Abstract
Background
Inflammation has been hypothesized to play an important etiological role in the initiation or progression of prostate cancer. Circulating levels of the systemic inflammation marker C-reactive protein (CRP) have been associated with increased risk of prostate cancer. We investigated the role of genetic variation in CRP and prostate cancer, under the hypothesis that variants may alter risk of disease.
Methods
We undertook a case-control study nested within the prospective Physicians' Health Study among 1,286 men with incident prostate cancer and 1,264 controls. Four single-nucleotide polymorphisms (SNPs) were selected to capture the common genetic variation across CRP (r2>0.8). We used unconditional logistic regression to assess the association between each SNP and risk of prostate cancer. Linear regression models explored associations between each genotype and plasma CRP levels.
Results
None of the CRP SNPs were associated with prostate cancer overall. Individuals with one copy of the minor allele (C) in rs1800947 had an increased risk of high-grade prostate cancer (OR: 1.7; 95% CI: 1.1–2.8), and significantly lower mean CRP levels (p-value <0.001), however, we found no significant association with lethal disease. Mean CRP levels were significantly elevated in men with one or two copies of the minor allele in rs3093075 and rs1417939, but these were unrelated to prostate cancer risk.
Conclusion
Our findings suggest that SNPs in the CRP gene are not associated with risk of overall or lethal prostate cancer. Polymorphisms in CRP rs1800947 may be associated with higher grade disease, but our results require replication in other cohorts.
Keywords: CRP SNPs, prostate cancer, inflammation
Introduction
Several lines of evidence point to an infectious or inflammatory etiology as a possible mechanism for the initiation or progression of prostate cancer(1). Inflammatory infiltrates are commonly found near prostate cancer precursor lesions and tumor cells(2). In addition, several factors associated with chronic inflammation, including obesity, infectious agents, hormonal variations/exposures, and dietary factors, have been associated with the risk or progression of prostate cancer(3).
C-reactive protein (CRP) is a sensitive marker of nonspecific systemic inflammation. It is produced by hepatocytes and transcriptionally regulated primarily by IL-6(4). Circulating levels of CRP have been associated with an increased risk of several cancer outcomes(5,6); other studies have found no association with prostate cancer(7,8). In the Physicians' Health Study (PHS), we found that elevated CRP measured prior to cancer diagnosis was associated with an increased risk of total prostate cancer in healthy weight men and there was a suggestive trend of increasing risk with high grade disease(9). Among patients with metastatic prostate cancer, elevated plasma CRP levels were independent predictors of poor disease-specific survival(10). In tumor tissue, cytoplasmic CRP expression was positively associated with metastases at diagnosis and nuclear CRP presence was associated with increased metastases at relapse(11).
The genetic variation in immune and inflammatory pathways offers another potentially important linkage with prostate cancer pathogenesis and progression. Most previous studies have found no association between CRP single-nucleotide polymorphisms (SNPs) and overall risk of prostate cancer(12–14). A recent study found that compared to men with 2 copies of the major allele, men with at least 1 copy of the minor allele (C) in CRP rs1800947 had a lower risk of recurrence (OR = 0.53, 95% CI: 0.36–0.79)(15). Other studies have shown that genetic variation in the CRP gene and adiposity measures such as waist-hip ratio (WHR) and BMI interact to modify the risk of endometrial, colon and rectal cancer(16,17). Few studies have evaluated the association between CRP and prostate cancer for more aggressive disease or evaluated whether adiposity modifies this association.
The role of CRP SNPs in relation to prostate cancer risk and circulating levels of CRP has not been fully elucidated. The aims of the present study were to investigate whether common variants in CRP are associated with prostate cancer risk and circulating levels of CRP; to determine whether variants in CRP were more important for the development of aggressive tumors; and to assess the role of adiposity in modifying these associations.
Materials and Methods
Study Population
We undertook a case-control study nested within the prospective Physicians' Health Study I (PHS). As described in detail elsewhere, PHS I began in 1982 as a randomized, double-blind, placebo-controlled trial of aspirin and beta-carotene for the primary prevention of cardiovascular disease and cancer(18,19). The trial included 22,071 U.S. male physicians aged 40–84 years at randomization and free of cardiovascular disease and cancer at randomization(18,19). The study population was selected from 14,916 PHS participants who provided a blood sample at baseline (pre-randomization)(19).
Our analytic cohort included 1,286 incident and histologically confirmed prostate cancer cases diagnosed between 1982 to 2005. In a nested case-control design, cases were matched to 1,264 controls on age at randomization (within 1 year for cases ≤ 55 years old and within 5 years for cases > 55 years), follow-up time, and smoking status (never, former, current). Although PHS is largely Caucasian (over 93%), we restricted this analysis to Caucasian men to decrease the likelihood of population stratification.
