxygen. The timing of each pulmonary condition was determined by age at first occurrence. Treatment information was obtained from medical records by trained abstractors. Radiation exposures to the heart and lungs were reviewed and quantified by a radiation physicist (MS) with maximum radiation doses estimated.
Given the known associations between cardiac and pulmonary dysfunction in childhood cancer survivors, cardiovascular event history was considered as a covariate in multiple variable models. Events including coronary heart disease, congestive heart failure, arrhythmia, heart valve problems, and stroke were obtained from participant report on the baseline, 2000, and 2007 questionnaires. In addition, age at primary diagnosis, current age, gender, and smoking status (ever, never, current, unknown) were considered as covariates in multiple variable models.
Statistical Analysis
Descriptive statistics, stratified by radiation exposure and location, were calculated to describe demographics, treatment characteristics, distributions of cardiac disease history, and smoking status among the study population. For each pulmonary condition, the prevalence rate was calculated at five years from primary CNS tumor diagnosis. Incidence rates were calculated for pulmonary conditions that occurred five or more years from the initial CNS tumor diagnosis. Incidence rates among survivors were compared to incidence rates among siblings using Generalized Estimating Equations (GEE) to account for within-family correlation, adjusted for age at follow-up and sex.
To assess the impact of CSI on each pulmonary condition, multivariable Poisson regression was used. The follow-up time started at a point five years from original diagnosis and ended at the earliest of the pulmonary condition of interest, second malignant neoplasm, late recurrence, or death. All models were adjusted for (1) age during follow-up (time-dependent), (2) history of cardiovascular disease (time-dependent), and (3) because of the large number of potential confounding variables, a propensity score,[19] which included gender, age at diagnosis, current smoking status, chemotherapy [nitrosoureas (yes/no), other alkylating agent (yes/no), antimetabolites (yes/no), antitumor antibiotics (yes/no), and other drugs that at least 2% of survivors had received] as covariates. Multiple imputation under the assumption of “missing at random”[20] was used to estimate the missing time of some of the reported pulmonary conditions using the method of Taylor et al.[21]
Results
Among 1,653 CNS tumor survivors who completed the baseline survey, 1,200 (72.6%) and 904 (54.7%) completed the 2000 and 2007 follow-up questionnaires, respectively. Among the 1,200 survivors who completed the 2000 questionnaire, 848 (70.7%) completed the 2007 questionnaire. Characteristics of the study population are shown in Table I by radiation exposure. Over one-half (54.7%) were male and 41.3% were diagnosed when six years of age or younger. Median age at diagnosis was 7.6 (range 0–21) years, and median age at last questionnaire completion was 31.2 (range 9.1–56.4) years. Among 4,023 siblings who completed the baseline survey, 2,540 (63.1%) and 2,373 (59.0%) completed the 2000 and 2007 follow-up questionnaires, respectively. Among the 2,540 siblings who completed the 2000 questionnaire, 1,746 (73.6%) completed the 2007 questionnaire. Almost one-half (48.1%) of siblings were male. The median age of siblings at last questionnaire was 33.9 (range 3.1–62.6) years. Among survivors, the median length of follow-up (from cohort entry) was 18.5 (range 3.3–33.9) years. Tumor histology was astrocytoma/glial tumor in 66.2%, and medulloblastoma/primitive neuroectodermal tumor in 21.4% of survivors. Nearly two thirds (64.6%) were exposed to CSI; 29.0% of survivors had no radiation exposure history. One fourth (25.1%) had received maximum lung radiation doses of more than 20 Gray (Gy). A similar percentage (25.2%) had maximum cardiac radiation exposures of more than 10 Gy.
Table I.
