Abstract
We report on a patient with early onset pediatric bilateral pheochromocytomas caused by mosaic chromosome 11p15 paternal uniparental isodisomy (UPD). Hemihyperplasia of the arm was diagnosed in a 4-month-old female and clinical methylation testing for 11p15 in the blood was normal, with a reported detection threshold for mosaicism of 20%. She was subsequently diagnosed at 18 months with bilateral pheochromocytomas. Single-nucleotide polymorphism (SNP) array analysis of pheochromocytoma tissue demonstrated mosaic deletions of 8p12pter, 21q21.1qter, 22q11.23qter; commonly seen in pheochromocytomas. In addition, mosaic 11p15.3pter homozygosity was noted. Molecular testing for other causes of pheochromocytomas was normal, suggesting that 11p15 homozygosity was the primary event. Subsequent SNP array analysis of skin fibroblasts from the hyperplastic side demonstrated 5% mosaic paternal UPD for 11p15. We have subsequently used SNP array analysis to identify four patients with subtle hemihyperplasia with low-level mosaic UPD that was not detected by methylation analysis. Given the increased sensitivity of SNP array analysis to detect UPD along with the increased incidence of tumorigenesis in these UPD patients, we suggest that it has high utility in the clinical work-up of hemihyperplasia. The present case also suggests that 11p15 paternal UPD may be an under-detected mechanism of sporadic pheochromocytoma in the pediatric population. Furthermore, a review of the literature suggests that patients with 11p15 paternal UPD may present after 8 years of age with pheochromocytoma and raises the possibility that ultrasound screening could be considered beyond 8 years of age in this subset of hemihyperplasia and Beckwith–Wiedemann syndrome patients.
Keywords: hemihyperplasia, hemihypertrophy, Beckwith–Wiedemann, uniparental disomy, isodisomy, mosaicism, methylation, pheochromocytoma
INTRODUCTION
Isolated hemihyperplasia (IH; OMIM 235000), also known as isolated hemihypertrophy, is a congenital overgrowth disorder mechanistically related to Beckwith–Wiedemann syndrome (BWS; OMIM 130650) but, by definition, displays asymmetric involvement of the body. It may also manifest few of the abnormal facial or other features associated with BWS. However, like BWS, IH patients have an increased risk for embryonal tumors, primarily Wilms tumor and hepatoblastoma, which are usually diagnosed before 10 years of age [Clericuzio and Martin, 2009]. Although tumor types in IH are similar to BWS, the mean age of tumor diagnosis in a BWS patient is 15 months while the average age of IH tumor diagnosis is 36 months [DeBaun and Tucker, 1998; Lapunzina, 2005]. An increased incidence of adrenal and renal masses is also seen in IH compared to BWS [Lapunzina, 2005]. Furthermore, BWS patients with hemihyperplasia have a fourfold increased tumor risk compared to BWS patients without hemihyperplasia, emphasizing the increased risk of tumors in IH DeBaun and Tucker, 1998]. Finally, a study of 168 patients with IH showed an overall tumor risk of 6% [Hoyme et al., 1998].
To date, molecular analysis of patients with IH and BWS has not proven to fully predict tumor risk. The three most common epigenotypes found in BWS are loss of methylation of KvDMR, uniparental isodisomy (UPD) 11p15, and hypermethylation of H19DMR [Weksberg et al., 2010]. Current clinical methylation testing techniques include methylation-sensitive restriction fragment analysis (MS-RFA), methylation-sensitive quantitative polymerase chain reaction (MS-PCR), and methylation-sensitive multiplex ligation-dependent probe amplification (MS-MLPA). MS-RFA uses restriction enzyme digestion with methylation-sensitive enzymes and quantification of the products to determine the ratio of methylated DNA. MS-PCR is based on polymerase chain reaction (PCR) amplification of methylated DNA to quantify the ratio of methylated DNA. MS-MLPA relies on probe hybridization to the DNA sample, followed by ligation and digestion with methylation-sensitive enzymes, then PCR and quantification of the products to determine the ratio of methylated DNA. A methylation index based on the ratio of methylated DNA to total DNA is calculated for all three clinically available techniques.
Both IH and BWS patients with and without molecular abnormalities detected by methylation testing have developed tumors, emphasizing the limitations of molecular diagnostics in identifying patients at increased tumor risk [Clericuzio and Martin, 2009]. This inability to molecularly classify patients with increased tumor risk is even more difficult in IH than in BWS, since the minority (less than a third) of IH patients have a molecular abnormality detected in the common epigenotypes found in BWS [Grundy et al., 1991; Hertel et al., 2003; West et al., 2003; Cooper et al., 2005; Martin et al., 2005; Shuman et al., 2006]. This compares with 70% of BWS patients who have an identifiable etiology. Because of this, current molecular testing is not sufficiently sensitive to effectively characterize which IH patients have increased tumor risk [Clericuzio and Martin, 2009]. We posit that this shortcoming is due to the combination of low-level mosaicism in the blood of patients and the lack of sensitivity of current methylation-based testing. As such, tumor surveillance is recommended based largely on clinical diagnosis for high-risk IH individuals to allow early identification of tumors when treatment may be most efficacious and minimally invasive.
Pheochromocytomas in Children
Pheochromocytomas are neuroendocrine tumors that arise from the paraganglial cells of the adrenal medulla. In the general population, about 70–80% of pheochromocytomas are thought to be sporadic and 20–30% are due to familial cancer syndromes [Neumann et al., 2002; Erlic and Neumann, 2009; Mannelli et al., 2009]. However, between 40% and 59% of pheochromocytomas in children <18 years of age are due to known genetic mutations [Neumann et al., 2002; De Krijger et al., 2006], with the percentage of hereditary disease as high as 70% in children <10 years of age [Neumann et al., 2002]. Reported annual rates are 0.3 per million [Waguespack et al., 2010]. Nearly 20% of all pheochromocytomas are diagnosed during childhood with 40% of these arising bilaterally [Vicha et al., 2011]. The average age at diagnosis in these children is 11 years [Hering et al., 2006; Waguespack et al., 2010]. The youngest reported patient with a pheochromocytoma was a boy who presented at 11 months of age with a secretory tumor [Kumar et al., 2010]. Because of the high frequency of a genetic etiology including von Hippel-Lindau disease (VHL), Neurofibromatosis Type 1 (NF), and Multiple Endocrine Neoplasia Type 2 (RET)] in patients with onset prior to 20 years of age, bilateral tumors, or malignant pheochromocytoma, molecular testing is recommended in these patients [Mannelli et al., 2009].