Each participant provided written informed consent, and The Physicians' Health Study receives ongoing approval from the Institutional Review Board at Partners Health Care.
Outcome Ascertainment
Incident prostate cancer cases were first identified via self-report and confirmed by review of hospital medical records and pathology reports. Data on tumor stage and metastases were also abstracted from medical records. Stage was classified by the TNM staging system from pathology and clinical reports. Gleason score was obtained from original pathology records or from histopathological re-review of tumor specimens from biopsy or radical prostatectomy. Data on mortality (overall and prostate cancer specific) was available through March 20, 2012 and cause of death was determined by an Endpoints Committee of physicians through review of death certificates, medical records, and information from families. High-grade prostate cancer was defined as tumors with total Gleason scores 8–10. Advanced stage disease was defined as stage T3/T4 or N1 or M1 at cancer diagnosis and lethal was defined as death from prostate cancer or development of bony or organ metastases. Follow-up information on mortality is over 99% complete and follow-up for nonfatal outcomes is over 97% complete.
Exposure Ascertainment
Genotyping and circulating CRP measures were undertaken on prediagnostic blood samples collected from August 1982 to December 1984, prior to randomization. The physician participants returned their drawn blood tubes to the laboratory via overnight mail on chill packs within 24 hours of being drawn. Once received the samples were aliquotted into separate tubes of whole blood and plasma and frozen for long-term storage. The median time between blood collection and cancer diagnosis among cases was 12.5 years (range: 0.2–21.9 years).
Genotype
Four SNPs were selected across the CRP gene using the `Tagger' program in the HapMap Release 27 CEPH panel to capture the common (minor allele frequency >5%) genetic variation across the 2.3 kb gene and 5 kb upstream and downstream, with the coefficient of determination, r2, >0.8. Genotyping was conducted blinded to case/control status using Sequenom GOLD iPlex panels at the Harvard Medical School-Partners Healthcare Center for Genetics and Genomics. Genotyping of blind duplicate samples across genotyping batches was conducted for quality control, with concordance greater than 98% for duplicate samples.
Biomarker
Circulating levels of inflammatory biomarkers were measured from prospectively collected plasma stored in the laboratory of Dr. Nader Rifai (Boston, MA). CRP levels were measured using a high-sensitivity assay (Dade Behring). IL-6 and TNFR2 levels were determined by a commercially available enzyme-linked immunosorbent assay test (R and D Systems)(20). The median coefficients of variation (CV) for CRP, IL-6 and TNFR2 were 0.5%, 5.3%, and 6.8%, respectively(9,20). All laboratory personnel were blinded from case/control status. Case/control pairs were assayed in adjoining wells, and 10% of samples were randomly distributed across the plates as pooled quality controls.
Statistical Analysis
Each CRP SNP was evaluated for deviation from Hardy-Weinberg Equilibrium (HWE) in controls using an exact test with 10,000 permutations, setting p<0.05 to indicate deviation from HWE.
To evaluate the association between CRP SNPs and overall prostate cancer, we conducted unconditional logistic regression controlling for the matching factors (age at randomization, follow-up time, and smoking status), conducting the analysis separately for each SNP. Odds ratios (OR) and 95% confidence intervals (CI) were calculated modeling each genotype as an ordinal or dichotomous variable with homozygous common genotype as the reference group. We used dominant models for those SNPs with rare homozygotes (<5% of individuals homozygous for the variant allele), combining rare homozygous and heterozygous individuals into one category; co-dominant models were used for SNPs with ≥5% of individuals homozygous for the minor allele. Statistical significance was determined using a likelihood ratio test (LRT) with 1 d.f. for dominant models and 2 d.f. for co-dominant models. To evaluate whether CRP SNPs were associated with the development of aggressive prostate cancer, we conducted analyses modeling several different outcomes of interest, including high grade disease (defined as Gleason 8–10), advanced stage (defined as stage T3/T4 or N1 or M1 at cancer diagnosis), lethal (defined as death from prostate cancer or development of bony or organ metastases), and a combination of advanced stage or lethal disease.
We also assessed the association between CRP SNPs and survival after prostate cancer diagnosis among the cases. We followed the men from date of prostate cancer diagnosis until death from prostate cancer or progression to bony or organ metastases, or until censoring (death from other causes or end of follow-up, March 20, 2012). We conducted Cox proportional hazards models and calculated hazard ratios adjusted for age at diagnosis, separately modeling each CRP SNP as the exposure of interest.
We also evaluated whether there was a differential effect of BMI on the association between each SNP and prostate cancer. Because there were few men with BMI ≥30 kg/m2, we combined `overweight' (BMI 25–30 kg/m2) and `obese' (BMI ≥30 kg/m2) men and compared them to `normal' (BMI <25 kg/m2) weight men. We evaluated interaction terms and conducted stratified analyses using each of the outcomes defined above. We also conducted each analysis stratified by PSA-era, which was defined as cases diagnosed before (pre-PSA) and after (post-PSA) January 1, 1992.