Characteristics of the study population
| Survivors by location of radiation (N=1,653)
| |||||||
|---|---|---|---|---|---|---|---|
| Craniospinal (N=406) |
Cranial RT only (N=648) |
Spinal RT only (N=14) |
Other RT (N=25) |
No RT (N=480) |
Unknown RT exposure (N=80) |
Siblings (N=4,023) |
|
|
|
|||||||
| Gender | |||||||
| Male | 242 (59.6%) | 343 (52.9%) | 8 (57.1%) | 11 (44.0%) | 258 (53.8%) | 42 (52.5%) | 1937 (48.1%) |
| Female | 164 (40.4%) | 305 (47.1%) | 6 (42.9%) | 14 (56.0%) | 222 (46.3%) | 38 (47.5%) | 2086 (51.9%) |
| Age at diagnosis | |||||||
| 0–3 yrs | 69 (17.0%) | 131 (20.2%) | 2 (14.3%) | 7 (28.0%) | 109 (22.7%) | 11 (13.8%) | |
| 4–6 yrs | 83 (20.4%) | 141 (21.8%) | 5 (35.7%) | 2 (8.0%) | 107 (22.3%) | 15 (18.8%) | |
| 7–14 yrs | 202 (49.8%) | 279 (43.1%) | 5 (35.7%) | 12 (48.0%) | 192 (40.0%) | 33 (41.3%) | |
| 15–21 years | 52 (12.8%) | 97 (15.0%) | 2 (14.3%) | 4 (16.0%) | 72 (15.0%) | 21 (26.3%) | |
| Age at last questionnaire | |||||||
| <20 yrs | 80 (19.7%) | 101 (15.6%) | 3 (21.4%) | 0 (0.0%) | 60 (12.5%) | 16 (20.0%) | 369 (9.2%) |
| 20–29 yrs | 125 (30.8%) | 193 (29.8%) | 6 (42.9%) | 5 (20.0%) | 166 (34.6%) | 24 (30.0%) | 1128 (28.0%) |
| 30–39 yrs | 147 (36.2%) | 243 (37.5%) | 3 (21.4%) | 9 (36.0%) | 168 (35.0%) | 24 (30.0%) | 1360 (33.8%) |
| 40–49 yrs | 53 (13.1%) | 99 (15.3%) | 2 (14.3%) | 11 (44.0%) | 79 (16.5%) | 14 (17.5%) | 925 (23.0%) |
| >=50 yrs | 1 (0.2%) | 12 (1.9%) | 0 (0.0%) | 0 (0.0%) | 7 (1.5%) | 2 (2.5%) | 241 (6.0%) |
| CNS type | |||||||
| Astrocytoma/glial tumor | 16 (3.9%) | 564 (87.0%) | 9 (64.3%) | 17 (68.0%) | 435 (90.6%) | 53 (66.3%) | |
| Medulloblastoma/PNET | 309 (76.1%) | 15 (2.3%) | 0 (0.0%) | 0 (0.0%) | 10 (2.1%) | 20 (25.0%) | |
| Ependymoma | 53 (13.1%) | 37 (5.7%) | 5 (35.7%) | 8 (32.0%) | 12 (2.5%) | 6 (7.5%) | |
| other CNS tumors | 28 (6.9%) | 32 (4.9%) | 0 (0.0%) | 0 (0.0%) | 23 (4.8%) | 1 (1.3%) | |
| Chest surgery | |||||||
| yes | 4 (1.0%) | 9 (1.4%) | 0 (0.0%) | 1 (4.0%) | 4 (0.8%) | 0 (0.0%) | |
| no | 401 (98.8%) | 639 (98.6%) | 14 (100.0%) | 24 (96.0%) | 474 (98.8%) | 80 (100.0%) | |
| unknown | 1 (0.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (0.4%) | 0 (0.0%) | |
| Lung radiation dose exposurea | |||||||
| No | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 480 (100.0%) | 0 (0.0%) | |
| <=20 Gy | 13 (3.2%) | 644 (99.4%) | 2 (14.3%) | 10 (40.0%) | 0 (0.0%) | 0 (0.0%) | |
| >20 Gy | 391 (96.3%) | 0 (0.0%) | 11 (78.6%) | 13 (52.0%) | 0 (0.0%) | 0 (0.0%) | |
| Unknown | 2 (0.5%) | 4 (0.6%) | 1 (7.1%) | 2 (8.0%) | 0 (0.0%) | 80 (100.0%) | |
| Heart radiation dose exposurea | |||||||
| No | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 480 (100.0%) | 0 (0.0%) | |
| <=10 Gy | 9 (2.2%) | 644 (99.4%) | 1 (7.1%) | 13 (52.0%) | 0 (0.0%) | 0 (0.0%) | |