In IH/BWS, pheochromocytoma presents as a rare tumor with only five cases reported, all of which were prior to current strategies for molecular analysis [Schnakenburg et al., 1976; Bemurat et al., 2002; van den Akker et al., 2002; Baldisserotto et al., 2005; Wilson et al., 2008].
MATERIALS AND METHODS
Clinical Presentation of Patients With IH
The patients were referred to the Genetics clinic or service at The Children’s Hospital of Philadelphia (CHOP) for evaluation of IH. Clinical methylation testing included MS-RFA, MS-PCR, or MS-MLPA. In some cases, methylation testing was sent prior to referral to our center. Single-nucleotide polymorphism (SNP) array analysis was sent on all patients to the Clinical CytoGenomics Laboratory at CHOP and processed as described below.
Arrays
DNA was purified from peripheral blood, fibroblasts cultured from skin, and tumor tissue. The quality of the DNA was monitored by analysis of OD260/OD280 and OD260/OD230 ratios. Acceptable values were between 1.8 and 2.0 and ratios >2.0, respectively. Thirty microliters of a 50 ng/μl solution of genomic DNA was aliquoted into 96-well plates and genotyped on the Illumina BeadStation. The samples were whole genome-amplified, fragmented, hybridized, fluorescently tagged, and scanned, as per standard protocols [Gunderson et al., 2005]. Array analysis was performed using IlluminaQuad610 BeadChip SNP arrays. For Quad610 analysis, a subset of probes was used for analysis that included all intensity-only probes on the Y chromosome and in the pseudoautosomal (XY) region, but excluding these probes elsewhere in the genome, for atotal of 594,906 probes. All samples had call rates >99.7% and a standard deviation of the LogR ratio across the autosomes of <0.210. The B-allele frequencies for each sample were examined for imbalance of A and B alleles as indicators of mosaicism. The percent of cells with homozygosity was calculated as previously described [Conlin et al., 2010]. We have found that strict adherence to reagent lots in generating reference data sets and development of in-house analytics has led to an improved detection of both mosaic copy number abnormalities as well as mosaic homozygosity (Conlin, unpublished data).
Histology
Tissue from bilateral pheochromocytomas and non-neoplastic adrenal gland were fixed in 10% neutral-buffered formalin, routinely processed and embedded in paraffin. Histologic sections for light microscopy were cut to a thickness of 4 μm and stained with hematoxylin and eosin (H&E). Representative sections for immunohistochemistry were treated with mouse monoclonal antibody to p57Kip2 (Ab-6, Clone57P06) (ThermoScientific, Fremont, CA), synaptophysin (Clone SY38; Dako, Carpinteria, CA), and neuronspecific enolase (NSE; Clone BBS/NC/VI-H14; Dako). Slides labeled with antibodies to p57Kip2 were scanned using the Aperio ScanScope ® CS slide scanner (Aperio Technologies, Vista, CA). The digitized slides were analyzed using the Aperio ImageScope software (version 10.0.1346.1807; Aperio Technologies) macro for determination of percent nuclear positivity optimized for immunohistochemistry.
RESULTS
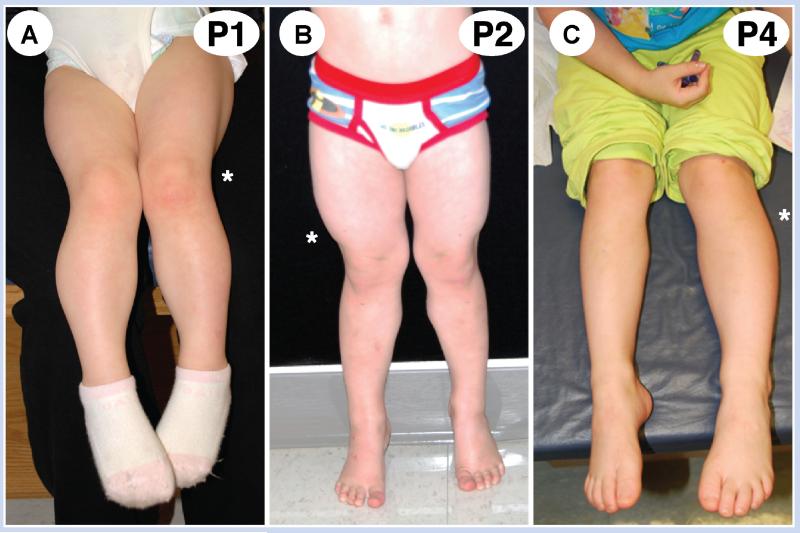
Patient 1 was diagnosed at 4 months of age with isolated hemihyperplasia, following referral for asymmetric arm size and a left elbow hemangioma. Following an unremarkable prenatal course, her birth history was notable for a birth weight of 98th centile at term. At 4 months of age, her weight was at the 93rd centile, her height was at the 40th centile, and her head circumference was at the 50th centile. She had a symmetric face with slightly upslanted palpebral fissures. She had a 4-cm deep cutaneous hemangioma on her left elbow and a cutaneous capillary vascular malformation at the nape of her neck. She had a small, reducible umbilical hernia. Her legs demonstrated a positive left-sided Galeazzi sign with the left thigh and calf palpably larger than the right (Fig. 1A). The hemihyperplasia in her upper arms showed a 7.5% larger girth in the right arm compared to the left arm. Due to her very subtle dysmorphic features, SNP array analysis (Fig. 2) in addition to MS-MLPA for methylation defects of KvDMR and H19DMR and copy number of the 11p15.5 region was performed in blood and each was normal (Table I).
