Abstract
Background
Higher intakes of cruciferous vegetables or their constituents have been shown to lower inflammation in animal studies. However, evidence for this anti-inflammatory effect of cruciferous vegetable consumption in humans is scarce.
Objective/Design
In this cross-sectional analysis, we evaluated associations of vegetable intake with a panel of inflammatory and oxidative stress markers among 1,005 middle-aged Chinese women. Dietary intake of foods was assessed by a food frequency questionnaire.
Results
Multivariable-adjusted circulating concentrations of tumor necrosis factor-α (TNF-α), interlukin-1β (IL-1β), and IL-6 were lower among women with higher intakes of cruciferous vegetables. The differences in concentrations of inflammatory biomarkers between extreme quintiles of cruciferous vegetable intake were 12.66% for TNF-α (Ptrend=0.01), 18.18% for IL-1β (Ptrend=0.02), and 24.68% for IL-6 (Ptrend=0.02). A similar, but less apparent, inverse association was found for intakes of all vegetables combined but not for noncruciferous vegetables. Levels of the urinary oxidative stress markers F2-isoprostanes and their major metabolite, 2,3-dinor-5,6-dihydro-15-F2t-IsoP, were not associated with intakes of cruciferous vegetables or all vegetables combined.
Conclusions
This study suggests that the previously observed health benefits of cruciferous vegetable consumption may be partly associated with the anti-inflammatory effects of these vegetables.
Keywords: Cruciferous vegetables, Inflammation, Oxidative stress, Biomarker
Increasing consumption of cruciferous vegetables, including cabbage, broccoli, bok choi, brussel sprouts, kale, and cauliflower, has been recommended as a key component of a healthy diet to reduce the risk of chronic diseases such as cancer and cardiovascular disease.1–3 Similar to many plant-based foods, cruciferous vegetables are rich in antioxidant vitamins and phytochemicals. Uniquely, they are the primary dietary source of isothiocyanates and indoles.4 This class of molecules is a potent inducer of phase II enzymes5,6 and may also reduce inflammation and oxidative stress by activating transcription factor Nrf2 or through inhibition of nuclear factor-kappa B activity, modulation of toll-like receptor 4 signaling, and modification of proinflammatory cytokines.7–11 Previous research on cruciferous vegetables has focused mainly on the tumor-inhibitory effect of isothiocyanates.12 Greater intake of cruciferous vegetables or their constituents has been shown to lower inflammation in in vivo animal studies.8 Evidence for this anti-inflammatory effect of cruciferous vegetables in humans is scarce.
Recent research suggests that genetic variations may influence the health properties of cruciferous vegetables in humans.13 Individuals who are homozygous for deletion of either the GSTM1 gene or the GSTT1 gene may metabolize and eliminate isothiocyanates and other glucosinolate breakdown products at a slower rate; therefore, they may have higher exposure to these compounds after consumption of cruciferous vegetables.14 Epidemiological studies have shown that the inverse association between isothiocyanate intake and risk of certain cancers is more pronounced among GSTM1-null and/or GSTT1-null individuals.15,16 On the other hand, it has been shown that some of the genes whose expression is induced by isothiocyanates regulate defenses against inflammation and oxidative stress.7,17 Therefore, individuals with a functional GSTM1 or GSTT1 allele may benefit from consumption of cruciferous vegetables through activation of detoxifying enzymes.
Asian populations are known to habitually consume large amounts of cruciferous vegetables and other plant-based foods, which provides a unique opportunity to address hypotheses related to the potential health properties of these foods. In this study, we evaluated the association of circulating levels of inflammatory and oxidative stress markers with cruciferous vegetable intake, overall and stratified by GSTM1 and GSTT1 genotype, among 1,005 middle-aged Chinese women. To compare the effects of cruciferous vegetable intake with intakes of other plant-based foods, we also evaluated associations of inflammatory markers with intakes of fruit and noncruciferous vegetables.
METHODS
Study Participants
This cross-sectional analysis was conducted among 1,005 women selected from among participants of the Shanghai Women’s Health Study (SWHS),16 an ongoing, population-based cohort study.18 Baseline questionnaire data and bio-specimens were used. Assays for inflammatory and oxidative stress markers were conducted as part of an ancillary study on colorectal cancer. All study participants were cancer-free at baseline. The SWHS was approved by the institutional review boards for human research at the Shanghai Cancer Institute in China and at Vanderbilt University and the National Cancer Institute in the United States. Written informed consent was obtained from all participants. The design and methods of the SWHS have been described in detail elsewhere.19 Briefly, between 1997 and 2000, 74,941 women aged 40 to 70 years from seven urban communities of Shanghai were recruited and completed the baseline survey (participation rate=92.7%). Trained interviewers conducted baseline surveys and collected anthropometric measurements at participants’ homes. Structured questionnaires were used to obtain information on demographics, diet and other lifestyle habits, medical history, and other characteristics.
Biospecimen Collection
At study enrollment, 76% of SWHS cohort members (n=56,832) donated blood and urine samples. An additional 12% of cohort members donated a urine sample during the first follow-up survey (approximately 2 years later). After collection, samples were kept at 0° to 4°C and processed within 6 hours. Immediately after processing, all samples were stored at −70° C until laboratory analyses were conducted.
Dietary Intake Assessment
Usual dietary intake during the 12 months before the interview was assessed using a comprehensive, quantitative, food-frequency questionnaire (FFQ).20 The FFQ captured 86% of the foods consumed by the study population. Five cruciferous vegetables commonly consumed in this population were listed as separate items in the questionnaire, including Chinese greens (bok choi), green cabbage, Chinese cabbage (napa), cauliflower, and white turnip/radish. During the in-person interview, each participant was first asked how often, on average, during the previous year she had consumed a specific food or food group (the possible responses were daily, weekly, monthly, yearly, or never), followed by a question on the amount consumed in liang (1 liang=50 g) per unit of time. The validity and reproducibility of the FFQ for assessing usual dietary intake have been described elsewhere.20 Overall, the estimates of food intakes derived from the FFQ and from multiple 24-hour dietary recalls correlated reasonably well. The correlation coefficients for total fruit and vegetable intakes were 0.55 and 0.41, respectively.