| >10 Gy | 395 (97.3%) | 0 (0.0%) | 12 (85.7%) | 10 (40.0%) | 0 (0.0%) | 0 (0.0%) | |
| Unknown | 2 (0.5%) | 4 (0.6%) | 1 (7.1%) | 2 (8.0%) | 0 (0.0%) | 80 (100.0%) | |
| Chemotherapy | |||||||
| Nitrosoureas | 137 (33.7%) | 112 (17.3%) | 3 (21.4%) | 0 (0.0%) | 9 (1.9%) | 18 (22.5%) | |
| Other alkylating agents | 139 (34.2%) | 90 (13.9%) | 1 (7.1%) | 0 (0.0%) | 13 (2.7%) | 21 (26.3%) | |
| Anti-metabolites | 74 (18.2%) | 68 (10.5%) | 0 (0.0%) | 2 (8.0%) | 4 (0.8%) | 13 (16.3%) | |
| Antitumor Antibiotics | 7 (1.7%) | 26 (4.0%) | 0 (0.0%) | 0 (0.0%) | 15 (3.1%) | 1 (1.3%) | |
| Other agentsb | 200 (49.3%) | 144 (22.2%) | 2 (14.3%) | 2 (8.0%) | 29 (6.0%) | 25 (31.3%) | |
| History of cardiac disease | |||||||
| Coronary heart disease | 6 (1.5%) | 6 (0.9%) | 0 (0.0%) | 0 (0.0%) | 3 (0.6%) | 0 (0.0%) | 26 (0.6%) |
| Congestive heart failure | 0 (0.0%) | 2 (0.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 12 (0.3%) |
| Arrhythmia | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.2%) | 0 (0.0%) | 9 (0.2%) |
| Heart valves problems | 0 (0.0%) | 0 (0.0%) | 1 (7.1%) | 0 (0.0%) | 1 (0.2%) | 0 (0.0%) | 3 (0.1%) |
| Stroke | 42 (10.3%) | 84 (13.0%) | 1 (7.1%) | 2 (8.0%) | 22 (4.6%) | 12 (15.0%) | 19 (0.5%) |
| Smoke | |||||||
| Never | 311 (76.6%) | 492 (75.9%) | 11 (78.6%) | 17 (68.0%) | 311 (64.8%) | 57 (71.3%) | 2141 (53.2%) |
| Ever | 23 (5.7%) | 46 (7.1%) | 1 (7.1%) | 2 (8.0%) | 53 (11.0%) | 10 (12.5%) | 590 (14.7%) |
| Current | 42 (10.3%) | 68 (10.5%) | 1 (14.3%) | 6 (24.0%) | 92 (19.2%) | 10 (12.5%) | 1167 (29.0%) |
| Unknown | 30 (7.4%) | 42 (6.5%) | 0 (0%) | 0 (0%) | 24 (5.0%) | 3 (3.8%) | 125 (3.1%) |
Maximum dose to any part of the lung or heart (values < 20 Gy to lungs and < 10 Gy to heart include scatter doses)
Other agents received by at least 2% of CNS patients
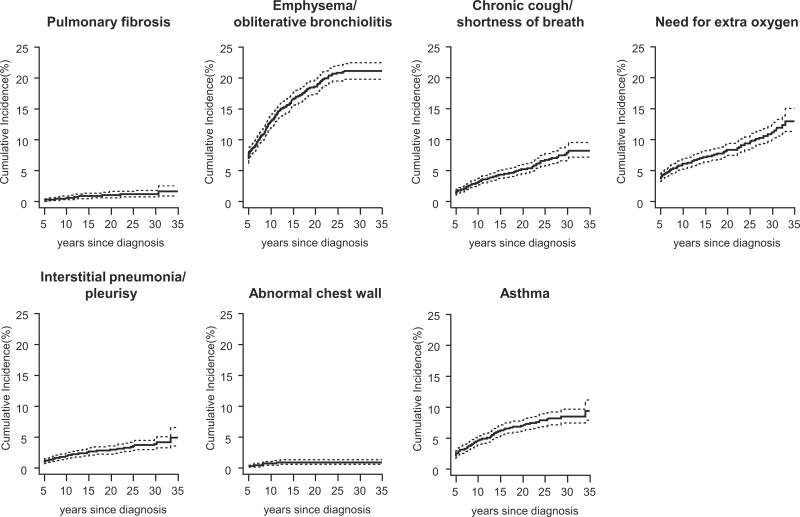
Incidence rates, per 1,000 person years, for pulmonary outcomes, are shown in Table II. The incidence of emphysema/obliterative bronchiolitis was 9.1, (95% CI 7.8–10.6 cases per 1,000 person years). Chronic cough/shortness of breath, need for supplemental oxygen and asthma had incidence rates > 3 cases per 1,000 person years. Pulmonary fibrosis, interstitial pneumonia/pleurisy and chest wall deformities were uncommon with incidence rates of 0.6, 1.6, and 0.4 per 1,000 person years, respectively. At 40 years from diagnosis, the cumulative incidence of emphysema/obliterative bronchiolitis was 20.8%, (95% CI 19.5–22.0), the cumulative incidence of chronic cough/shortness of breath was 7.3% (95% CI 6.4–8.4), and the cumulative incidence of asthma was 8.0% (95% CI 7.0–9.0). Survivors had higher rates than siblings of pulmonary fibrosis, chest wall deformities, chronic cough/shortness of breath and need for extra oxygen.