FIG. 1.
Photographs of patients. A: Patient 1 legs. B: Patient 2 legs. C: Patient 4 legs. The asterisk indicates the clinically larger side.
FIG. 2.
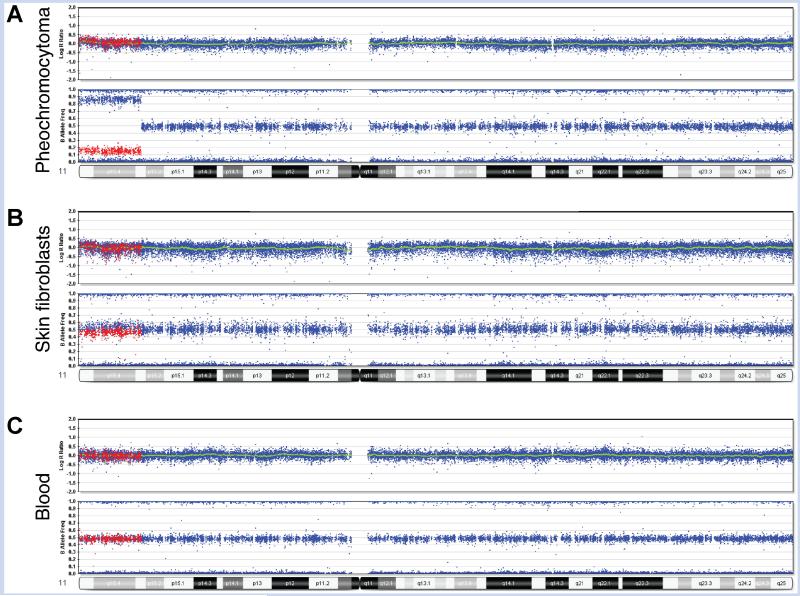
SNP array analysis data from multiple tissues of Patient 1. Illumina Human Quad 610 BeadChip results for chromosome 11. A: Pheochromocytoma demonstrates 70% mosaic isodisomy. B: Skin fibroblasts from hyperplastic leg demonstrate 5% mosaicism. C: Blood lymphocytes show no mosaicism. For illustration purposes, SNPs mosaic for heterozygosity and homozygosity of the minor allele in the pheochromocytoma are colored in red to demonstrate comparison of these same SNPs between the different tissues. In cells with normal levels of heterozygosity (peripheral blood), these red SNPs appear at the B allele frequency of 50%. In the cells with mosaic homozygosity, these red SNPs show a shift toward 0%, or an AA genotype (fibroblasts and tumor). Using this shift, the percent of cells with homozygosity was calculated as 5% in fibroblasts and 70% in tumor. In the tumor, heterozygous deletions in 8p12pter, 21q21.1qter, and 22q11.23qter were detected at lower percentages than the loss of heterozygosity (LOH) of 11p15.3pter, suggesting that the LOH was the primary event (data not shown).
TABLE I.
Summary of Testing Results of Patients With Mosaic Paternal UPD 11p15 and Hemihyperplasia in This Study
| Tissue | Methylation testing method |
Methylation Index | Methylation testing interpretation |
Level of mosaicism detected by SNP array |
|
|---|---|---|---|---|---|
| Patient 1 | Lymphocytes | MS-MLPA | ND | Normal | 0 |
| Fibroblasts | NP | N/A | N/A | 5% | |
| Pheochromocytoma | NP | N/A | N/A | 70% | |
| Patient 2 | Lymphocytes | MS-PCR | 0.63 | Normal | 8% |
| Patient 3 | Lymphocytes | MS-PCR | 0.63 | Normal | 7% |
| Patient 4 | Lymphocytes | MS-RFA | 0.67 | Isolated H19hypermethylation | 20% |
| Patient 5 | Lymphocytes | MS-RFA | ND | Normal | 0 |
| Fibroblasts | NP | N/A | N/A | 11% |
MS-MLPA, methylation-sensitive multiplex ligation-dependent probe amplification; MS-PCR, methylation-sensitive PCR; MS-RFA, methylation-sensitive restriction fragment analysis; NP, not performed; ND, not determined; N/A, not applicable.
For MS-PCR, the methylation index (MI) reported for H19DMR by the clinical laboratory had a normal range of 0.28–0.68. For MS-RFA, the MI reported for H19DMR had a normal range of <0.64.
Based on the diagnosis of IH, screening abdominal ultrasounds were initiated. At 18 months, bilateral suprarenal soft tissue masses were noted by ultrasound and confirmed by MRI to be 2.5- and 0.9- cm focal masses within the left and right adrenal glands, respectively. A complete metabolic panel, complete blood count, homovanillic acid/vanillylmandelic acid, plasma normetanephrine and metanephrine, deoxycortisol, androstenedione, dehydroepian-drosterone, 17-OH pregnenolone, 17-alpha-hydroxyprogesterone, testosterone, calcitonin, cortisol, and alpha-fetoprotein (AFP) were all within normal limits. Whole body scanning showed no evidence of MIBG avidity. The masses were resected surgically via left adrenalectomy and right adrenal mass excision with preservation of residual adrenal tissue. MRI imaging at 19 months showed no residual disease. The patient is currently 48 months old, tumor-free, undergoing abdominal ultrasounds every 3 months. She demonstrated normal adrenal stimulation testing and shows no signs of adrenal insufficiency.