Inflammatory Marker Measurement
Millipore’s MILLIPLEX MAP High Sensitivity Human Cytokine multiplex kits were used to measure plasma concentrations of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and IL-6. Using the manufacturer’s instructions, these assays were conducted at the Hormone Assay & Analytical Services Core at Vanderbilt University. Plasma samples and standards were assayed in duplicate. High-sensitivity C-reactive protein (CRP) measurements were done using the ACE High Sensitivity C-Reactive Protein Reagent (ACI-22) for the first batch and the CRP (HS) Wide Range kit (Pointe Scientific) for the second batch on an ACE Clinical Chemistry System (Alfa Wassermann, Inc). We adjusted for batch in all analyses. The limits of detection were as follows: TNF-α, 0.05 pg/mL; IL-1β, 0.06 pg/mL; IL-6, 0.10 pg/mL; and CRP, 0.1 mg/L. Intra-assay coefficients of variation for the plasma inflammatory markers studied were less than 17.4%; inter-assay coefficients of variation were less than 21%.21
Oxidative Stress Marker Measurement
Oxidative stress markers F2-isoprostanes (F2-IsoP) and their major metabolite 2,3-dinor-5,6-dihydro-15-F2t-IsoP (F2-IsoP-M) in urine were measured by using gas chromatography/negative ion chemical ionization mass spectrometry (GC/NICI MS) at the Vanderbilt Eicosanoid Core Laboratory. Details of this method have been reported elsewhere.22–24 Both F2-IsoP and F2-IsoP-M concentrations were expressed as ng/mg creatinine. The lower limit of sensitivity was approximately 5 pg. The precision of the assay was ±6% and its accuracy was 96%.23
GSTM1 and GSTT1 Genotyping
Methods used for the GSTM1 and GSTT1 genotyping are described in detail in Figure 1 (available online at www.andjrnl.org). Briefly, after DNA was extracted from baseline blood samples, copy number for the GSTM1 and GSTT1 genes was determined by our lab by using a duplex real-time quantitative polymerase chain reaction–based assay, according to the method described by the National Cancer Institute’s SNP500 Cancer project with modifications.15 The sequences used in the assay design were obtained from GenBank (GSTM1, NM_000561 and GSTT1, NM_000853). The concordance rate for quality control samples, including water, Coriell DNA, and blinded DNA samples, was 100%.
Figure 1.
GSTM1 and GSTT1 genotyping method.
Statistical Analysis
Participants with less than the detectable limits of inflammatory and oxidative stress markers were excluded from corresponding analyses (TNF-α, n=4; IL-1β, n=102; IL-6, n=71; CRP, n=90; F2-IsoP, n=0; and F2-IsoP-M, n=0), as were those with outliers according to a box plot (F2-IsoP, n=2and F2-IsoP-M, n=2). Log-transformation was conducted to normalize the distribution of the markers studied. Geometric means of these markers were obtained based on the least square means estimated using a general linear regression model according to quintiles of intake of vegetables and fruits after adjusting for potential confounding factors. Tests for linear trend were done by entering categorical variables as continuous variables in the linear regression model. A restricted cubic spline linear regression model was also used to evaluate the association of inflammatory biomarkers with cruciferous vegetable intake on a continuous basis and to account for possible nonlinear effects.25 Knots were placed at the 5th, 50th, and 95th percentiles of the distribution of these measures. We excluded participants whose cruciferous vegetable intakes were more than the 99.5th percentile from the spline model to minimize the influence of outliers.
Potential confounders adjusted for in multivariable models included age; education; occupation; cigarette smoking; alcohol consumption; menopausal status; body mass index (BMI); Charlson comorbidity score26; history or presence of other infectious or inflammation-related diseases that were not included in the Charlson comorbidity score (hypertension, tuberculosis, gastritis, chronic pancreatitis); regular use of aspirin and other nonsteroidal anti-inflammatory drugs (NSAIDs); regular use of vitamin supplements; and dietary intakes of total energy, total fruits, noncruciferous vegetables, and red meat. We also adjusted for assay batch and use of antibiotics, vitamin supplements, and NSAIDs in the 24 hours before sample collection.
In sensitivity analyses, participants with less than the detectable limits of markers were included to check whether nondetection differed by intake of cruciferous vegetables. To reduce the potential influence of acute inflammation on the study results, we excluded participants with CRP more than 10 mg/L and participants reporting use of antibiotics or NSAIDs in the 24 hours before sample collection.27 Effect modification was evaluated in analyses stratified by GST genotype and health conditions (Figure 2, available online at www.andjrnl.org). Multi-collinearity was evaluated with the variance inflation factor (VIF).28 The VIF for the variables under study was approximately 1 except for age (VIF=3.68) and menopausal status (VIF=3.26). All statistical tests were two-sided and were done using Statistical Analysis Software (version 9.2, 2010, SAS Institute, Inc).
Figure 2.
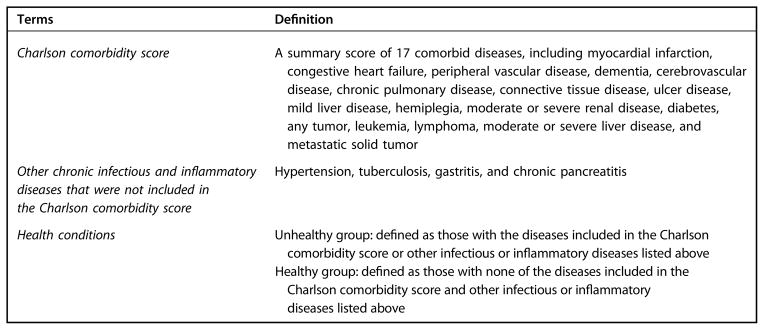
Definition of terms used in the cross-sectional analysis evaluating associations of vegetable intake with a panel of inflammatory and oxidative stress markers among 1,005 middle-aged Chinese women.
RESULTS
Characteristics of Study Participants
In this study of 1,005 women, the mean age (±standard deviation) was 58.1 (±8.8) years. The median intake of cruciferous vegetables was 82.9 g/day (interquartile range, 48.9 to 138.1). Characteristics of study participants by quintiles of cruciferous vegetable intake are shown in Table 1. Women with higher intakes of cruciferous vegetables were likely to have higher intakes of fruits and noncruciferous vegetables and to be more physically active. Cruciferous vegetable intake was not associated with age, BMI, cigarette smoking, NSAID use, vitamin supplement use, or history of infectious or inflammatory diseases.
Table 1.