Table III shows the association between CSI and pulmonary dysfunction. After adjustment for current age, history of cardiac disease, and propensity score, risk for a chest wall deformity among survivors who had received CSI was 10.4-times (95% CI 7.6 to 14.4) higher than among those who had not. There was not an increased risk for other pulmonary conditions among individuals who received CSI when compared to those who had not. Although we did not have statistical power to include individual chemotherapy agents in our models, Table IV shows the percentages with adverse pulmonary outcomes among survivors exposed to specific chemotherapy agents.
Discussion
Pulmonary toxicity is a well-established long-term complication of exposure to certain anticancer therapies in childhood[13] and can vary from subclinical to life threatening. Previous studies have enumerated pulmonary complications among survivors of childhood and adolescent cancer and have reported specific risk factors for poor outcomes.[22–25] Our study adds to current knowledge by documenting adverse pulmonary outcomes specifically among survivors of CNS malignancy, with a focus on evaluating the effects of CSI. This is one of the first studies to quantify reported pulmonary conditions among childhood and adolescent CNS tumor survivors followed nearly 25 years after their original diagnoses. Based on self-report, we found that asthma, emphysema/obliterative bronchiolitis, and chronic cough/shortness of breath were present at rates greater than 3 cases per 1,000 person years. Moreover, our data showed that CNS survivors continue to be at risk for new onset pulmonary conditions beyond five years following diagnosis.
The reported incidence of asthma (3.5 per 1,000 person years) after treatment of childhood CNS malignancy is similar to a previous report from the CCSS.[23] Mertens et al, using data from the baseline questionnaire, reported asthma rates five or more years post-diagnosis among survivors of all diagnoses at 3.8 per 1,000 person years. These rates were higher than rates among siblings (Relative Risk of 1.3; 95% CI: 1.1–1.5).[23] Diagnosis specific rates were not reported in this manuscript. Data from two other childhood cancer survivor cohorts report cross-sectional prevalence of asthma at 6.2% among 1,065 adult survivors (10.2% CNS tumor survivors) in Switzerland, [26] and 23.0% among 102 children treated for acute myeloid leukemia (AML) in the Nordic countries.[27] Although the Swiss cohort included CNS tumor survivors likely treated with CSI, the authors did not report associations between treatment and asthma, but rather examined the association between asthma prevalence and concurrent acetaminophen use. The Nordic study did not include survivors treated with radiation.
Our findings of an association between CSI exposure and an abnormal chest wall are consistent with two investigations that included survivors of childhood medulloblastoma. Gerosa et al reported kyphoscoliosis in 25% of 28 survivors whose treatment included spinal irradiation (30 to 35 Gy),[9] and Gaspar et al reported scoliosis in 18% of 11 survivors 4 to 18 years after radiation treatment (electrons) that included 35 to 37.5 Gy to the spine.[8] Radiation induced scoliosis and rib cage deformities alter mechanical properties of the chest wall and interfere with lung function. Hartley et al measured vertebral body growth rates in 61 children with CNS tumors (40 boys; median age, 7 years) before and after CSI (23.4 or 36–39.6 Gy; median follow-up 44 months) and found that spinal growth rates were inversely proportional to CSI dose.[7]
Data from an earlier study among children treated for Wilms tumor indicate that in addition to its effect on bones of the chest wall,[28] radiation of the thorax affects lung parenchyma, reducing lung volume, and impairing dynamic compliance, contributing to deformity of both the lung and chest wall.[29–31] Although our data are from self-report and do not include traditional pulmonary function tests, the association between CSI and chest wall deformity in this study may be a mechanism to help explain the findings of restrictive lung disease previously reported among 24–50% of childhood CNS tumor survivors treated with CSI.[11,12] Endicott et al measured pulmonary function and reported restrictive lung disease in five and diminished diffusion capacity in one of 21 survivors of CNS malignancies.[11] Jakacki et al, in a group of 28 CNS tumor survivors, reported a 4.3 increase in risk for restrictive lung disease among individuals exposed to CSI when compared to those who were not exposed.[12]