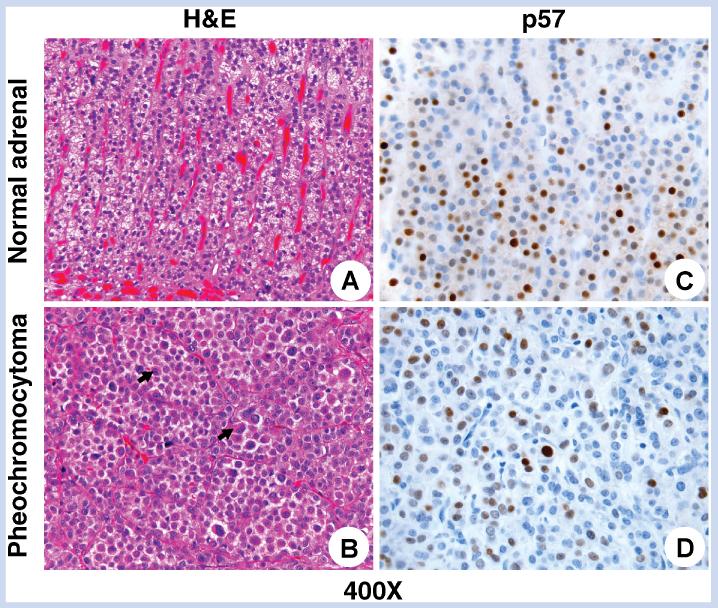
Gross pathology of the lesions demonstrated pheochromocytoma combined with adrenal cortical nodularity and focal cortical cytomegaly. Tumor histology demonstrated eccentric round nuclei, eosinophilic cytoplasm that was diffusely positive for NSE (Fig. 3), with some nodules positive for synaptophysin, all consistent with pheochromocytoma (Fig. 3). Both lesions were surrounded by thin fibrous capsules. The lesion involving the left adrenal gland demonstrated focal infiltration of tumor into the capsule but no definitive extracapsular or lymphovascular invasion was seen. No other featuresassociated with more aggressivebiologic behavior, such as necrosis or increased mitotic activity, were identified in either lesion. Pheochromocytoma of the Adrenal Gland Scaled Score (PASS), a determinant of benign versus malignant pheochromocytoma, indicated a benign lesion with a score of 1 out of 10 based only on the presence of several mitotic figures, with no other adverse features [Thompson, 2002]. Taken together, these findings lead us to conclude that these lesions arose independently. Immunostaining for CDKN1C/p57 and IGF2, looking for altered expression of epigenetically regulated genes on 11p15 was performed. CDKN1C/p57 immunostaining demonstrated a normal, variable degree of reactivity with some cells showing strongly positive, intermediate, and negative nuclear staining in both tumor and adjacent normal adrenal gland (Fig. 3). In addition, IGF2 immunostaining showed no difference between tumor and normal adrenal tissue (data not shown).
FIG. 3.
Histologic features of pheochromocytoma. Hematoxylin- and eosin-stained sections of normal architecture of adrenal cortex (A: H&E, original magnification 400 ×) in contrast to the bilateral pheochromocytomas which show a trabecular to solid arrangement of large cells with abundant eosinophilic cytoplasm (arrows) and nuclear pleomorphism including occasional pseudo-inclusions (B: H&E, original magnification 400). p57 immunostaining demonstrated a variable degree of reactivity with some cells showing strongly positive, intermediate, and negative nuclear × staining iwhich was similar in the non-neoplastic parts of the corresponding adrenal glands, particularly in the zonae fasciculata and reticularis (C: IHC, original magnification 400 ×) and within the tumor (D: IHC, original magnification 400 ×).
Given the high prevalence of germline mutations in early onset pheochromocytoma that would predispose to future disease, we screened for germline mutations in genes associated with pheochromocytomas including VHL, SDHB, SDHD, and RET, all of which were negative in blood. SNP array analysis, using an Illumina Human Quad 610 BeadChip SNP Array, in the tumor showed mosaic heterozygous deletions in 8p12pter, 21q21.1qter, 22q11.23qter, each consistent with pheochromocytoma as well as an 11.75 Mb region of homozygosity of 11p15.3pter, detected in 70% of cells. This is in contrast to the blood, which showed no detectable homozygosity for 11p. To support the model that 11p homozygosity was likely the predisposing factor for her pheochromocytoma, which would carry a very low adult cancer or recurrence risk, a skin biopsy of the larger leg was performed and subsequently demonstrated homozygosity of 11p15.3pter in 5% of cells (Fig. 2).
Prompted by the detection of low-level mosaic paternal UPD of 11p15 in this patient, we subsequently tested several additional 11p15 methylation testing-negative patients by SNP array and identified four patients with low-level mosaic 11p UPD (Fig. 1). Patient 2 was a 3-year-old boy with average birth weight who presented with a 2-cm leg length discrepancy. On examination, he had slight right-sided facial hemihyperplasia and his right calf and thigh showed 6% increased girth. Patient 3 was a 10-month-old girl with 85th centile birth weight and transient neonatal hypoglycemia. She had facial and tongue asymmetry with cutaneous capillary vascular malformations on her right thigh and nape of neck. Her right biceps, forearm, and calf all showed increased girth of 15%. Patient 4 presented initially at 9 months of age with macroglossia and leg length discrepancy. At 4 years of age, she has slight left facial fullness, a 2-cm leg length discrepancy and showed increased girth of 16% of her biceps and calves on the left. Patient 5 presented at birth with hypoglycemia, macroglossia, and hemihyperplasia. He had right-sided facial fullness and his right thigh girth was 8% larger than his left.
Testing of peripheral blood DNA by clinical methylation analysis of 11p15 demonstrated normal indices for Patients 2 (MS-PCR), 3 (MS-PCR), and 5 (MS-RFA) and Patient 4 had isolated hypermethylation of H19DMR (MS-RFA; Table I). SNP array testing of blood for Patient 2 demonstrated an 8% mosaic region of homozygosity at 11p15.5p15.3 of 13.87 Mb and for Patient 3 a 7% mosaic region of homozygosity at 11p15.5p13 of 32.71 Mb. Patient 5 had no homozygosity in blood but testing of skin fibroblasts demonstrated an 11% mosaic region of homozygosity of 46.08 Mb at 11p15.5p11.2. In contrast to her methylation results, Patient 4 had a 20% mosaic region of homozygosity of 11.49 Mb at 11p15.5p15.3 in blood.