For a subset of the men (n=1,171) we had CRP SNP data and circulating levels of CRP. We excluded men with greater than a six day delay in returning the blood sample to our laboratory (n=24), and cases diagnosed less than 5 years after the blood draw (n=70) to reduce the likelihood of pre-clinical disease affecting CRP levels; however, results were similar when these men were included (data not shown). We conducted linear regression analysis separately modeling log10-transformed CRP as the outcome of interest and each CRP SNP as the ordinal or dichotomous exposure of interest, adjusting for dichotomous BMI, age at randomization, and smoking status. We conducted the same analyses stratified by BMI, adjusting for age at randomization and smoking.
We tested the global association across the 4 SNPs in the CRP gene using a kernel machine method. This approach groups SNPs together into `SNP-sets' based on biological significance (i.e. genes) and allows one to perform tests for SNP-sets rather than evaluating SNPs individually(21). The logistic kernel machine test was used to test the global association of the CRP gene with each of the prostate cancer outcomes listed above; we conducted a linear kernel machine test the global association of the CRP gene with CRP levels.
We then created a `genetic score' combining the CRP SNPs to determine if having multiple variant alleles was associated with increased CRP levels. The r2 between each tag SNP pair was <0.30. Any SNP not significantly associated with mean CRP levels was excluded from the score. Alleles associated with higher mean CRP received a score of 1 and those associated with lower mean CRP received a score of 0; each allele score within each SNP was summed. The SNP scores were then summed together into a `genetic score' and evaluated as the exposure of interest, with log10-transformed plasma CRP and prostate cancer (overall, high grade, advanced stage, and lethal) as the outcomes of interest.
Finally, using linear regression analysis and adjusting for dichotomous BMI, age at randomization, and smoking status, we assessed whether circulating levels of log10-transformed IL-6, TNFR2, or baseline PSA levels were associated with any of the four CRP SNPs, the genetic score, or circulating levels of CRP.
All analyses were conducted using SAS v.9.2 (SAS Institute, Cary, NC) and all p-values were 2-tailed with statistical significance at α < 0.05.
Results
The study population consisted of 1,286 incident histologically confirmed prostate cancer cases and 1,264 controls. The distribution of BMI was similar between cases and controls, as was the mean plasma CRP level at baseline (Table 1). There were 142 cases with advanced stage and 172 cases with high grade disease. Of the 618 cases who died, 30% (n=183) died from prostate cancer; 25 additional men developed bony or organ metastases over follow-up. 70% of the cases (n=899) were identified during the PSA era.
Table 1.
Characteristics of study population, Physicians' Health Study (1982–2012)
| Cases (n= 1,286) | Controls (n=1,264) | |
|---|---|---|
|
| ||
| Age at enrollment, years | ||
| Mean (± SD) | 57.9 (8.4) | 57.5 (8.4) |
|
| ||
| Follow-up time, years | ||
| Mean (± SD) | 12.2 (5.1) | 12.1 (5.0) |
|
| ||
| Smoking Status, N (%) | ||
| Never | 625 (49%) | 634 (50%) |
| Past | 553 (43%) | 531 (42%) |
| Current | 106 (8%) | 99 (8%) |
|
| ||
| BMI, N (%) | ||
| < 25 kg/m2 | 746 (58%) | 759 (60%) |
| 25 to < 30 kg/m2 | 499 (39%) | 465 (37%) |
| ≥ 30 kg/m2 | 41 (3%) | 40 (3%) |
|
| ||
| Randomized to Aspirin Treatment, N (%) | ||
| Yes | 650 (51%) | 630 (50%) |
| No | 636 (49%) | 634 (50%) |
|
| ||
| Plasma CRP, mg/L | ||
| Mean (± SD) | 1.6 (3.3) | 1.7 (3.8) |
|
| ||
| Among cases only (n= 1,286) | ||
|
| ||
| Age at diagnosis, years | ||
| Mean (± SD) | 70.0 (7.4) | |
|
| ||
| Stage, N (%) | ||
| T1/T2 | 1079 (84%) | |
| T3 | 63 (5%) | |
| T4/N1/M1 | 79 (6%) | |
| Missing | 65 (5%) | |
|
| ||
| Gleason score*, N (%) | ||
| 2–6 | 753 (59%) | |
| 7 | 300 (23%) | |
| 8–10 | 172 (13%) | |
| Missing | 61 (5%) | |
|
| ||
| Outcomes, N | ||
| Bony Metastases | 23 | |
| Organ Metastases | 2 | |
| Death from Prostate Cancer | 183 | |
| Death from other causes | 435 | |
For those missing Gleason data, Gleason 8–10 includes “poorly differentiated” and Gleason 2–6 includes “well differentiated” tumors
We evaluated 4 CRP SNPs (rs3093075, rs2808630, rs1800947, rs1417938) on chromosome 1 in the locations listed in Table 2. All 4 CRP SNPs conformed to Hardy-Weinberg equilibrium among controls and the frequency of each SNP in this population was similar to those from the HapMap CEPH set (data not shown).