We did not find associations between CSI and severe lung disease, such as fibrosis, in these mostly adult survivors of childhood CNS tumors. This is likely because, in most cases, the volume of the lung (<10%) exposed to radiation was small. It is believed that pulmonary effects of radiotherapy including pneumonitis and fibrosis are not only dose-, but also volume- dependent.[32–34]
These data should be considered in the context of several study limitations. First, there were relatively few late-onset pulmonary conditions in our cohort, which precluded evaluation of a radiation dose gradient and pulmonary outcomes. The sparsity of outcomes also made it impossible to evaluate independent or additive contributions of specific chemotherapeutic agents to pulmonary toxicity among survivors who received CSI. Additionally, our data are from self-report. Pulmonary dysfunction may be underestimated because our questionnaire required that survivors have significant enough symptoms to visit a clinician to have their condition diagnosed. Respiratory insufficiency in early stages is likely managed by subtle and unrecognized lifestyle changes, like avoiding steps and driving instead of walking. Our data do not include disease outcomes of a subclinical nature. Finally, radiation techniques have changed considerably since the time when the patients in this cohort were treated. Treatment options today include conformal and intensity modulated techniques and proton therapy, approaches designed to spare normal tissue. The results of this analysis may not be applicable to future survivors.
Conclusions
This study describes the rates of adverse pulmonary outcomes specific to a cohort of survivors of childhood onset CNS tumors nearly 25 years after diagnosis of their primary malignancy. Pulmonary dysfunction continues to manifest more than five years after diagnosis and craniospinal irradiation is likely a risk factor for chest wall deformity.
Fig. 1.
Cumulative incidence percentages of adverse pulmonary outcomes among survivors of childhood CNS tumors.
Table II.
Reported pulmonary conditions and rate per 1000 person-years
| Reported first occurrence of pulmonary conditions | Pulmonary conditions | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Pulmonary fibrosis | Emphysema/obliterative bronchiolitis | Interstitial pneumonia/pleurisy | Abnormal chest wall | Chronic cough/shortness of breath | Need for extra oxygen | Asthma | |
|
|
|||||||
| Survivors | |||||||
| Overall | 19 | 297 | 54 | 15 | 100 | 149 | 118 |
| Diagnosis to 5 years after diagnosis | 4 | 103 | 13 | 5 | 20 | 57 | 33 |
| 5+ years after diagnosis | 15 | 194 | 41 | 10 | 80 | 92 | 85 |
| No | 1,633 | 1,273 | 1,589 | 1,629 | 1,535 | 1,487 | 1,491 |
| Prevalence at 5 years since diagnosis (95% CI)a | 0.00 (0.00 – 0.01) | 0.07 (0.06 – 0.09) | 0.01 (0.00 – 0.01) | 0.00 (0.00 – 0.01) | 0.01 (0.01 – 0.02) | 0.04 (0.03 – 0.05) | 0.02 (0.01–0.03) |
| Siblings | |||||||
| Overall | 36 | 1206 | 164 | 11 | 273 | 216 | 598 |
| No | 3,987 | 2,817 | 3,859 | 4,012 | 3,750 | 3,807 | 3,425 |
| Incidence Rate (95% CI) | |||||||
| Survivors | 0.6 (0.3 – 1.0) | 9.1 (7.8 – 10.6) | 1.6 (1.1 – 2.2) | 0.4 (0.2 –0.7) | 3.2 (2.5 – 4.0) | 3.7 (3.0 – 4.6) | 3.5 (2.8 – 4.3) |
| Siblings | 0.3 (0.2 – 0.4) | 10.7 (10.1 – 11.3) | 1.1 (0.9 – 1.3) | 0.1 (0.0 –0.2) | 1.8 (1.6 – 2.1) | 1.6 (1.4 – 1.9) | 4.8 (4.4 – 5.1) |
| Rate Ratiob | 2.0 (1.0 – 3.9) | 0.9 (0.8 – 1.1) | 1.2 (0.8 – 1.7) | 19.0 (4.2 – 85.7) | 1.6 (1.2 – 2.1) | 2.5 (1.9 – 3.3) | 0.8 (0.6 – 1.1) |
Prevalence at 5 years from diagnosis could not be calculated for siblings because they have no childhood cancer diagnosis.