Age-adjusted characteristics of study participants according to cruciferous vegetable intake (the Shanghai Women’s Health Studya)
| Quintiles of Cruciferous Vegetable Intake (g/day)
|
P trendb | |||||
|---|---|---|---|---|---|---|
| ≤ 42.5 (n=201) | 42.6–68.4 (n=201) | 68.5–98.8 (n=201) | 98.9–140.5 (n=201) | >140.6 (n=201) | ||
| mean±standard deviation | ||||||
| Age (y) | 57.4 ±9.0 | 58.1±8.5 | 58.8±8.7 | 57.4±8.7 | 58.7±9.0 | 0.29 |
| % | ||||||
| Education, ≥high school | 33.4 | 33.3 | 32.7 | 30.8 | 31.5 | 0.96 |
| Occupation, manual laborer | 62.6 | 59.7 | 57.2 | 56.3 | 51.7 | 0.15 |
| Cigarette smoker | 5.6 | 3.1 | 2.9 | 3.2 | 3.6 | 0.57 |
| Regular alcohol consumption | 3.7 | 1.6 | 4.1 | 3.1 | 2.3 | 0.59 |
| Postmenopausal | 72.5 | 72.5 | 76.5 | 75.3 | 77.3 | 0.13 |
| History of infectious/inflammatory disease | 55.8 | 61.7 | 58.1 | 59.4 | 66.1 | 0.25 |
| Aspirin and other nonsteroidal anti-inflammatory drug use | 2.6 | 2.2 | 4.9 | 2.0 | 5.4 | 0.17 |
| Multivitamin supplement use | 17.4 | 17.7 | 17.1 | 20.9 | 21.1 | 0.64 |
| Season of interview | ||||||
| Spring | 21.4 | 26.3 | 21.9 | 23.7 | 19.8 | 0.47 |
| Summer | 33.3 | 26.4 | 26.1 | 26.5 | 23.3 | |
| Fall | 22.5 | 22.6 | 20.0 | 21.1 | 24.9 | |
| Winter | 22.8 | 24.9 | 32.1 | 28.7 | 32.0 | |
| mean±standard error | ||||||
| Body mass index | 24.5±0.2 | 24.9±0.2 | 24.8 ±0.2 | 24.4±0.2 | 24.6±0.2 | 0.75 |
| Charlson comorbidity score | 0.38±0.05 | 0.25±0.05 | 0.32±0.05 | 0.23±0.05 | 0.30±0.05 | 0.23 |
| Physical activity (METc hour/week) | 4.4±0.8 | 6.3±0.8 | 6.9±0.8 | 7.8±0.8 | 8.8±0.8 | <0.0001 |
| Dietary intake | ||||||
| Total calories (kcal/d) | 1,619.0±28.5 | 1,664.5±28.5 | 1,703.7±28.5 | 1,728.3±28.5 | 1,601.4±28.5 | 0.75 |
| All fruits combined (g/d) | 184.7±11.2 | 245.3±11.2 | 236.1±11.2 | 249.2±11.2 | 272.9±11.2 | <0.0001 |
| All vegetables combined (g/d) | 150.5±8.2 | 217.5±8.2 | 265.3±8.2 | 324.8±8.2 | 463.7±8.2 | <0.0001 |
| Noncruciferous vegetables (g/d) | 123.7±7.3 | 161.1±7.3 | 182.5±7.3 | 206.0±7.3 | 259.3±7.3 | <0.0001 |
| Red meats (g/d) | 42.2±2.0 | 42.2±2.0 | 53.7±2.0 | 47.4±2.0 | 43.6±2.0 | 0.21 |
Except for the mean age, data were standardized to the age distribution.
Generalized linear models were used for continuous variables, and the Cochran-Mantel-Haenszel χ2 test was used for categorical variables.
MET=metabolic equivalent.
Association of Cruciferous Vegetable Intake with Markers of Inflammation and Oxidative Stress
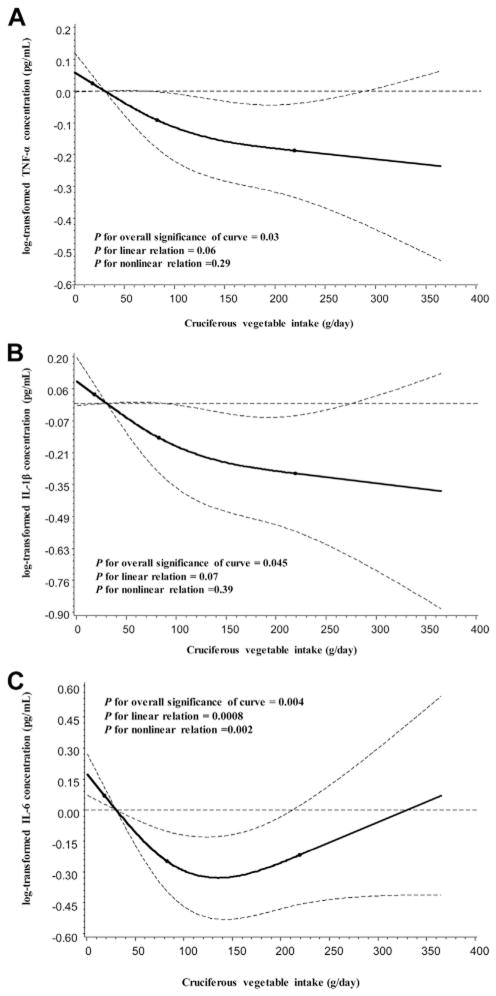
After adjustment for age and assay batch, higher intakes of cruciferous vegetables were inversely associated with concentrations of TNF-α (Ptrend=0.001), IL-1β (Ptrend=0.004), and IL-6 (Ptrend=0.02) (data not shown). Further adjustment for BMI, Charlson comorbidity score, history of other chronic infections or inflammatory disease, NSAID and supplemental vitamin use, intakes of noncruciferous vegetables, fruits and total energy intake, and other lifestyle factors did not appreciably alter the results (Table 2). The differences in multivariable-adjusted mean concentrations of biomarkers between extreme quintiles of cruciferous vegetable intake were 12.66% for TNF-α, 18.18% for IL-1β, and 24.68% for IL-6. Results were essentially unchanged when cruciferous vegetable intake was analyzed on a continuous basis in a restricted cubic spline linear regression model (P for overall significance of 0.03 for TNF-α and 0.045 for IL-1β; P for linearity of 0.06 for TNF-α and 0.07 for IL-1β; Figure 3, panels A and B). An L-shaped association between cruciferous vegetable intake and IL-6 levels was suggested (P for overall significance of 0.004; P for nonlinearity of 0.002; Figure 3, panel C). A similar, but less apparent, inverse association was found for intake of all vegetables combined but not for noncruciferous vegetables. Fruit intake was inversely correlated with levels of circulating IL-6, but the association was not statistically significant. Except for an inverse association between F2-IsoP-M and fruit intake (Ptrend=0.03), no statistically significant associations were observed between oxidative stress markers and intakes of total, cruciferous, or noncruciferous vegetables.
Table 2.