Table 2.
Association between CRP SNPs and overall incident prostate cancer, Physicians' Health Study (1982 2012)
| Overall Prostate Cancer Risk*,± | ||||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| SNP | Genotype | Location | Population Frequency* | Cases n (%) | Controls n (%) | OR** | 95% CI | P*** |
|
| ||||||||
| rs3093075 | CC | 3' Flanking | 87% | 1077 (87%) | 1065 (88%) | Referent | Referent | 0.55 |
| AC | 12% | 167 (13%) | 151 (12%) | 1.1 | 0.9–1.4 | |||
| AA | 0.5% | |||||||
|
| ||||||||
| rs2808630 | AA | 3' Flanking | 52% | 622 (53%) | 600 (52%) | Referent | Referent | 0.11 |
| AG | 40% | 455 (39%) | 489 (42%) | 0.9 | 0.8–1.1 | |||
| GG | 8% | 100 (8%) | 76 (6%) | 1.3 | 0.9–1.7 | |||
|
| ||||||||
| rs1800947 | GG | Exon 2 | 88% | 1007 (88%) | 1010 (89%) | Referent | Referent | 0.23 |
| CG | 12% | 140 (12%) | 123 (11%) | 1.2 | 0.9–1.5 | |||
| CC | 0% | |||||||
|
| ||||||||
| rs1417938 | AA | Intron 1 | 50% | 616 (50%) | 603 (50%) | Referent | Referent | 0.89 |
| AT | 41% | 496 (40%) | 501 (41%) | 1.0 | 0.8–1.2 | |||
| TT | 9% | 118 (10%) | 111 (9%) | 1.0 | 0.8–1.4 | |||
Excluded missing genotype data; missing genotype data ranged from 3–11% per SNP
Dominant Model where MAF <5%: rs3093075 (AA/AC vs. CC) and rs1800947 (GG/GC vs. CC) Codominant Model where MAF >5%: rs2808630 and rs1417938
OR adjusted for matching factors: age at randomization, follow-up time, and smoking status
p-value from LRT
None of the four CRP SNPs evaluated were significantly associated with overall prostate cancer risk (Table 2). The association between each SNP and overall prostate cancer risk did not differ substantially when stratified by BMI or PSA-era (data not shown).
To evaluate whether CRP SNPs were associated with more aggressive prostate cancer, we assessed 172 men with high grade disease, 142 men with advanced stage, 195 men with lethal, and 262 men with advanced stage or lethal disease. As shown in Table 3, men with one copy of the variant allele (C) in rs1800947 and men with two copies of the variant allele (C) in rs2808630 had 1.7 times the odds of high grade disease compared to men with no copies of the variant allele (GG genotype and TT genotype, respectively; OR: 1.7; 95% CI: 1.1–2.8 and OR: 1.7; 95% CI: 1.0–3.0, respectively). Although the multiplicative interaction term was not statistically significant (p-value 0.25), the association between rs1800947 and high grade disease appeared stronger in healthy weight men (OR: 2.2; 95% CI: 1.2–4.0) than overweight men (OR: 1.2; 95% CI: 0.6–2.7). Results were not significantly altered for the other SNPs when stratified by BMI (data not shown).
Table 3.