Rate ratio is survivor compared to sibling incidence rate, adjusted for sex and age during follow up.
Table III.
Summary of treatment factors by reported pulmonary conditions 5 or more years from diagnosisa
| Reported pulmonary conditions | |||||||
|---|---|---|---|---|---|---|---|
| RR (95% CI)b | Pulmonary fibrosis | Emphysema/obliterative bronchiolitis | Interstitial pneumonia/pleurisy | Abnormal chest wall | Chronic cough/shortness of breath | Need for extra oxygen | Asthma |
| Craniospinal radiation | 1.0 (0.3 – 3.6) | 0.9 (0.6 – 1.4) | 0.7 (0.3 – 1.7) | 10.4 (7.6 – 14.4) c | 0.9 (0.5 – 1.7) | 0.7 (0.4 – 1.2) | 1.1 (0.6 – 1.8) |
Because of the small number of outcomes, a propensity score was used to represent demographic and treatment variables other than CSI. All the models are adjusted for time independent variables age (cubic spline), gender, age at diagnosis, current smoking status, nitrosoureas (yes/no), other alkylating agent (yes/no), antimetabolites(yes/no), antitumor antibiotics (yes/no), the other drugs that at least 2% of the CNS patients had received (yes/no), and cardiac disease (time dependent).
RR=rate ratio compared to those who did not receive craniospinal radiation, CI=Confidence Interval.
P<0.05.
Table IV.
Adverse pulmonary outcomes by chemotherapy exposure
| Chemotherapy | Pulmonary fibrosis (N=19) | Emphysema/obliterative bronchiolitis (N=297) | Interstitial pneumonia/pleurisy (N=54) | Abnormal chest wall (N=15) | Chronic cough/shortness of breath (N=100) | Need for extra oxygen (N=149) | Asthma (N=149) | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Agent | Total N | Row % | Row % | Row % | Row % | Row % | Row % | Row % |
| BCNU | 33 | 1 (3.0%) | 8 (24.2%) | 2 (6.1%) | 0 (0%) | 2 (6.1%) | 4 (12.1%) | 2 (6.1%) |
| CCNU | 256 | 2 (0.8%) | 42 (16.4%) | 19 (7.4%) | 4 (1.6%) | 25 (9.8%) | 37 (14.5%) | 16 (6.3%) |
| Cyclophosphamide | 100 | 1 (1.0%) | 19 (19.0%) | 0 (0.0%) | 3 (3.0%) | 8 (8.0%) | 9 (9.0%) | 4 (4.0%) |
| Other chemotherapy | 138 | 3 (2.2%) | 23 (16.7%) | 5 (3.6%) | 2 (1.5%) | 9 (6.5%) | 11 (8.0%) | 7 (5.1%) |
Acknowledgments
FUNDING: This work was supported by the National Cancer Institute (grant number U24-CA55727 to L.L. Robison, Principal Investigator). Support to St Jude Children’s Research Hospital also provided by the Cancer Center Support (CORE) grant (CA21765) and the American Lebanese-Syrian Associated Charities (ALSAC).
The authors thank Ms. Kathy Laub for manuscript preparation, and the hundreds of cancer survivors and family members who participated in this study. Financial support was provided by the National Cancer Institute (grant number U24-CA55727 to L.L. Robison, Principal Investigator). Support to St Jude Children’s Research Hospital also provided by the Cancer Center Support (CORE) grant (CA21765) and the American Lebanese-Syrian Associated Charities (ALSAC). The sponsors had no role in study design, data collection, data analysis, data interpretation, manuscript preparation, manuscript review or in the decision to submit the manuscript to Pediatric Blood & Cancer.
Abbreviations list
- CNS
Central Nervous System
- CSI
Craniospinal Irradiation
- CCSS
Childhood Cancer Survivor Study
- CI
Confidence Interval
- BCNU
Carmustine
- CCNU
Lomustine
- Gy
Gray
Footnotes
Author contributions
Drs. Huang, Stovall, Leisenring, Mertens, Green, Robison, and Ness conceived of the project and designed the study. Drs. Robison, Mertens, Armstrong, and Stovall acquired the data. Drs. Chen, Yasui, Leisenring, and Ness designed and conducted the analysis. Drs. Huang, Chen, Dietz, Yasui, Donaldson, Stokes, Stovall, Leisenring, Sklar, Diller, Mertens, Armstrong, Green, Robison, Ness interpreted the data. Drs. Huang, Chen, Yasui, and Ness drafted the manuscript. Drs. Huang, Chen, Dietz,, Yasui, Donaldson, Stokes, Stovall, Leisenring, Sklar, Diller, Mertens, Armstrong, Green, Robison, and Ness made revisions for important intellectual content. All authors provided final approval of the submitted version.