Multivariable-adjusted concentrations of biomarkers according to quintiles of intake for all vegetables combined, cruciferous vegetables, noncruciferous vegetables, and all fruits combined (the Shanghai Women’s Health Studyab)
| Quintiles of Dietary Intakec | By All Vegetables Combined
|
By Cruciferous Vegetables
|
By Noncruciferous Vegetables
|
By All Fruits Combined | ||||
|---|---|---|---|---|---|---|---|---|
| Geometric mean±SEEd | Difference (%)e | Geometric mean±SEEd | Difference (%)e | Geometric mean±SEEd | Difference (%)e | Geometric mean±SEEd | Difference (%)e | |
| TNF-αf (pg/mL) | ||||||||
| Q1 | 6.40±1.05 | Reference | 6.40±1.05 | Reference | 5.74±1.05 | Reference | 5.92±1.05 | Reference |
| Q2 | 6.44±1.05 | 0.63 | 6.21±1.05 | −2.97 | 6.29±1.05 | 9.58 | 6.28±1.05 | 6.08 |
| Q3 | 5.62±1.05 | −12.19 | 6.22±1.05 | −2.81 | 5.66±1.05 | −1.39 | 5.81±1.05 | −1.86 |
| Q4 | 5.57±1.05 | −12.97g | 5.24±1.05 | −18.12g | 5.97±1.05 | 4.01 | 5.98±1.05 | 1.01 |
| Q5 | 5.61±1.05 | −12.34g | 5.59±1.05 | −12.66 | 5.94±1.05 | 3.48 | 5.61±1.05 | −5.24g |
| Ptrend | 0.02 | 0.01 | 0.92 | 0.38 | ||||
| IL-1βh (pg/mL) | ||||||||
| Q1 | 1.32±1.09 | Reference | 1.43±1.09 | Reference | 1.17±1.09 | Reference | 1.17±1.09 | Reference |
| Q2 | 1.51±1.09 | 14.39 | 1.40±1.09 | −2.10 | 1.40±1.09 | 19.66 | 1.35±1.09 | 15.38 |
| Q3 | 1.17±1.09 | −11.36 | 1.19±1.09 | −16.78 | 1.21±1.09 | 3.42 | 1.27±1.09 | 8.55 |
| Q4 | 1.12±1.09 | −15.15 | 1.10±1.09 | −23.08g | 1.21±1.09 | 3.42 | 1.35±1.09 | 15.38 |
| Q5 | 1.16±1.09 | −12.12 | 1.17±1.10 | −18.18 | 1.28±1.09 | 9.40 | 1.14±1.09 | 2.56 |
| Ptrend | 0.048 | 0.02 | 0.93 | 0.88 | ||||
| IL-6 (pg/mL) | ||||||||
| Q1 | 4.07±1.09 | Reference | 4.74±1.09 | Reference | 3.64±1.09 | Reference | 4.05±1.09 | Reference |
| Q2 | 3.63±1.09 | ±10.81 | 3.80±1.08 | −19.83 | 3.57±1.09 | −1.92 | 4.08±1.09 | 0.74 |
| Q3 | 3.46±1.09 | −14.99 | 3.31±1.08 | −30.17 | 3.76±1.09 | 3.30 | 3.64±1.09 | −10.12 |
| Q4 | 3.68±1.09 | −9.58 | 3.43±1.09 | −27.64g | 3.63±1.09 | −0.27 | 3.53±1.09 | −12.84 |
| Q5 | 3.89±1.09 | −4.42 | 3.57±1.09 | −24.68g | 4.15±1.09 | 14.01 | 3.45±1.09 | −14.81 |
| Ptrend | 0.79 | 0.02 | 0.35 | 0.10 | ||||
| CRPi (mg/L) | ||||||||
| Q1 | 1.26±1.09 | Reference | 1.37±1.09 | Reference | 1.08±1.09 | Reference | 1.23±1.09 | Reference |
| Q2 | 1.21±1.09 | −3.97 | 1.07±1.09 | −21.90g | 1.29±1.09 | 19.44 | 1.18±1.09 | −4.07 |
| Q3 | 1.12±1.09 | −11.11 | 1.24±1.09 | −9.49 | 1.28±1.09 | 18.52 | 1.21±1.09 | −1.63 |
| Q4 | 1.33±1.09 | 5.56 | 1.13±1.09 | −17.52 | 1.14±1.09 | 5.56 | 1.22±1.09 | −0.81 |
| Q5 | 1.26±1.09 | 0.00 | 1.18±1.09 | −13.87 | 1.20±1.09 | 11.11 | 1.12±1.09 | −8.94g |
| Ptrend | 0.88 | 0.41 | 0.77 | 0.60 | ||||
| F2-IsoPj (ng/mg creatinine) | ||||||||
| Q1 | 1.51±1.04 | Reference | 1.54±1.04 | Reference | 1.47±1.04 | Reference | 1.57±1.04 | Reference |
| Q2 | 1.61±1.04 | 6.62 | 1.56±1.04 | 1.30 | 1.63±1.04 | 10.88 | 1.51±1.04 | −3.82 |
| Q3 | 1.52±1.04 | 0.66 | 1.48±1.04 | −3.90 | 1.50±1.04 | 2.04 | 1.56±1.04 | −0.64 |
| Q4 | 1.51±1.04 | 0.00 | 1.51±1.04 | −1.95 | 1.57±1.04 | 6.80 | 1.51±1.04 | −3.82 |
| Q5 | 1.58±1.04 | 4.64 | 1.65±1.04 | 7.14 | 1.58±1.04 | 7.48 | 1.58±1.04 | 0.64 |
| Ptrend | 0.77 | 0.45 | 0.45 | 0.91 | ||||
| F2-IsoP-Mk (ng/mg creatinine) | ||||||||
| Q1 | 0.57±1.04 | Reference | 0.55±1.04 | Reference | 0.61±1.04 | Reference | 0.62±1.04 | Reference |
| Q2 | 0.63±1.04 | 10.53 | 0.61±1.04 | 10.91 | 0.61±1.04 | 0.00 | 0.58±1.04 | −6.45 |
| Q3 | 0.57±1.04 | 0.00 | 0.56±1.04 | 1.82 | 0.57±1.04 | −6.56 | 0.59±1.04 | −4.84 |
| Q4 | 0.59±1.04 | 3.51 | 0.57±1.04 | 3.64 | 0.58±1.04 | −4.92 | 0.55±1.04 | −11.29g |
| Q5 | 0.56±1.04 | −1.75 | 0.62±1.04 | 12.73g | 0.55±1.04 | −9.84 | 0.56±1.04 | −9.68g |
| Ptrend | 0.44 | 0.22 | 0.06 | 0.03 | ||||
Number of participants included in the analysis: 998 for TNF-α, 900 for IL-1β, 931 for IL-6, 791 for CRP, 904 for F2-IsoPs and 876 for F2-IsoP-M.
Adjusted for age; education; occupation; cigarette smoking; alcohol consumption; physical activity; body mass index; menopausal status; vitamin supplement use; nonsteroidal anti-inflammatory drug (NSAID) use; total intake of all fruits combined (in the model for all vegetables combined), noncruciferous vegetables (in the model for cruciferous vegetables), cruciferous vegetables (in the model for noncruciferous vegetables), red meat, and total energy; Charlson comorbidity score and history or presence of other infectious/inflammatory diseases; use of antibiotics, vitamin supplements, or NSAIDs in the 24 hours before sample collection; and assay batch using general linear regression models.
The quintile distribution of dietary intakes was used to categorize participants into five groups. Quintile cutoffs for intake of all vegetables combined were 160.1, 222.5, 291.2, and 386.2 g/d; quintile cutoffs for cruciferous vegetable intake were 42.5, 68.4, 98.8, and 140.5 g/d; quintile cutoffs for noncruciferous vegetable intake were 97.3, 140.0, 186.7 and 265.1 g/d; quintile cutoffs for intake of all fruits combined were 99.3, 175.0, 249.1, and 360.1 g/d.
SEE=standard error of estimation.
Difference (%)=(geometric mean of the biomarker in each higher quintile of cruciferous vegetable intake−geometric mean in the lowest quintile)/geometric mean in the lowest quintile.
TNF-α=tumor necrosis factor-α.
Compared with Q1 (the lowest quintile), P<0.05.
IL-1β=interleukin-1β.
CRP=C-reactive protein.
F2-IsoP=F2-isoprostanes.
F2-IsoP-M=2,3-dinor-5,6-dihydro-15-F2t-IsoP.
Figure 3.
Smoothed plot of logarithmically transformed concentrations of tumor necrosis factor-α (TNF-α) (A), interleukin-1β (IL-β) (B), and IL-6 (C) according to cruciferous vegetable intake. The median value of the biomarkers for participants in the first quintile of cruciferous vegetable intake was treated as the reference point. The differences in biomarker concentrations relative to the reference were estimated by restricted cubic-spline linear regression models after adjustment for potential confounding factors (see footnotes to Table 2). Point estimates are indicated by the solid line and 95% CIs by the dashed lines. The P for overall significance of the curve was 0.03 for TNF-α, 0.045 for IL-1β, and 0.004 for IL-6. The P for linear relation was 0.06 for TNF-α, 0.07 for IL-1β, and 0.0008 for IL-6. The P for nonlinear relation was 0.29 for TNF-α, 0.39 for IL-1β, and 0.002 for IL-6.