Association between CRP SNPs and high grade or lethal prostate cancer, Physicians' Health Study (1982–2012)
| High grade disease* (n=172 cases; n=1,264 controls) | Lethal** (n=195 cases; n=1,264 controls) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| SNP | Genotype | Cases (n) | OR | 95% CI | P*** | Cases (n) | OR | 95% CI | P*** |
|
| |||||||||
| rs3093075 | CC | 146 | Referent | Referent | 0.95 | 158 | Referent | Referent | 0.06 |
| AC/AA | 21 | 1.0 | 0.6–1.7 | 33 | 1.5 | 1.0–2.3 | |||
|
| |||||||||
| rs2808630 | TT | 87 | Referent | Referent | 0.03 | 87 | Referent | Referent | 0.16 |
| CT | 51 | 0.7 | 0.5–1.1 | 64 | 0.9 | 0.7–1.4 | |||
| CC | 19 | 1.7 | 1.0–3.0 | 19 | 1.7 | 0.9–3.0 | |||
|
| |||||||||
| rs1800947 | GG | 126 | Referent | Referent | 0.03 | 153 | Referent | Referent | 0.56 |
| CG/CC | 25 | 1.7 | 1.1–2.8 | 20 | 1.2 | 0.7–2.0 | |||
|
| |||||||||
| rs1417938 | TT | 87 | Referent | Referent | 0.80 | 87 | Referent | Referent | 0.65 |
| AT | 64 | 0.9 | 0.6–1.3 | 82 | 1.2 | 0.8–1.6 | |||
| AA | 15 | 0.9 | 0.5–1.7 | 19 | 1.2 | 0.7–2.0 | |||
High grade: Gleason 8–10 and if missing Gleason data, “poorly” differentiated tumors
Lethal Prostate cancer (n=195): n=170 prostate cancer deaths, n=12 developed bone or organ metastases, and n=13 developed bone or organ metastases and died from prostate cancer
P-value from LRT
None of the four CRP SNPs were associated with the combined endpoint of advanced stage and lethal prostate cancer (data not shown). Restricted to lethal prostate cancer alone (Table 3), men who had at least one copy of the variant allele (A) in rs3093075 had a borderline statistically significant 1.5 fold higher odds of lethal prostate cancer compared to men with no copies of the variant allele (OR: 1.5; 95% CI:1.0–2.3). The association was significant when restricted to overweight men (OR: 1.9; 95% CI: 1.1–3.4; p-value 0.03). Men with two copies of the variant allele (C) in rs2808630 had a borderline statistically significant 1.7 fold higher odds of lethal prostate cancer compared to men with no copies of the variant allele (OR: 1.7; 95% CI:0.9–3.0). There were no significant associations between any of the four CRP SNPs and advanced stage disease alone or survival following prostate cancer diagnosis (data not shown).
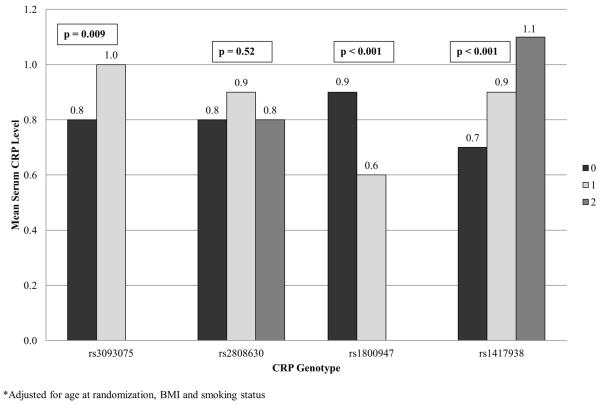
CRP SNPs rs3093075, rs1800947, and rs1417938 were significantly associated with mean plasma CRP levels after adjusting for BMI, smoking status and age at randomization (Figure 1). Mean CRP levels were significantly elevated in men with one copy of the variant allele in rs3093075 compared to those with no copies (p-value 0.009). Men with one or two copies of the variant allele in rs1417938 had significantly elevated mean CRP levels compared to those men with no copies of the variant allele (trend p-value <0.001). Conversely, men with one copy of the variant allele of rs1800947 had significantly lower mean CRP levels than those men with no copies (p-value <0.0001). Variation in rs2808630 was not associated with circulating CRP (p-value 0.52).
Figure 1.
Mean Plasma CRP Levels by CRP SNP Genotype
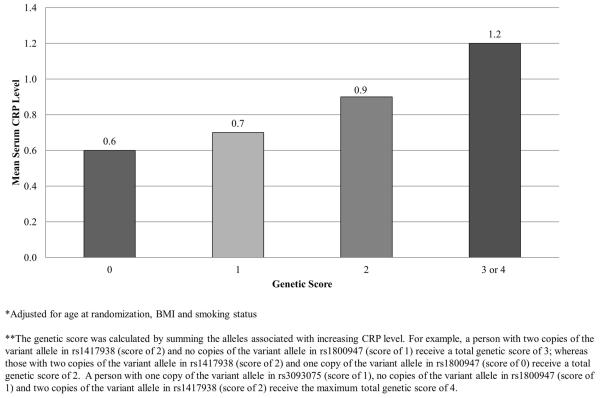
We then assessed the global test for association between common genetic variation in CRP and each of the outcomes. We found a significant association of common variation in CRP and CRP levels (p = 0.009); however, we found no association for any of the prostate cancer outcomes (data not shown). Increasing genetic score was significantly associated with increasing mean CRP (p-value <0.0001). Men with 3 or 4 “risk” alleles had a mean CRP level of 1.2 mg/L compared to a mean CRP level of 0.6 mg/L among men with no risk alleles (Figure 2). Genetic score was not associated with prostate cancer, overall (p-value 0.78) or among high grade (p-value 0.19), advanced stage (p-value 0.55), or lethal cancers (p-value 0.26)
Figure 2.