SUMMARY CONFLICT OF INTERESTS: The authors have no conflicts of interest to disclose.
References
- 1.Smith MA, Seibel NL, Altekruse SF, et al. Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol. 2010;28(15):2625–2634. doi: 10.1200/JCO.2009.27.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong GT. Long-term survivors of childhood central nervous system malignancies: the experience of the Childhood Cancer Survivor Study. Eur J Paediatr Neurol. 2010;14(4):298–303. doi: 10.1016/j.ejpn.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gleeson HK, Shalet SM. The impact of cancer therapy on the endocrine system in survivors of childhood brain tumours. Endocr Relat Cancer. 2004;11(4):589–602. doi: 10.1677/erc.1.00779. [DOI] [PubMed] [Google Scholar]
- 4.Mulhern RK, Merchant TE, Gajjar A, et al. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5(7):399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- 5.Pietila S, Korpela R, Lenko HL, et al. Neurological outcome of childhood brain tumor survivors. J Neurooncol. 108(1):153–161. doi: 10.1007/s11060-012-0816-5. [DOI] [PubMed] [Google Scholar]
- 6.Ghafoori P, Marks LB, Vujaskovic Z, et al. Radiation-induced lung injury. Assessment, management, and prevention. Oncology (Williston Park) 2008;22(1):37–47. discussion 52–33. [PubMed] [Google Scholar]
- 7.Hartley KA, Li C, Laningham FH, et al. Vertebral body growth after craniospinal irradiation. Int J Radiat Oncol Biol Phys. 2008;70(5):1343–1349. doi: 10.1016/j.ijrobp.2007.08.085. [DOI] [PubMed] [Google Scholar]
- 8.Gaspar LE, Dawson DJ, Tilley-Gulliford SA, et al. Medulloblastoma: long-term follow-up of patients treated with electron irradiation of the spinal field. Radiology. 1991;180(3):867–870. doi: 10.1148/radiology.180.3.1871308. [DOI] [PubMed] [Google Scholar]
- 9.Gerosa MA, di Stefano E, Olivi A, et al. Multidisciplinary treatment of medulloblastoma: a 5-year experience with the SIOP trial. Childs Brain. 1981;8(2):107–118. doi: 10.1159/000119972. [DOI] [PubMed] [Google Scholar]
- 10.Fossati P, Ricardi U, Orecchia R. Pediatric medulloblastoma: toxicity of current treatment and potential role of protontherapy. Cancer Treat Rev. 2009;35(1):79–96. doi: 10.1016/j.ctrv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Endicott TJ, Fisher BJ, Wong E, et al. Pulmonary sequelae after electron spinal irradiation. Radiother Oncol. 2001;60(3):267–272. doi: 10.1016/s0167-8140(01)00380-2. [DOI] [PubMed] [Google Scholar]
- 12.Jakacki RI, Schramm CM, Donahue BR, et al. Restrictive lung disease following treatment for malignant brain tumors: a potential late effect of craniospinal irradiation. J Clin Oncol. 1995;13(6):1478–1485. doi: 10.1200/JCO.1995.13.6.1478. [DOI] [PubMed] [Google Scholar]
- 13.Huang TT, Hudson MM, Stokes DC, et al. Pulmonary outcomes in survivors of childhood cancer: a systematic review. Chest. 2011 doi: 10.1378/chest.10-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lohani S, O’Driscoll BR, Woodcock AA. 25-year study of lung fibrosis following carmustine therapy for brain tumor in childhood. Chest. 2004;126(3):1007. doi: 10.1378/chest.126.3.1007. [DOI] [PubMed] [Google Scholar]
- 15.O’Driscoll BR, Hasleton PS, Taylor PM, et al. Active lung fibrosis up to 17 years after chemotherapy with carmustine (BCNU) in childhood. N Engl J Med. 1990;323(6):378–382. doi: 10.1056/NEJM199008093230604. [DOI] [PubMed] [Google Scholar]
- 16.O’Driscoll BR, Kalra S, Gattamaneni HR, et al. Late carmustine lung fibrosis. Age at treatment may influence severity and survival. Chest. 1995;107(5):1355–1357. doi: 10.1378/chest.107.5.1355. [DOI] [PubMed] [Google Scholar]