Pheochromocytoma in IH/BWS
Here, we describe low-level mosaic paternal UPD in an 18-monthold girl resulting in IH and bilateral pheochromocytomas. Upon review of the literature, we have identified five previously reported patients with overgrowth and pheochromocytoma. Two of these had BWS, hemihyperplasia, and bilateral pheochromocytomas presenting at 6 and 20 years of age (Table II) [Bemurat et al., 2002; Baldisserotto et al., 2005]. In addition, two patients had IH and pheochromocytomas presenting at 11 and 12 years of age, without other features diagnostic of BWS (Table II) [Schnakenburg et al., 1976; van den Akker et al., 2002]. Of these, detailed molecular testing was reported for only one case and revealed a normal karyotype, no detectable mutations in RET or NF in the blood or VHL or RET in the tumor, and a normal methylation pattern of KvDMR and H19DMR [van den Akker et al., 2002]. In addition to these patients with asymmetry, one patient with BWS without hemihyperplasia was diagnosed with bilateral pheochromocytomas at 8 years of age [Wilson et al., 2008]. Molecular testing in this patient showed loss of methylation at KvDMR, hypermethylation at H19DMR, and microsatellite genotyping showed paternal UPD for chromosome 11. Further analysis using a 250 kb SNP array demonstrated high level mosaic genome-wide paternal UPD in blood [Wilson et al., 2008]. This patient had biparental inheritance in skin fibroblasts although genetic analysis of the pheochromocytomas was not reported [Wilson et al., 2008]. Four of five previously reported patients have had elevated urinary catecholamines although only two were clinically symptomatic.
TABLE II.
Clinical Features and Testing Results of Patients With Pheochromocytoma and Isolated Hemihyperplasia or Beckwith–Wiedemann Syndrome
| This report | Schnakenburg et al. [1976] | van den Akker et al. [2002] | Bemurat et al. [2002] | Baldisserotto et al. [2005] | Wilson et al. [2008] | |
|---|---|---|---|---|---|---|
| Gender | F | M | F | F | M | F |
| Birth weight | 4,347 g | 4,600 g | 1,310 g at 34 weeks | Not reported | 3,700 g | <97th |
| Clinical diagnosis | IH | IH | IH | Asymmetric BWS | Asymmetric BWS | BWS |
| Age at IH/BWS diagnosis | 4 months | Birth | Birth | Birth | Birth | Not reported |
| Hemihyperplasia (side) | Right | Right | Right | Right | Right | Not reported |
| Age at diagnosis of pheochromocytoma | 1 year, 6 months | 11 years | 12 years | 20 years | 6 years | 8 years |
| Pheochromocytoma | Bilateral | Right unilateral | Bilateral | Bilateral | Bilateral | Bilateral |
| Pheochromocytoma | MEN2A/B, VHL, RET | None | MEN2A/B (blood) negative | None | None | Not reported |
| testing | (blood) negative | VHL, RET (tumor) negative | ||||
| BWS testing | Methylation negative | None | Methylation negative | None | None | KvDMR Hypomethylation H19DMR Hypermethylation |
| 11p UPD | Yes | Unknown | Unknown | Unknown | Unknown | Yes—genome-wide |
| Other features | Umbilical hernia | Macroglossia | Hypoglycemia | Hypoglycemia | Hypoglycemia | |
| Hemangioma | Metopic ridge | Omphalocele Hepatomegaly |
Nephromegaly Umbilical hernia |
Macroglossia Umbilical hernia Hepatosplenomegaly |
F, female; M, male; BWS, Beckwith–Wiedemann syndrome; IH, isolated hemihyperplasia.
Mechanism of Pheochromocytomas in IH/BWS
Although pheochromocytomas are uncommon in BWS, alterations of chromosome 11 have been associated with malignant pheochromocytomas. In fact, loss of heterozygosity (LOH) of chromosome 11 is seen with VHL-associated pheochromocytomas, and paternal UPD at 11p15 is speculated to be an initial step in the pathogenesis of both sporadic and VHL-associated pheochromocytomas [Lui et al., 2002; Margetts et al., 2005; Hering et al., 2006; Vicha et al., 2011]. Genes on 11p hypothesized to be involved in this process include WT1, CDKN1C, IGF2, and H19. CDKN1C encodes p57, a cell cycle inhibitor/tumor suppressor gene and epigenetic dysregulation of p57 has been proposed to have a role in the pathogenesis of several embryonal tumors including hepatoblastomas and rhabdomyosarcomas [Weksberg et al., 2001; Diaz-Meyer et al., 2005; Hering et al., 2006; Algar et al., 2009]. Somewhat notably, staining for p57 in our patient’s pheochromocytomas showed a normal pattern, suggesting that it was not differentially regulated, at least at the time of tumor resection. Another potentially relevant gene in this region is IGF2, which has been shown to be overexpressed in adrenal carcinomas and pheochromocytomas [Mircescu et al., 2001; Lui et al., 2002; Hering et al., 2006]. Again, in our patient’s tumor, we did not see a difference in protein expression as assessed by immunohistochemistry.
Increased Tumor Risk in Patients With Paternal UPD 11p15
Paternal UPD 11p15 has consistently been associated with increased tumor risk in BWS and IH. In a study of 51 patients with IH screened by methylation analysis alone, 8 were identified as having a pattern consistent with mosaic paternal UPD 11p15 (with a threshold of detection of 20%) [Shuman et al., 2006]. Notably, in this cohort, patients with paternal UPD 11p15 had a tumor incidence of 50%, while the patients without paternal UPD 11p15 had a tumor incidence of 15% [Shuman et al., 2006]. It is possible that test-negative patients with tumors could also have had paternal UPD 11p15, but were below the detection threshold of that assay. Consistent with inadequate detection of UPD by methylation analysis alone, another study of 200 BWS patients screened by both MS-RFA as well as microsatellite marker-based UPD analysis, convincingly demonstrated that 25% had paternal UPD 11p15 [Cooper et al., 2005]. By 5 years of age, 24% of the paternal UPD 11p15 patients had tumors [Cooper et al., 2005], while the overall tumor rate for all patients with BWS in this meta analysis, independent of molecular findings, was 9%, suggesting that paternal UPD 11p15 is a major factor in driving tumor risk in both IH and BWS.