In sensitivity analyses, we included participants with less than the detectable limits of markers by assigning them a value one half of the detectable limit; this did not markedly change the association of cruciferous vegetable intake with concentrations of TNF-α (Ptrend=0.01), IL-1β (Ptrend=0.02), or IL-6 (Ptrend=0.02). Similar inverse associations were also found for TNF-α (Ptrend=0.03), IL-1β (Ptrend=0.01), and IL-6 (Ptrend=0.03) when we excluded participants with CRP more than 10 mg/L (n=29, 2.9%) to reduce the potential influence of acute infection on measures of these biomarkers. Moreover, cruciferous vegetable intake was still significantly correlated with TNF-α (Ptrend=0.03), IL-1β (Ptrend=0.02), and IL-6 (Ptrend=0.02) after further exclusion of participants who had used antibiotics or NSAIDs in the 24 hours before sample collection.
We further evaluated whether the cruciferous vegetable and inflammation association differed by health conditions (with or without diseases included in the Charlson comorbidity score and other infectious/inflammatory diseases). The inverse associations between cruciferous vegetable intake and levels of TNF-α, IL-6, and IL-1β seemed to be more pronounced in the “healthy” group than in the “unhealthy” group (Table 3, available online at www.andjrnl.org). The P for heterogeneity between the two groups was of borderline significance for IL-1β (P=0.045) and IL-6 (P=0.07).
Table 3.
Multivariable-adjusted concentrations of biomarkers in women according to quintiles of cruciferous vegetable intake stratified by health conditions, the Shanghai Women’s Health Studya
| By quintles of cruciferous vegetable intakec | Unhealthy Womenb (n=566)
|
Healthy Womenb (n=439)
|
||
|---|---|---|---|---|
| Geometric mean±SEd | Difference (%)e | Geometric mean±SEEd | Difference (%)e | |
| TNF-αf (pg/mL) | ||||
| Q1 | 6.14±1.08 | Reference | 6.56±1.08 | Reference |
| Q2 | 6.20±1.07 | 0.98 | 6.32±1.08 | −3.66 |
| Q3 | 6.82±1.07 | 11.07 | 5.48±1.08 | −16.46 |
| Q4 | 5.47±1.07 | −10.91 | 4.98±1.08 | −24.09g |
| Q5 | 5.87±1.07 | −4.40 | 5.33±1.09 | −18.75 |
| Ptrend | 0.38 | 0.01 | ||
| Pheterogeneity | 0.32 | |||
| IL-1βh (pg/mL) | ||||
| Q1 | 1.24±1.13 | Reference | 1.70±1.13 | Reference |
| Q2 | 1.30±1.12 | 4.84 | 1.56±1.13 | −8.24 |
| Q3 | 1.26±1.13 | 1.61 | 1.11±1.13 | −34.71g |
| Q4 | 1.13±1.13 | −8.87 | 1.06±1.12 | −37.65g |
| Q5 | 1.22±1.13 | −1.61 | 1.08±1.15 | −36.47g |
| Ptrend | 0.67 | 0.002 | ||
| Pheterogeneity | 0.045 | |||
| IL-6 (pg/mL) | ||||
| Q1 | 4.21±1.13 | Reference | 5.45±1.13 | Reference |
| Q2 | 3.58±1.12 | −14.96 | 4.11±1.13 | −24.59 |
| Q3 | 3.59±1.12 | −14.72 | 3.03±1.12 | −44.40g |
| Q4 | 3.40±1.13 | −19.24 | 3.52±1.12 | −35.41g |
| Q5 | 3.64±1.12 | −13.54 | 3.38±1.15 | −37.98 |
| Ptrend | 0.40 | 0.01 | ||
| Pheterogeneity | 0.07 | |||
Adjusted for age; education; occupation; cigarette smoking; alcohol consumption; physical activity; body mass index; menopausal status; vitamin supplement use; nonsteroidal anti-inflammatory drug (NSAID) use; intakes of all fruits combined, noncruciferous vegetables, red meat, and total energy; use of antibiotics, vitamin supplements or NSAIDs in the 24 hours before sample collection; and assay batch using general linear regression models.
Definitions presented in Figure 2.
Quintile distribution of cruciferous vegetable intake was used to categorize participants into five groups. Quintile cutoffs for cruciferous vegetable intake were 42.5, 68.4, 98.8, and 140.5 g/day.
SEE=standard error of estimation.
Difference (%)=(geometric mean of the biomarker in each higher quintile of cruciferous vegetable intake –geometric mean in the lowest quintile)/geometric mean in the lowest quintile.
TNF-α=tumor necrosis factor-α.
Compared with group Q1 (the lowest quintile), P<0.05.
IL-1β=interleukin-1β.
Analyses Stratified by GSTM1 and GSTT1 Genotype
The GSTM1 and GSTT1 genotypes conformed to Hardy-Weinberg equilibrium. For both genes, the proportion of individuals with the null genotype was comparable to other Asian populations.29 Because only a small number of women carried two copies of the GSTM1 or GSTT1 gene and because there was no significant allelic dosage effect (one vs two copies) for either gene on circulating levels of the biomarkers studied (data not shown), women with one or two copies of these genes were combined into a single group for each gene (GSTT1 present and GSTM1 present). Neither GSTM1 nor GSTT1 genotype distribution was associated with cruciferous vegetable intake (data not shown). With the exception of IL-6, the observed inverse association between cruciferous vegetable intake and levels of TNF-α and IL-1β seemed to be stronger among women with the GSTT1-present or GSTM1-present genotype compared with the null genotypes (Table 4), although tests for interaction were not statistically significant.
Table 4.