Mean Plasma CRP Levels by Genetic Score
In general, obese men had higher mean levels of plasma CRP than normal weight men (μ: 1.04 mg/L vs. 0.65 mg/L, respectively). Although interactions between BMI and each CRP SNP were not significant (p-interaction >0.05), the association between mean CRP level and rs1800947 was significant among healthy weight men (p-value 0.008), but not among overweight men (p-value 0.09). Similarly for rs1417938, mean plasma CRP was significantly associated with variation in rs1417938 in healthy weight (p-value <0.001), but not overweight men (p-value 0.06). In contrast, the association between rs3093075 and mean plasma CRP was significant in overweight men, but not healthy weight (p-value 0.04 and 0.08, respectively). There remained no significant association between rs2808630 and mean CRP levels stratified by BMI.
There were no significant associations between any of the four CRP SNPs or genetic score and circulating levels of IL-6 or TNFR2 (data not shown). There was also no association between baseline PSA levels and the SNPs or circulating CRP levels (data not shown).
Discussion
We evaluated the association between four SNPs in the CRP gene in a case-control study nested within the large prospective Physicians' Health Study. Similar to previous reports, none of the CRP SNPs we evaluated were associated with overall risk of prostate cancer(13,14,22). It has been hypothesized that CRP plays a role in the progression of prostate cancer and is therefore thought to be associated with more aggressive forms of the disease. Our findings of a borderline significant association between the variant allele in rs1800947 and increased risk of high Gleason grade disease, and between rs3093075 and lethal prostate cancer support this hypothesis and warrant further investigation. In addition, our study adds to accumulating evidence supporting the hypothesis that aggressive and indolent prostate cancer may have distinct etiologies(23).
Most previous studies on the association between polymorphisms in the CRP gene and prostate-specific disease endpoints found no association overall and did not present findings by grade or stage(12–14,22). In addition, each study had fewer than 300 incident prostate cancer cases. Dluzniewski et al. recently reported an inverse association between CRP rs1800947 and risk of recurrence(15); we found men with one copy of the minor allele (C) in CRP rs1800947 had an increased risk of high grade prostate cancer compared with men with 2 copies of the major allele (G). A study by Pierce et al. on 215 men in the Cardiovascular Health Study found no association between rs1800947, rs1417938, and rs2808630 and plasma CRP levels or risk of prostate cancer(22). In contrast, we found having two copies of the variant allele (CC) in rs2808630 was associated with an increased risk of high grade prostate cancer, as was having at least one copy of the variant allele in rs1800947 (C). At least one copy of the variant allele in rs3093075 (A) was associated with a borderline statistically significant increased risk of lethal prostate cancer, and increasing variant alleles in rs3093075 and rs1417938 were significantly associated with elevated plasma CRP. We found no association between rs2808630 and plasma CRP, and found carriers of the C allele in rs1800947 had lower plasma CRP, similar to previously published reports(5,24).
The association between common variation across CRP and between individual CRP SNPs rs3093075, rs1800947, and rs1417938, and circulating levels of CRP, perhaps suggest these SNPs, or others in linkage disequilibrium (LD), alter the expression or ability to produce an inflammatory response in the blood. Interestingly, we found one polymorphism (rs3093075) was associated with elevated mean plasma CRP and borderline increased risk of lethal disease, while another (rs1800947) was associated with lower plasma CRP levels and an increased risk of high Gleason grade. Thus, our findings may point to a different pathway acting to influence tumor progression.
We hypothesized that the association between CRP SNPs and prostate cancer and between CRP SNPs and circulating CRP would vary according to BMI (BMI < 25 kg/m2 vs. BMI ≥ 25 kg/m2). Circulating levels of CRP have been associated with adipose tissue and a previous report on 2,300 participants in the AGES-Reykjavik study found an interaction between adiposity and CRP genotypes on circulating CRP(25). Slattery et al. reported a significant interaction between CRP rs1800947 and BMI in modifying the risk of both rectal and colon cancer(16). Our previous analysis in PHS showed elevated plasma CRP levels were associated with an increased risk of overall prostate cancer only among healthy weight men(9). Similarly, we found the association between CRP rs1800947 and risk of high Gleason grade disease and CRP levels was stronger among healthy weight than overweight men. Conversely, we found the association between CRP rs3093075 and risk of lethal prostate cancer and CRP levels was significant only among overweight men. Thus, our findings suggest that BMI and CRP genotypes may interact to affect circulating CRP levels and the risk of aggressive prostate cancer.
The functional significance of rs1800947, rs2808630 and rs3093075 is unknown at this time. SNP rs3093075 and rs2808630 are polymorphisms located in the 3' flanking region. The A allele in rs3093075 has been previously associated with a reduced risk of rectal cancer(16) and showed a significant association with microangiopathic stroke(26). SNP rs1800947 is a synonymous base polymorphism in the coding region of exon 2, an evolutionarily conserved coding region(27) and was previously found associated with reduced risk of prostate cancer recurrence(15). Thus, it remains unclear what functional role, if any, these SNPs play in relation to progression of prostate cancer.