Increased Paternal UPD Detection in IH/BWS Can be Obtained With SNP Arrays
Based on the epigenetic etiology of IH and BWS, molecular techniques including MS-RFA, MS-PCR, and MS-MLPA have been utilized for methylation assessment in patients. The detection of both hypomethylation at KvDMR and hypermethylation at H19DMR has been used as a surrogate marker for paternal UPD 11p15. However, because of both biological and technical limitations, this approach typically can only detect methylation abnormalities in patients with >15–20% deviation from normal [Shuman et al., 2006]. Although the calculation of a methylation index based on the percentage of methylated DNA compared to the total DNA in the sample helps to quantify methylation changes and allow interpretation of results at the edge of normal based on the clinical context, it is also not definitive (as seen in Patients 2 and 3, [Table I). Because of the large number of data points sampled on a SNP array (e.g., 11363 probes on chromosome 11p in the 610K SNP array we used here), coupled with our efforts to minimize back-ground signal, we have been able to reliably detect5% mosaicism for deletions and UPD [Conlin et al., 2010]. As noted above, this led us to consider that patients with previously undetected methylation abnormalities could be assayed for low-level mosaic UPD using this SNP array analysis, as we subsequently noted in Patients 2–4 with low-level mosaic paternal UPD at 11p15 in blood previously undetected by methylation analysis. Although we have only tested skin fibroblasts in two patients, we have tested blood samples from 60 patients who presented with IH by both methylation and SNP array analysis. Of these, 12 patients had paternal UPD detected in blood and in both patients for whom skin biopsies were performed (Patients 1 and 4). Of the 14 patients with paternal UPD, methylation testing identified paternal UPD in the blood of nine patients (all with >20% mosaicism based on SNP array). The five patients where paternal UPD was not detected by methylation are Patients 1–5 presented here. The overall rate of paternal UPD detection is lower than we expected (23%) but consistent with the published molecular diagnosis rate for patients with isolated hemihyperplasia of <30% [Martin et al., 2005].
Accordingly, we feel the use of SNP array analysis affords a notable increase in the sensitivity of mosaic paternal UPD 11p15 diagnosis for patients with IH and BWS over MS-RFA, MS-PCR, or MS-MLPA analysis alone. Most importantly, this approach increases the sensitivity of detection for the subset of patients with a clear increased tumor risk. Whether the detection of low-level mosaic paternal UPD 11p15 in IH and BWS will advance the understanding of tumor risk in these patients remains to be seen, and will require concerted efforts of testing and follow-up clinical evaluation.
Screening Recommendations
These cases emphasize the longstanding recommendations for screening with full abdominal ultrasounds and AFP levels for children with both IH and BWS [Tan and Amor, 2006; Clericuzio and Martin, 2009; Weksberg et al., 2010]. However, they also raise several additional questions: (1) With more sensitive testing, can we better stratify tumor risk and obviate the need for screening some subsets of children? While there is not sufficient data at this time to make definitive conclusions, we are hopeful that testing and outcomes analysis will improve to facilitate this. (2) Since there are patients with paternal UPD 11p15 who present with pheochromocytomas at ages older than we currently screen, should abdominal ultrasounds or other screening be considered for these patients into adolescence? (3) If we are able to effectively stratify risk based on molecular basis to the point where it would be clinically useful, should skin fibroblast testing from the hyperplastic side be used as an adjunct in the diagnosis?
Given our experience, we now perform sensitive SNP array analysis with a focus on low-level mosaic paternal UPD 11p15 in the assessment of all patients with IH or BWS, even those with subtle asymmetry. While we have not altered our diagnostic approach to include routine methylation and SNP array analysis on skin biopsies from the hyperplastic side, we have become more proactive in testing samples collected at the time of tumor surgery or other unrelated procedures requiring sedation, particularly in those patients where testing has been normal in the blood. We have not yet altered our screening practice from standard guidelines[Tan and Amor, 2006; Clericuzio and Martin, 2009; Weksberg et al., 2010] regarding frequency or length of surveillance for tumors in patients with paternal UPD 11p15 as further longitudinal study of these low-level mosaic patients is needed. However, when following these patients we recommend tight adherence to screening guidelines and careful consideration of any variant screening results.
These findings reinforce and raise many questions regarding tumor risk in IH/BWS and emphasize the need for longitudinal assessment to clarify the prognostic ability of current diagnostic testing, including sensitive mosaicism analysis, in the screening guidelines.
In conclusion, we highlight the utility of SNP array analysis in clinical diagnosis and risk assessment of IH in the context of reporting the youngest patient with bilateral pheochromocytomas without a hereditary cancer predisposition syndrome. This is also the only analysis of an IH/BWS patient with pheochromocytomas using contemporary cytogenetic analysis to definitively demonstrate paternal UPD asa cause. We suggest thatSNP array analysisto detect low-level mosaic paternal UPD is a sensitive adjunct to identify and stratify patients at higher risk for malignancy. The lower level of detection afforded by SNP array analysis with our methodology can be used to detect patients with low-level mosaic pUPD 11 and further longitudinal study of these patients will help elicit their tumor risk and allow modification of screening recommendations to lead to improved clinical outcomes.
ACKNOWLEDGMENTS
We are grateful to the families for their participation in this study. This work was supported, in part, by the following NIH grants: T32GM008638 (J.M.K.) and NIH/NICHD K08HD055488 (M.A.D.).
Grant sponsor: NIH; Grant numbers: T32GM008638, NIH/NICHD K08HD055488.
Footnotes
Conflicts of interest: nothing to declare.