- 17.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38(4):229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 19.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127(8 Pt 2):757–763. doi: 10.7326/0003-4819-127-8_part_2-199710151-00064. [DOI] [PubMed] [Google Scholar]
- 20.Little RRD. Statistical analysis with missing data. New York, NY: John Wiley & Sons; 2002. [Google Scholar]
- 21.Taylor JM, Munoz A, Bass SM, et al. Estimating the distribution of times from HIV seroconversion to AIDS using multiple imputation. Multicentre AIDS Cohort Study. Stat Med. 1990;9(5):505–514. doi: 10.1002/sim.4780090504. [DOI] [PubMed] [Google Scholar]
- 22.Laverdiere C, Cheung NK, Kushner BH, et al. Long-term complications in survivors of advanced stage neuroblastoma. Pediatr Blood Cancer. 2005;45(3):324–332. doi: 10.1002/pbc.20331. [DOI] [PubMed] [Google Scholar]
- 23.Mertens AC, Yasui Y, Liu Y, et al. Pulmonary complications in survivors of childhood and adolescent cancer. A report from the Childhood Cancer Survivor Study. Cancer. 2002;95(11):2431–2441. doi: 10.1002/cncr.10978. [DOI] [PubMed] [Google Scholar]
- 24.Mulder RL, Thonissen NM, van der Pal HJ, et al. Pulmonary function impairment measured by pulmonary function tests in long-term survivors of childhood cancer. Thorax. 2011;66(12):1065–1071. doi: 10.1136/thoraxjnl-2011-200618. [DOI] [PubMed] [Google Scholar]
- 25.Nysom K, Holm K, Hertz H, et al. Risk factors for reduced pulmonary function after malignant lymphoma in childhood. Med Pediatr Oncol. 1998;30(4):240–248. doi: 10.1002/(sici)1096-911x(199804)30:4<240::aid-mpo6>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.Marquis A, Strippoli MP, Spycher BD, et al. Paracetamol, nonsteroidal anti-inflammatory drugs, and risk of asthma in adult survivors of childhood cancer. J Allergy Clin Immunol. 2011;127(1):270–272. doi: 10.1016/j.jaci.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 27.Molgaard-Hansen L, Glosli H, Jahnukainen K, et al. Quality of health in survivors of childhood acute myeloid leukemia treated with chemotherapy only: a NOPHO-AML study. Pediatr Blood Cancer. 2011;57(7):1222–1229. doi: 10.1002/pbc.22931. [DOI] [PubMed] [Google Scholar]
- 28.Probert JC, Parker BR. The effects of radiation therapy on bone growth. Radiology. 1975;114(1):155–162. doi: 10.1148/114.1.155. [DOI] [PubMed] [Google Scholar]
- 29.Benoist MR, Lemerle J, Jean R, et al. Effects of pulmonary function of whole lung irradiation for Wilm’s tumour in children. Thorax. 1982;37(3):175–180. doi: 10.1136/thx.37.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Littman P, Meadows AT, Polgar G, et al. Pulmonary function in survivors of Wilm’s tumor. Patterns of impairment. Cancer. 1976;37(6):2773–2776. doi: 10.1002/1097-0142(197606)37:6<2773::aid-cncr2820370631>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Wohl ME, Griscom NT, Traggis DG, et al. Effects of therapeutic irradiation delivered in early childhood upon subsequent lung function. Pediatrics. 1975;55(4):507–516. [PubMed] [Google Scholar]
- 32.Krasin MJ, Constine LS, Friedman DL, et al. Radiation-related treatment effects across the age spectrum: differences and similarities or what the old and young can learn from each other. Semin Radiat Oncol. 2010;20(1):21–29. doi: 10.1016/j.semradonc.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marks LB, Munley MT, Bentel GC, et al. Physical and biological predictors of changes in whole-lung function following thoracic irradiation. Int J Radiat Oncol Biol Phys. 1997;39(3):563–570. doi: 10.1016/s0360-3016(97)00343-x. [DOI] [PubMed] [Google Scholar]
- 34.Oetzel D, Schraube P, Hensley F, et al. Estimation of pneumonitis risk in three-dimensional treatment planning using dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 1995;33(2):455–460. doi: 10.1016/0360-3016(95)00009-N. [DOI] [PubMed] [Google Scholar]