Multivariable-adjusted concentrations of biomarkers according to quintiles of cruciferous vegetable intake stratified by GST genotype (the Shanghai Women’s Health Studya)
| Quintiles of Cruciferous Vegetable Intakeb |
GSTT1 Genotype
|
GSTM1 Genotype
|
||||||
|---|---|---|---|---|---|---|---|---|
| Null (n =508)
|
Present (n=494)
|
Null (n =597)
|
Present (n=407)
|
|||||
| Geometric mean±SEEc | Difference (%)d | Geometric mean±SEEc | Difference (%)d | Geometric mean±SEEc | Difference (%)d | Geometric mean±SEEc | Difference (%)d | |
| TNF-αe (pg/mL) | ||||||||
| Q1 | 6.38±1.08 | Reference | 6.63±1.08 | Reference | 6.71±1.08 | Reference | 5.99±1.08 | Reference |
| Q2 | 6.15±1.07 | −3.61 | 6.22±1.08 | −6.18 | 5.91±1.07 | −11.92 | 6.86±1.08 | 14.52 |
| Q3 | 6.22±1.07 | −2.51 | 6.25±1.08 | −5.73 | 6.58±1.07 | −1.94 | 5.87±1.08 | −2.00 |
| Q4 | 5.53±1.08 | −13.32 | 4.99±1.08 | −24.74f | 5.04±1.07 | −24.89f | 5.66±1.09 | −5.51 |
| Q5 | 5.43±1.08 | −14.89 | 5.61±1.08 | −15.38 | 6.09±1.07 | −9.24 | 4.86±1.09 | −18.86 |
| Ptrend | 0.09 | 0.02 | 0.13 | 0.03 | ||||
| Pinteraction | 0.50 | 0.79 | ||||||
| IL-1βg (pg/mL) | ||||||||
| Q1 | 1.57±1.15 | Reference | 1.41±1.13 | Reference | 1.48±1.14 | Reference | 1.38±1.14 | Reference |
| Q2 | 1.28±1.13 | −18.47 | 1.52±1.12 | 7.80 | 1.39±1.12 | −6.08 | 1.45±1.14 | 5.07 |
| Q3 | 1.14±1.13 | −27.39 | 1.23±1.13 | −12.77 | 1.21±1.13 | −18.24 | 1.16±1.13 | −15.94 |
| Q4 | 1.26±1.13 | −19.75 | 0.93±1.13 | −34.04f | 1.04±1.12 | −29.73 | 1.18±1.16 | −14.49 |
| Q5 | 1.26±1.14 | −19.75 | 1.05±1.13 | −25.53 | 1.33±1.13 | −10.14 | 0.98±1.15 | −28.99 |
| Ptrend | 0.33 | 0.01 | 0.20 | 0.05 | ||||
| Pinteraction | 0.17 | 0.63 | ||||||
| IL-6 (pg/mL) | ||||||||
| Q1 | 4.87±1.14 | Reference | 4.76±1.24 | Reference | 5.29±1.13 | Reference | 4.04±1.14 | Reference |
| Q2 | 3.76±1.12 | −22.79 | 3.93±1.12 | −17.44 | 3.68±1.11 | −30.43 | 4.12±1.14 | 1.98 |
| Q3 | 3.38±1.12 | −30.60 | 3.19±1.13 | −32.98f | 3.97±1.12 | −24.95 | 2.66±1.13 | −34.16 |
| Q4 | 3.80±1.12 | −21.97 | 3.00±1.12 | −36.97f | 3.38±1.11 | −36.11f | 3.54±1.16 | −12.38 |
| Q5 | 3.32±1.13 | −31.83 | 3.78±1.13 | −20.59 | 3.50±1.12 | −33.84 | 3.71±1.15 | −8.17 |
| Ptrend | 0.08 | 0.06 | 0.02 | 0.38 | ||||
| Pinteraction | 0.82 | 0.48 | ||||||
Adjusted for age; education; occupation; cigarette smoking; alcohol consumption; physical activity; body mass index; menopausal status; vitamin supplement use; nonsteroidal anti-inflammatory drug (NSAID) use; intakes of all fruits combined, noncruciferous vegetables, red meat, and total energy; Charlson comorbidity score; history or presence of other infectious/inflammatory diseases; use of antibiotics, vitamin supplements, or NSAIDs in the 24 hours before sample collection; and assay batch using general linear regression models.
The quintile distribution of cruciferous vegetable intake was used to categorize participants into five groups. Quintile cutoffs for cruciferous vegetable intake were 42.5, 68.4, 98.8, and 140.5 g/d.
SEE=standard error of estimation.
Difference (%)=(geometric mean of the biomarker in each higher quintile of cruciferous vegetable intake–geometric mean in the lowest quintile)/geometric mean in the lowest quintile.
TNF-α=tumor necrosis factor-α.
Compared with Q1 (the lowest quintile), P<0.05.
IL-1β=interleukin-1β.
DISCUSSION
In this study of 1,005 middle-aged Chinese women, we found that higher intake of cruciferous vegetables was associated with significantly lower circulating concentrations of the proinflammatory markers TNF-α, IL-1β, and IL-6, after accounting for a wide range of potential confounding variables, including socioeconomic status, dietary and nondietary lifestyle factors, BMI, health conditions, and medication use. The inverse association was more pronounced among healthy women without a prior history of infectious or inflammatory disease. We also found a similar, but less apparent, inverse association with intakes of all vegetables combined but not with noncruciferous vegetables.
Fruits and vegetables are rich in antioxidant vitamins and various phytochemicals and may affect health through anti-inflammatory activity.30,31 There is growing evidence that certain groups of fruits and vegetables, such as cruciferous vegetables, may be particularly beneficial.32–34 We recently reported that consumption of fruits and vegetables, particularly cruciferous vegetables, was associated with lower mortality in two prospective cohorts of 134,796 Chinese adults.2 Individuals with the highest intake of cruciferous vegetables had 22% lower total mortality and 31% lower cardiovascular disease mortality compared with the group with the lowest intake.2 The present study’s finding of an inverse association between cruciferous vegetable intake and circulating concentrations of inflammatory markers provides clues to the potential underlying biological mechanisms behind the health benefits of cruciferae.
After stratifying by health conditions, we found that the inverse association between cruciferous vegetable intake and inflammatory cytokines seemed more evident among healthy women without chronic inflammatory diseases than among women with these diseases. It is possible that the association between cruciferous vegetable consumption and inflammation status among unhealthy people may be masked by the severity and heterogeneity of chronic inflammation-related diseases, as these factors were not accounted for in our study. In addition, people with health conditions may have altered their lifestyle, including changing their dietary habits, or may have been taking medication for their conditions, all of which could dilute the association between cruciferous vegetable consumption and inflammation.
Many previous epidemiological studies have failed to find a link between higher intakes of fruits and vegetables and lower levels of oxidative stress,35,36 nor have recent clinical trials supported the notion that diets rich in antioxidants reduce endogenous oxidative stress.37,38 In the present study, we used a mass spectrometry–based method to measure urinary F2-IsoPs and their major metabolite (F2-IsoP-M), both of which are now well-accepted as accurate and reliable biomarkers of oxidative stress in vivo.39 In line with previous studies,35,36 none of these markers were associated with intake of total or cruciferous vegetables. It has been speculated that in vivo oxidative balance is conservatively controlled in humans, and dietary intake of antioxidants may not have a significant influence on oxidative stress in well-nourished individuals, such as the women in our study population.
Several reports have suggested that the health benefits of cruciferous vegetables may be modified by genetic variations in the GST enzymes that are involved in the metabolism and elimination of isothiocyanates and other glucosinolate breakdown products of cruciferae.16,40–43 In this study, we found that the inverse association between cruciferous vegetable intake and inflammation seemed to be stronger among women carrying the GSTT1 or GSTM1 genes. This finding is supported by a recent study that cruciferous vegetable consumption was associated with lower risk of myocardial infarction only among individuals with a functional GSTT1*1 allele.44 However, the present finding is in contrast to our previous report that the inverse association between cruciferous vegetable intake and colorectal cancer risk was observed only in the GSTM1-null group.16 Possible explanations include that the up-regulation of phase II enzymes such as GSTs by isothiocyanates and other constituents of cruciferae may play an important role in the modulation of systemic inflammation or that GSTM1 and GSTT1 may not be the major determinants of the bioavailability of the inflammation-lowering constituents of cruciferae.