REFERENCES
- Algar EM, Muscat A, Dagar V, Rickert C, Chow CW, Biegel JA, Ekert PG, Saffery R, Craig J, Johnstone RW, Ashley DM. Imprinted CDKN1C is a tumor suppressor in rhabdoid tumor and activated by restoration of SMARCB1 and histone deacetylase inhibitors. PLoS ONE. 2009;4:e4482. doi: 10.1371/journal.pone.0004482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldisserotto M, Peletti AB, Angelo de Araujo M, Pertence AP, Dora MD, Maciel EO, Gaiger AM. Beckwith-Wiedemann syndrome and bilateral adrenal pheochromocytoma: Sonography and MRI findings. Pediatr Radiol. 2005;35:1132–1134. doi: 10.1007/s00247-005-1518-3. [DOI] [PubMed] [Google Scholar]
- Bemurat L, Gosse P, Ballanger P, Tauzin-Fin P, Barat P, Lacombe D, Lemetayer P, Clementy J. Successful laparoscopic operation of bilateral pheochromocytoma in a patient with Beckwith-Wiedemann syndrome. J Hum Hypertens. 2002;16:281–284. doi: 10.1038/sj.jhh.1001378. [DOI] [PubMed] [Google Scholar]
- Clericuzio CL, Martin RA. Diagnostic criteria and tumor screening for individuals with isolated hemihyperplasia. Genet Med. 2009;11:220–222. doi: 10.1097/GIM.0b013e31819436cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlin LK, Thiel BD, Bonnemann CG, Medne L, Ernst LM, Zackai EH, Deardorff MA, Krantz ID, Hakonarson H, Spinner NB. Mechanisms of mosaicism, chimerism and uniparental disomy identified by single nucleotide polymorphism array analysis. Hum Mol Genet. 2010;19:1263–1275. doi: 10.1093/hmg/ddq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper WN, Luharia A, Evans GA, Raza H, Haire AC, Grundy R, Bowdin SC, Riccio A, Sebastio G, Bliek J, Schofield PN, Reik W, Macdonald F, Maher ER. Molecular subtypes and phenotypic expression of Beckwith-Wiedemann syndrome. Eur J Hum Genet. 2005;13:1025–1032. doi: 10.1038/sj.ejhg.5201463. [DOI] [PubMed] [Google Scholar]
- De Krijger RR, Petri BJ, Van Nederveen FH, Korpershoek E, De Herder WW, De Muinck Keizer-Schrama SM, Dinjens WN. Frequent genetic changes in childhood pheochromocytomas. Ann N Y Acad Sci. 2006;1073:166–176. doi: 10.1196/annals.1353.017. [DOI] [PubMed] [Google Scholar]
- DeBaun MR, Tucker MA. Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry. J Pediatr. 1998;132:398–400. doi: 10.1016/s0022-3476(98)70008-3. [DOI] [PubMed] [Google Scholar]
- Diaz-Meyer N, Yang Y, Sait SN, Maher ER, Higgins MJ. Alternative mechanisms associated with silencing of CDKN1C in Beckwith-Wiedemann syndrome. J Med Genet. 2005;42:648–655. doi: 10.1136/jmg.2004.030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlic Z, Neumann HP. When should genetic testing be obtained in a patient with phaeochromocytoma or paraganglioma? Clin Endocrinol. 2009;70:354–357. doi: 10.1111/j.1365-2265.2008.03480.x. [DOI] [PubMed] [Google Scholar]
- Grundy P, Telzerow P, Paterson MC, Haber D, Berman B, Li F, Garber J. Chromosome 11 uniparental isodisomy predisposing to embryonal neoplasms. Lancet. 1991;338:1079–1080. doi: 10.1016/0140-6736(91)91937-p. [DOI] [PubMed] [Google Scholar]
- Gunderson KL, Steemers FJ, Lee G, Mendoza LG, Chee MS. A genome-wide scalable SNP genotyping assay using microarray technology. Nat Genet. 2005;37:549–554. doi: 10.1038/ng1547. [DOI] [PubMed] [Google Scholar]
- Hering A, Guratowska M, Bucsky P, Claussen U, Decker J, Ernst G, Hoeppner W, Michel S, Neumann H, Parlowsky T, Loncarevic I. Characteristic genomic imbalances in pediatric pheochromocytoma. Genes Chromosomes Cancer. 2006;45:602–607. doi: 10.1002/gcc.20323. [DOI] [PubMed] [Google Scholar]
- Hertel NT, Carlsen N, Kerndrup G, Pedersen IL, Clausen N, Hahnemann JM, Jacobsen BB. Late relapse of adrenocortical carcinoma in Beckwith-Wiedemann syndrome. Clinical, endocrinological and genetic aspects Acta Paediatr. 2003;92:439–443. doi: 10.1111/j.1651-2227.2003.tb00575.x. [DOI] [PubMed] [Google Scholar]
- Hoyme HE, Seaver LH, Jones KL, Procopio F, Crooks W, Feingold M. Isolated hemihyperplasia (hemihypertrophy): Report of a prospective multicenter study of the incidence of neoplasia and review. Am J Med Genet. 1998;79:274–278. [PubMed] [Google Scholar]
- Kumar M, Kumar V, Talukdar B, Mohta A, Khurana N. Cushing syndrome in an infant due to cortisol secreting adrenal pheochromocytoma: A rare association. J Pediatr Endocrinol Metab. 2010;23:621–625. doi: 10.1515/jpem.2010.102. [DOI] [PubMed] [Google Scholar]
- Lapunzina P. Risk of tumorigenesis in overgrowth syndromes: A comprehensive review. Am J Med Genet Part C. 2005;137C:53–71. doi: 10.1002/ajmg.c.30064. [DOI] [PubMed] [Google Scholar]
- Lui WO, Chen J, Glasker S, Bender BU, Madura C, Khoo SK, Kort E, Larsson C, Neumann HP, Teh BT. Selective loss of chromosome 11in pheochromocytomas associated with the VHL syndrome. Oncogene. 2002;21:1117–1122. doi: 10.1038/sj.onc.1205149. [DOI] [PubMed] [Google Scholar]