One potential limitation of our study is that CRP is a systemic inflammation marker and is not a specific marker for inflammation in the prostate, nor is it a cause of inflammation. In addition, we only had one measure of plasma CRP and although the four-year ICC was estimated to be 0.66 in another cohort(28), multiple measurements may be a more accurate representation of true CRP level over time. We also cannot rule out false-positive findings in this analysis, especially the paradoxical result that rs1800947 was associated with an increased risk of high grade disease, but decreased levels of CRP. Finally, over half the men were missing plasma CRP data and thus our stratified analysis of circulating levels may have been underpowered.
The main strengths of our study are the prospective nature of our nested case/control design, detailed information on clinical and physical characteristics, and high follow-up rates. Moreover, this nested study included 1,286 cases matched to 1,264 controls and thus, permitted analyses stratified by BMI, and allowed us to evaluate the associations restricted to more aggressive prostate cancer endpoints.
In summary, our findings suggest that SNPs in the CRP gene are not associated with overall prostate cancer risk. Polymorphisms in CRP SNPs rs1800947, rs2808630, and rs3093075 may be associated with more aggressive forms of prostate cancer, but our results require replication in other cohorts. In addition, the functional significance of these SNPs in prostate carcinogenesis remains unknown and warrants further study.
Acknowledgments
Funding National Institutes of Health research training grant (NIH, R25 CA098566 to S.C.M. and M.M.E. T32 CA09001-35 to S.C.M.). L.A.M., K.L.P., and J.R.R. are supported by the Prostate Cancer Foundation. The Physicians' Health Study is supported by the National Cancer Institute (CA42182, CA34944, CA40360, CA141298, and CA097193); the National Heart, Lung, and Blood Institute (HL26490, HL34595), Bethesda, MD.
Footnotes
Conflict of Interest Statement None of the authors has a conflict of interest relevant to this study.
References
- 1.Platz EA, De Marzo AM. Epidemiology of inflammation and prostate cancer. J Urol. 2004;171:S36–40. doi: 10.1097/01.ju.0000108131.43160.77. [DOI] [PubMed] [Google Scholar]
- 2.Sutcliffe S, Platz EA. Inflammation in the etiology of prostate cancer: an epidemiologic perspective. Urol Oncol. 2007;25:242–9. doi: 10.1016/j.urolonc.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 3.De Marzo AM, Platz EA, Sutcliffe S, Xu J, Grönberg H, Drake CG, et al. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–69. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaturvedi AK, Caporaso NE, Katki HA, Wong H-L, Chatterjee N, Pine SR, et al. C-reactive protein and risk of lung cancer. J Clin Oncol. 2010;28:2719–26. doi: 10.1200/JCO.2009.27.0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heikkilä K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J Epidemiol Community Health. 2007;61:824–33. doi: 10.1136/jech.2006.051292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Platz EA, De Marzo AM, Erlinger TP, Rifai N, Visvanathan K, Hoffman SC, et al. No association between pre-diagnostic plasma C-reactive protein concentration and subsequent prostate cancer. Prostate. 2004;59:393–400. doi: 10.1002/pros.10368. [DOI] [PubMed] [Google Scholar]
- 8.Van Hemelrijck M, Jungner I, Walldius G, Garmo H, Binda E, Hayday A, et al. Risk of prostate cancer is not associated with levels of C-reactive protein and other commonly used markers of inflammation. Int J Cancer. 2011;129:1485–92. doi: 10.1002/ijc.25773. [DOI] [PubMed] [Google Scholar]
- 9.Stark JR, Li H, Kraft P, Kurth T, Giovannucci EL, Stampfer MJ, et al. Circulating prediagnostic interleukin-6 and C-reactive protein and prostate cancer incidence and mortality. Int J Cancer. 2009;124:2683–9. doi: 10.1002/ijc.24241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McArdle PA, Mir K, Almushatat ASK, Wallace AM, Underwood MA, McMillan DC. Systemic inflammatory response, prostate-specific antigen and survival in patients with metastatic prostate cancer. Urol Int. 2006;77:127–9. doi: 10.1159/000093905. [DOI] [PubMed] [Google Scholar]
- 11.Elsberger B, Lankston L, McMillan DC, Underwood MA, Edwards J. Presence of tumoural C-reactive protein correlates with progressive prostate cancer. Prostate Cancer Prostatic Dis. 2011;14:122–8. doi: 10.1038/pcan.2011.5. [DOI] [PubMed] [Google Scholar]