Our study population is well-suited to the investigation of the association between cruciferous vegetable consumption and inflammation, because of its high, yet diverse, levels of intake. The availability of detailed information on a wide range of factors related to inflammation and oxidative stress allowed us to control for these potential confounding factors. However, this study had some methodological limitations that should be considered in the interpretation of our results. The study was cross-sectional in design; thus, no causal relationships can be inferred. Measurement error in the assessment of dietary intakes or biomarkers is another concern, although the SWHS FFQ has been previously evaluated to have reasonably good validity for the measurement of usual intakes of fruits and vegetables by comparing the estimates of food intake derived from the FFQ and from multiple 24-hour dietary recalls.20 An objective measurement, such as repeated doubly labeled water assessments for total energy expenditure, should be considered in future research. It is unlikely that intra- or inter-assay variations in the measurement of plasma inflammatory markers would significantly differ by vegetable intake, suggesting that the assay-related variations would attenuate the true association between inflammatory cytokines and cruciferous vegetable intake. In addition, we could not completely rule out the possibility of residual confounding due to unmeasured or inadequately measured covariates.
In summary, this study suggests that higher intake of cruciferous vegetables is significantly associated with lower circulating levels of inflammatory markers in women. Further investigation into the specific constituents of cruciferae that are responsible for reducing systemic inflammation and their health effects is clearly warranted.
Acknowledgments
FUNDING/SUPPORT
This study was supported by Public Health Service grants from the US National Cancer Institute, including grant number R01CA122364 (to G. Yang) for the inflammation and oxidative stress biomarker assays, R37 CA070867 (to W. Zheng) for the baseline data collection and genotyping, and a contract, NO2-CP-11010-66 (to X.-O. Shu) for biospecimen collection. Y. Jiang was supported by a training grant from the Fogarty International Center (D43 TW008313 to X.-O. Shu). G. L. Milne acknowledges support from the Vanderbilt University Center in Molecular Toxicology (NIH P30 ES000267).
The authors thank the participants and research staff of the Shanghai Women’s Health Study for their contributions to the study. We thank Bethanie Rammer for her assistance in editing the manuscript. We also thank Regina Courtney and Rodica Cal-Chris for sample preparation.
Footnotes
Supplementary materials:
Figure 1, Figure 2, and Table 3 available at www.andjrnl.org
STATEMENT OF POTENTIAL CONFLICT OF INTEREST
No potential conflict of interest was reported by the authors.
Contributor Information
Yu Jiang, Division of Chronic Disease Control and Prevention, Changning Center for Disease Control and Prevention, Shanghai, China, and a research fellow trainee, Division of Epidemiology, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN.
Sheng-Hui Wu, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN.
Xiao-Ou Shu, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN.
Yong-Bing Xiang, Shanghai Cancer Institute, Shanghai, China.
Bu-Tian Ji, Division of Cancer Epidemiology and Genetics, National Cancer Institute, National Institutes of Health, Department of Health and Human Services, Bethesda, MD.
Ginger L. Milne, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN.
Qiuyin Cai, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN.
Xianglan Zhang, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN.
Yu-Tang Gao, Shanghai Cancer Institute, Shanghai, China.
Wei Zheng, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN.
Gong Yang, Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN.
References
- 1.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: A review of the epidemiological evidence. Nutr Cancer. 1992;18(1):1–29. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Shu XO, Xiang YB, et al. Cruciferous vegetable consumption is associated with a reduced risk of total and cardiovascular disease mortality. Am J Clin Nutr. 2011;94(1):240–246. doi: 10.3945/ajcn.110.009340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Cancer Research Fund, American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: AICR; 2007. [Google Scholar]
- 4.Fenwick GR, Heaney RK, Mullin WJ. Glucosinolates and their breakdown products in food and food plants. Crit Rev Food Sci Nutr. 1983;18(2):123–201. doi: 10.1080/10408398209527361. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Talalay P. Anticarcinogenic activities of organic isothiocyanates: Chemistry and mechanisms. Cancer Res. 1994;54(7 suppl):S1976–S1981. [PubMed] [Google Scholar]
- 6.Fahey JW, Zhang Y, Talalay P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc Natl Acad Sci U S A. 1997;94(19):10367–10372. doi: 10.1073/pnas.94.19.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mi L, Hood BL, Stewart NA, et al. Identification of potential protein targets of isothiocyanates by proteomics. Chem Res Toxicol. 2011;24(10):1735–1743. doi: 10.1021/tx2002806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Youn HS, Kim YS, Park ZY, et al. Sulforaphane suppresses oligomerization of TLR4 in a thiol-dependent manner. J Immunol. 2010;184(1):411–419. doi: 10.4049/jimmunol.0803988. [DOI] [PubMed] [Google Scholar]
- 9.Xue M, Qian Q, Adaikalakoteswari A, Rabbani N, Babaei-Jadidi R, Thornalley PJ. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes. 2008;57(10):2809–2817. doi: 10.2337/db06-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zakkar M, Van der Heiden K, Luong LA, et al. Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler Thromb Vasc Biol. 2009;29(11):1851–1857. doi: 10.1161/ATVBAHA.109.193375. [DOI] [PubMed] [Google Scholar]
- 11.Brown KK, Blaikie FH, Smith RA, et al. Direct modification of the proinflammatory cytokine macrophage migration inhibitory factor by dietary isothiocyanates. J Biol Chem. 2009;284(47):32425–32433. doi: 10.1074/jbc.M109.047092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navarro SL, Li F, Lampe JW. Mechanisms of action of isothiocyanates in cancer chemoprevention: An update. Food Function. 2011;2(10):579–587. doi: 10.1039/c1fo10114e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lampe JW, Peterson S. Brassica, biotransformation and cancer risk: Genetic polymorphisms alter the preventive effects of cruciferous vegetables. J Nutr. 2002;132(10):2991–2994. doi: 10.1093/jn/131.10.2991. [DOI] [PubMed] [Google Scholar]
- 14.Lam TK, Gallicchio L, Lindsley K, et al. Cruciferous vegetable consumption and lung cancer risk: A systematic review. Cancer Epidemiol Biomarkers Prev. 2009;18(1):184–195. doi: 10.1158/1055-9965.EPI-08-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moore LE, Huang WY, Chatterjee N, et al. GSTM1, GSTT1, and GSTP1 polymorphisms and risk of advanced colorectal adenoma. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1823–1827. doi: 10.1158/1055-9965.EPI-05-0037. [DOI] [PubMed] [Google Scholar]