- Mannelli M, Castellano M, Schiavi F, Filetti S, Giacche M, Mori L, Pignataro V, Bernini G, Giache V, Bacca A, Biondi B, Corona G, Di Trapani G, Grossrubatscher E, Reimondo G, Arnaldi G, Giacchetti G, Veglio F, Loli P, Colao A, Ambrosio MR, Terzolo M, Letizia C, Ercolino T, Opocher G. Clinically guided genetic screening in a large cohort of Italian patients with pheochromocytomas and/or functional or nonfunctional paragangliomas. J Clin Endocrinol Metab. 2009;94:1541–1547. doi: 10.1210/jc.2008-2419. [DOI] [PubMed] [Google Scholar]
- Margetts CD, Astuti D, Gentle DC, Cooper WN, Cascon A, Catchpoole D, Robledo M, Neumann HP, Latif F, Maher ER. Epigenetic analysis of HIC1, CASP8, FLIP, TSP1, DCR1, DCR2, DR4, DR5, KvDMR1, H19 and preferential 11p15.5 maternal-allele loss in von Hippel-Lindau and sporadic phaeochromocytomas. Endocr Relat Cancer. 2005;12:161–172. doi: 10.1677/erc.1.00865. [DOI] [PubMed] [Google Scholar]
- Martin RA, Grange DK, Zehnbauer B, Debaun MR. LIT1 and H19 methylation defects in isolated hemihyperplasia. Am J Med Genet Part A. 2005;134A:129–131. doi: 10.1002/ajmg.a.30578. [DOI] [PubMed] [Google Scholar]
- Mircescu H, Wilkin F, Paquette J, Oligny LL, Decaluwe H, Gaboury L, Nolet S, Van Vliet G, Deal C. Molecular characterization of a pediatric pheochromocytoma with suspected bilateral disease. J Pediatr. 2001;138:269–273. doi: 10.1067/mpd.2001.111316. [DOI] [PubMed] [Google Scholar]
- Neumann HP, Bausch B, McWhinney SR, Bender BU, Gimm O, Franke G, Schipper J, Klisch J, Altehoefer C, Zerres K, Januszewicz A, Eng C, Smith WM, Munk R, Manz T, Glaesker S, Apel TW, Treier M, Reineke M, Walz MK, Hoang-Vu C, Brauckhoff M, Klein-Franke A, Klose P, Schmidt H, Maier-Woelfle M, Peczkowska M, Szmigielski C. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346:1459–1466. doi: 10.1056/NEJMoa020152. [DOI] [PubMed] [Google Scholar]
- Schnakenburg KV, Muller M, Dorner K, Harms D, Schwarze EW. Congenital hemihypertrophy and malignant giant pheochromocytoma —A previously undescribed coincidence. Eur J Pediatr. 1976;122:263–273. doi: 10.1007/BF00481506. [DOI] [PubMed] [Google Scholar]
- Shuman C, Smith AC, Steele L, Ray PN, Clericuzio C, Zackai E, Parisi MA, Meadows AT, Kelly T, Tichauer D, Squire JA, Sadowski P, Weksberg R. Constitutional UPD for chromosome 11p15 in individuals with isolated hemihyperplasia is associated with high tumor risk and occurs following assisted reproductive technologies. Am J Med Genet Part A. 2006;140A:1497–1503. doi: 10.1002/ajmg.a.31323. [DOI] [PubMed] [Google Scholar]
- Tan TY, Amor DJ. Tumour surveillance in Beckwith-Wiedemann syndrome and hemihyperplasia: A critical review of the evidence and suggested guidelines for local practice. J Paediatr Child Health. 2006;42:486–490. doi: 10.1111/j.1440-1754.2006.00908.x. [DOI] [PubMed] [Google Scholar]
- Thompson LD. Pheochromocytoma of the Adrenal gland Scaled Score (PASS) to separate benign from malignant neoplasms: A clinico-pathologic and immunophenotypic study of 100 cases. Am J Surg Pathol. 2002;26:551–566. doi: 10.1097/00000478-200205000-00002. [DOI] [PubMed] [Google Scholar]
- van den Akker E, de Krijger R, de Herder W, Drop S. Congenital hemihypertrophy and pheochromocytoma, not a coincidental combination? Eur J Pediatr. 2002;161:157–160. doi: 10.1007/s00431-001-0901-9. [DOI] [PubMed] [Google Scholar]
- Vicha A, Holzerova M, Krepelova A, Musil Z, Prochazka P, Sumerauer D, Kodet R, Eckschlager T, Jarosova M. Molecular cytogenetic characterization in four pediatric pheochromocytomas and paragangliomas. Pathol Oncol Res. 2011;17:801–808. doi: 10.1007/s12253-011-9385-8. [DOI] [PubMed] [Google Scholar]
- Waguespack SG, Rich T, Grubbs E, Ying AK, Perrier ND, Ayala-Ramirez M, Jimenez C. A current review of the etiology, diagnosis, and treatment of pediatric pheochromocytoma and paraganglioma. J Clin Endocrinol Metab. 2010;95:2023–2037. doi: 10.1210/jc.2009-2830. [DOI] [PubMed] [Google Scholar]
- Weksberg R, Nishikawa J, Caluseriu O, Fei YL, Shuman C, Wei C, Steele L, Cameron J, Smith A, Ambus I, Li M, Ray PN, Sadowski P, Squire J. Tumor development in the Beckwith-Wiedemann syndrome is associated with a variety of constitutional molecular 11p15 alterations including imprinting defects of KCNQ1OT1. Hum Mol Genet. 2001;10:2989–3000. doi: 10.1093/hmg/10.26.2989. [DOI] [PubMed] [Google Scholar]
- Weksberg R, Shuman C, Beckwith JB. Beckwith-Wiedemann syndrome. Eur J Hum Genet. 2010;18:8–14. doi: 10.1038/ejhg.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West PM, Love DR, Stapleton PM, Winship IM. Paternal uniparental disomy in monozygotic twins discordant for hemihypertrophy. J Med Genet. 2003;40:223–226. doi: 10.1136/jmg.40.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Peters G, Bennetts B, McGillivray G, Wu ZH, Poon C, Algar E. The clinical phenotype of mosaicism for genome-wide paternal uniparental disomy: Two new reports. Am J Med Genet Part A. 2008;146A:137–148. doi: 10.1002/ajmg.a.32172. [DOI] [PubMed] [Google Scholar]