- 12.Siemes C, Visser LE, Coebergh J-WW, Splinter TAW, Witteman JCM, Uitterlinden AG, et al. C-reactive protein levels, variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study. J Clin Oncol. 2006;24:5216–22. doi: 10.1200/JCO.2006.07.1381. [DOI] [PubMed] [Google Scholar]
- 13.Wang M-H, Helzlsouer KJ, Smith MW, Hoffman-Bolton JA, Clipp SL, Grinberg V, et al. Association of IL10 and other immune response- and obesity-related genes with prostate cancer in CLUE II. Prostate. 2009;69:874–85. doi: 10.1002/pros.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heikkilä K, Silander K, Salomaa V, Jousilahti P, Koskinen S, Pukkala E, et al. C-reactive protein-associated genetic variants and cancer risk: findings from FINRISK 1992, FINRISK 1997 and Health 2000 studies. Eur J Cancer. 2011;47:404–12. doi: 10.1016/j.ejca.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 15.Dluzniewski PJ, Wang M-H, Zheng SL, De Marzo AM, Drake CG, Fedor HL, et al. Variation in IL10 and other genes involved in the immune response and in oxidation and prostate cancer recurrence. Cancer Epidemiol Biomarkers Prev. 2012;21:1774–82. doi: 10.1158/1055-9965.EPI-12-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slattery ML, Curtin K, Poole EM, Duggan DJ, Samowitz WS, Peters U, et al. Genetic variation in C-reactive protein in relation to colon and rectal cancer risk and survival. Int J Cancer. 2011;128:2726–34. doi: 10.1002/ijc.25721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen W, Cai Q, Xiang Y-B, Xu W-H, Ruan ZX, Cheng J, et al. The modifying effect of C-reactive protein gene polymorphisms on the association between central obesity and endometrial cancer risk. Cancer. 2008;112:2409–16. doi: 10.1002/cncr.23453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hennekens CH, Buring JE, Manson JE, Stampfer M, Rosner B, Cook NR, et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N Engl J Med. 1996;334:1145–9. doi: 10.1056/NEJM199605023341801. [DOI] [PubMed] [Google Scholar]
- 19.Final report on the aspirin component of the ongoing Physicians' Health Study Steering Committee of the Physicians' Health Study Research Group. N Engl J Med. 1989;321:129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 20.Meyer MS, Penney KL, Stark JR, Schumacher FR, Sesso HD, Loda M, et al. Genetic variation in RNASEL associated with prostate cancer risk and progression. Carcinogenesis. 2010;31:1597–603. doi: 10.1093/carcin/bgq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu MC, Kraft P, Epstein MP, Taylor DM, Chanock SJ, Hunter DJ, et al. Powerful SNP-set analysis for case-control genome-wide association studies. Am J Hum Genet. 2010;86:929–42. doi: 10.1016/j.ajhg.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pierce BL, Biggs ML, DeCambre M, Reiner AP, Li C, Fitzpatrick A, et al. C-reactive protein, interleukin-6, and prostate cancer risk in men aged 65 years and older. Cancer Causes Control. 2009;20:1193–203. doi: 10.1007/s10552-009-9320-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007;121:1571–8. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zee RYL, Ridker PM. Polymorphism in the human C-reactive protein (CRP) gene, plasma concentrations of CRP, and the risk of future arterial thrombosis. Atherosclerosis. 2002;162:217–9. doi: 10.1016/s0021-9150(01)00703-1. [DOI] [PubMed] [Google Scholar]
- 25.Eiriksdottir G, Smith AV, Aspelund T, Hafsteinsdottir SH, Olafsdottir E, Launer LJ, et al. The interaction of adiposity with the CRP gene affects CRP levels: age, gene/environment susceptibilty-Reykjavik study. Int J Obes (Lond) 2009;33:267–72. doi: 10.1038/ijo.2008.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhlenbaeumer G, Huge A, Berger K, Kessler C, Voelzke H, Funke H, et al. Genetic variants in the C-reactive protein gene are associated with microangiopathic ischemic stroke. Cerebrovasc Dis. 2010;30:476–82. doi: 10.1159/000319021. [DOI] [PubMed] [Google Scholar]
- 27.Crawford DC, Sanders CL, Qin X, Smith JD, Shephard C, Wong M, et al. Genetic variation is associated with C-reactive protein levels in the Third National Health and Nutrition Examination Survey. Circulation. 2006;114:2458–65. doi: 10.1161/CIRCULATIONAHA.106.615740. [DOI] [PubMed] [Google Scholar]
- 28.Platz EA, Sutcliffe S, De Marzo AM, Drake CG, Rifai N, Hsing AW, et al. Intra-individual variation in serum C-reactive protein over 4 years: an implication for epidemiologic studies. Cancer Causes Control. 2010;21:847–51. doi: 10.1007/s10552-010-9511-z. [DOI] [PMC free article] [PubMed] [Google Scholar]