- 16.Yang G, Gao YT, Shu XO, et al. Isothiocyanate exposure, glutathione S-transferase polymorphisms, and colorectal cancer risk. Am J Clin Nutr. 2010;91(3):704–711. doi: 10.3945/ajcn.2009.28683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu L, Noyan AM, Facci M, et al. Dietary approach to attenuate oxidative stress, hypertension, and inflammation in the cardiovascular system. Proc Natl Acad Sci U S A. 2004;101(18):7094–7099. doi: 10.1073/pnas.0402004101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu SH, Shu XO, Chow WH, et al. Soy food intake and circulating levels of inflammatory markers in Chinese women. J Acad Nutr Diet. 2012;112(7):996–1004. doi: 10.1016/j.jand.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng W, Chow WH, Yang G, et al. The Shanghai Women’s Health Study: Rationale, study design, and baseline characteristics. Am J Epidemiol. 2005;162(11):1123–1131. doi: 10.1093/aje/kwi322. [DOI] [PubMed] [Google Scholar]
- 20.Shu XO, Yang G, Jin F, et al. Validity and reproducibility of the food frequency questionnaire used in the Shanghai Women’s Health Study. Eur J Clin Nutr. 2004;58(1):17–23. doi: 10.1038/sj.ejcn.1601738. [DOI] [PubMed] [Google Scholar]
- 21.Lee SA, Kallianpur A, Xiang YB, et al. Intra-individual variation of plasma adipokine levels and utility of single measurement of these biomarkers in population-based studies. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2464–2470. doi: 10.1158/1055-9965.EPI-07-0374. [DOI] [PubMed] [Google Scholar]
- 22.Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LN. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci U S A. 1990;87(23):9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milne GL, Sanchez SC, Musiek ES, Morrow JD. Quantification of F2-isoprostanes as a biomarker of oxidative stress. Nat Protoc. 2007;2(1):221–226. doi: 10.1038/nprot.2006.375. [DOI] [PubMed] [Google Scholar]
- 24.Milne GL, Yin H, Brooks JD, Sanchez S, Jackson RLN, Morrow JD. Quantification of F2-isoprostanes in biological fluids and tissues as a measure of oxidant stress. Methods Enzymol. 2007;433:113–126. doi: 10.1016/S0076-6879(07)33006-1. [DOI] [PubMed] [Google Scholar]
- 25.Harrell FJ, Shih YC. Using full probability models to compute probabilities of actual interest to decision makers. Int J Technol Assess Health Care. 2001;17(1):17–26. doi: 10.1017/s0266462301104034. [DOI] [PubMed] [Google Scholar]
- 26.D’Hoore W, Bouckaert A, Tilquin C. Practical considerations on the use of the Charlson comorbidity index with administrative data bases. J Clin Epidemiol. 1996;49(12):1429–1433. doi: 10.1016/s0895-4356(96)00271-5. [DOI] [PubMed] [Google Scholar]
- 27.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 28.Belsley DA, Kuh E, Welsch RE. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. New York, NY: Wiley; 1980. [Google Scholar]
- 29.Cotton SC, Sharp L, Little J, Brockton N. Glutathione S-transferase polymorphisms and colorectal cancer: A HuGE review. Am J Epidemiol. 2000;151(1):7–32. doi: 10.1093/oxfordjournals.aje.a010124. [DOI] [PubMed] [Google Scholar]
- 30.Holt EM, Steffen LM, Moran A, et al. Fruit and vegetable consumption and its relation to markers of inflammation and oxidative stress in adolescents. J Am Diet Assoc. 2009;109(3):414–421. doi: 10.1016/j.jada.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esfahani A, Wong JM, Truan J, et al. Health effects of mixed fruit and vegetable concentrates: A systematic review of the clinical interventions. J Am Coll Nutr. 2011;30(5):285–294. doi: 10.1080/07315724.2011.10719971. [DOI] [PubMed] [Google Scholar]
- 32.Jayakumar T, Chen WF, Lu WJ, et al. A novel antithrombotic effect of sulforaphane via activation of platelet adenylatecyclase: Ex vivo and in vivo studies. J Nutr Biochem. 2013;24(6):1086–1095. doi: 10.1016/j.jnutbio.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Tsai JT, Liu HC, Chen YH. Suppression of inflammatory mediators by cruciferous vegetable-derived indole-3-carbinol and phenyl-ethylisothiocyanate in lipopolysaccharide-activated macrophages. Mediators Inflamm. 2010;293642:1–5. doi: 10.1155/2010/293642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiku AB, Abraham SK, Kale RK. Protective effect of the cruciferous vegetable mustard leaf (Brassica campestris) against in vivo chromosomal damage and oxidative stress induced by gamma-radiation and genotoxic chemicals. Environ Mol Mutagen. 2008;49(5):335–342. doi: 10.1002/em.20383. [DOI] [PubMed] [Google Scholar]
- 35.van den Berg R, van Vliet T, Broekmans WM, et al. A vegetable/fruit concentrate with high antioxidant capacity has no effect on biomarkers of antioxidant status in male smokers. J Nutr. 2001;131(6):1714–1722. doi: 10.1093/jn/131.6.1714. [DOI] [PubMed] [Google Scholar]
- 36.Paterson E, Gordon MH, Niwat C, et al. Supplementation with fruit and vegetable soups and beverages increases plasma carotenoid concentrations but does not alter markers of oxidative stress or cardiovascular risk factors. J Nutr. 2006;136(11):2849–2855. doi: 10.1093/jn/136.11.2849. [DOI] [PubMed] [Google Scholar]
- 37.Hopkins MH, Fedirko V, Jones DP, Terry PD, Bostick RM. Antioxidant micronutrients and biomarkers of oxidative stress and inflammation in colorectal adenoma patients: Results from a randomized, controlled clinical trial. Cancer Epidemiol Biomarkers Prev. 2010;19(3):850–858. doi: 10.1158/1055-9965.EPI-09-1052. [DOI] [PubMed] [Google Scholar]
- 38.Braga M, Bissolati M, Rocchetti S, Beneduce A, Pecorelli N, Di Carlo V. Oral preoperative antioxidants in pancreatic surgery: A double-blind, randomized, clinical trial. Nutrition. 2012;28(2):160–164. doi: 10.1016/j.nut.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28(4):505–513. doi: 10.1016/s0891-5849(99)00264-6. [DOI] [PubMed] [Google Scholar]
- 40.London SJ, Yuan JM, Chung FL, et al. Isothiocyanates, glutathione S-transferase M1 and T1 polymorphisms, and lung-cancer risk: A prospective study of men in Shanghai, China. Lancet. 2000;356(9231):724–729. doi: 10.1016/S0140-6736(00)02631-3. [DOI] [PubMed] [Google Scholar]
- 41.Seow A, Yuan JM, Sun CL, Van Den Berg D, Lee HP, Yu MC. Dietary isothiocyanates, glutathione S-transferase polymorphisms and colorectal cancer risk in the Singapore Chinese Health Study. Carcinogenesis. 2002;23(12):2055–2061. doi: 10.1093/carcin/23.12.2055. [DOI] [PubMed] [Google Scholar]
- 42.Lin HJ, Probst-Hensch NM, Louie AD, et al. Glutathione transferase null genotype, broccoli, and lower prevalence of colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 1998;7(8):647–652. [PubMed] [Google Scholar]
- 43.Tijhuis MJ, Wark PA, Aarts JM, et al. GSTP1 and GSTA1 polymorphisms interact with cruciferous vegetable intake in colorectal adenoma risk. Cancer Epidemiol Biomarkers Prev. 2005;14(12):2943–2951. doi: 10.1158/1055-9965.EPI-05-0591. [DOI] [PubMed] [Google Scholar]
- 44.Cornelis MC, El-Sohemy A, Campos H. GSTT1 genotype modifies the association between cruciferous vegetable intake and the risk of myocardial infarction. Am J Clin Nutr. 2007;86:752–758. doi: 10.1093/ajcn/86.3.752. [DOI] [PubMed] [Google Scholar]