Abstract
The presence of druggable, topographically distinct allosteric sites on a wide range of receptor families has offered new paradigms for small molecules to modulate receptor function. Moreover, ligands that target allosteric sites offer significant advantages over the corresponding orthosteric ligands in terms of selectivity, including subtype selectivity within receptor families, and can also impart improved physicochemical properties. However, allosteric ligands are not a panacea. Many chemical issues (e.g., flat structure-activity relationships) and pharmacological issues (e.g., ligand-biased signaling) that are allosteric centric have emerged. Notably, the fact that allosteric sites are less evolutionarily conserved leads to improved selectivity; however, this can also lead to species differences that can hinder safety assessment. Many allosteric ligands possess molecular switches, wherein a small structural change (chemical or metabolic) can modulate the mode of pharmacology or receptor subtype selectivity. As the field has matured, as described here, key principles and strategies have emerged for the design of ligands/drugs for allosteric sites.
Keywords: G protein–coupled receptors, GPCRs, kinases, phospholipases, molecular switch, drug discovery, structure-activity relationship, SAR
INTRODUCTION
Although allosteric regulation of proteins by small molecules was first proposed in the midtwentieth century, the concept took several decades to come to prominence (1-8). Many point to 1965 as the year that allosterism was formalized, as the Monod-Wyman-Changeux model proposed conformational selection mechanisms to describe the action of ligands with bacterial regulatory enzymes (9, 10). However, attention became more formally focused on allosteric drug discovery as a viable therapeutic strategy upon the clinical success of the benzodiazepines, which are allosteric ligands that potentiate the effect of the neurotransmitter γ-aminobutyric acid (GABA) at the ionotropic GABAA receptor and overcome the potentially deadly effects of direct-acting GABAA agonists (4, 11). Since this discovery, interest in the development of allosteric ligands as medications has been steadily increasing. In fact, the number of publications that reference allosteric receptor modulators has grown at a nearly exponential rate between 1985 and the present day (Figure 1), and this growth is paralleled in the patent literature. This increase in both publication and patent activity reflects a generalized spread of interest in the development of allosteric ligands across a broad range of targets, including ion channels, kinases, caspases, G protein–coupled receptors (GPCRs), and phospholipases. Each of these classes contains many therapeutically relevant targets, and a wide variety of academic and commercial groups have pursued allosteric drug discovery efforts, some of which are discussed here. The development of this field reveals that a new smallmolecule design strategy, as well as more pharmacological and disposition scrutiny, is required to effectively develop safe and effective allosteric ligands as potential therapeutics (6, 11, 12).
Figure 1.
Number of publications per year that reference the concept of “allosteric receptor modulators” from 1985 to 2012, as reported by the Web of Knowledge database (http://www.webofknowledge.com/) (13). The trend is similar within the United States Patent and Trademark Office database (http://patft.uspto.gov/).
ORTHOSTERIC VERSUS ALLOSTERIC REGULATION
Each receptor/protein possesses a distinctive binding site for its respective endogenous ligand(s) that is defined as the orthosteric binding site, and both native and synthetic ligands that bind to this site are termed orthosteric ligands (5). For kinases, the orthosteric ligand is adenosine triphosphate (ATP) (1, Figure 2; bold numbers in parentheses refer to structures), and for GPCRs and ion channels, the orthosteric ligands may be small neurotransmitters [e.g., glutamate (2), GABA (3)] or large peptides [e.g., orexin (4)] (Figure 2) (1-11, 14). Classical synthetic orthosteric ligands, typically identified through radioligand binding assays, compete with these ligands for occupancy of the target and display a wide range of pharmacology (as inhibitor, agonist, antagonist, or inverse agonist). Historically, almost all of the compounds that have been FDA-approved for therapeutic use bind at a receptor/protein’s orthosteric site; however, these ligands can suffer from a lack of efficacy, decreased efficacy upon chronic administration, limited or poor selectivity, and/or resistance (1-14).
Figure 2.
Structures of endogenous orthosteric ligands 1-4 and marketed allosteric ligands 5 and 6. Abbreviations: ATP, adenosine triphosphate; GABA, γ-aminobutyric acid.
GPCRs, ion channels, caspases, kinases, and phospholipases have been found to possess, in addition to orthosteric binding sites, allosteric (from the Greek for “other site”) binding sites that are topologically and often functionally distinct from their orthosteric counterparts (1-14). The presence of allosteric sites allows for numerous additional ligand-receptor interactions and signaling phenomena beyond those associated with the orthosteric sites. This new allosteric approach has been heralded by the evolution of high-throughput screening (HTS) and functional assays that enable the identification of molecules that affect target function irrespective of the site of binding. Although the pharmacology has many target-specific caveats, receptors and proteins that are conformationally dynamic can be modulated by small molecules that bind at allosteric sites, either alone or in the presence of the endogenous orthosteric ligand, to stabilize either an active or inactive conformation of the receptor/protein. An active conformation elicits target activation/signaling; an inactive one blocks it (1-15). As the allosteric ligand stabilizes a unique conformation of the protein, the protein-ligand complex is in essence a new receptor that has a propensity for unique pharmacology (e.g., ligand-biased signaling) (1-14, 16). Many efforts have failed to produce highly selective orthosteric compounds that would be suitable as drug leads for GPCRs, kinases, and ion channels owing to the highly conserved orthosteric/ATP site and/or to unfavorable physicochemical and drug metabolism/pharmacokinetic properties of synthetic orthosteric ligands. In many cases, direct-acting agonists have proved either to be toxic or to lead to receptor desensitization, internalization, or downregulation because they are “switched on” for prolonged periods. Allosteric ligands, by binding at sites that are under less evolutionary pressure for conservation across a receptor family, typically afford unprecedented levels of selectivity. Moreover, allosteric ligands have a ceiling effect in that once allosteric sites are occupied, no additional effects are observed (that is, their effects are saturable). In addition, an allosteric modulator that lacks agonistic activity exerts its effects only when the endogenous agonist is present, preserving the temporal and spatial activity of the endogenous ligand; numerous works have demonstrated improved chemical tractability/physicochemical properties of allosteric versus orthosteric ligands (1-16).
For GPCRs and ion channels, allosteric ligands can possess a diversity of modes of pharmacology and include positive allosteric modulators (PAMs), which potentiate agonist-mediated receptor response, and negative allosteric modulators (NAMs), which noncompetitively decrease activity. In addition to PAMs and NAMs, silent allosteric modulators (SAMs; also known as neutral allosteric ligands) bind at allosteric sites and can block the activity of PAMs and NAMs, yet they have no effect on orthosteric ligand responses (1-14). More elaborate modes of allosteric modulation have also been reported. Significant efforts have been directed at the development of allosteric agonists (allosteric compounds that activate the receptor in the absence of the orthosteric ligand); however, many reported allosteric agonists may actually be bitopic ligands, that is, hybrid orthosteric/allosteric ligands that bind to both the orthosteric and allosteric sites. These bitopic ligands display receptor expression-dependent pharmacology, ligand-biased signaling, and confounding structure-activity relationships (SAR) (12, 17). Other allosteric ligands are partial antagonists (ligands that fully occupy the NAM site but only partially block receptor signaling) as well as ago-PAMs (PAMs that have inherent allosteric agonist activity). However, SAR analysis has been challenging, as these modes of pharmacology are highly variable within a given chemical series. For kinases and phospholipases, allosteric ligands access remote sites on the proteins, far removed from the orthosteric (catalytic) site(s), and stabilize unique, inactive conformations. Targeting these sites has afforded numerous advantages in terms of subtype and kinome selectivity and in terms of safety (14, 15).
Although many advantages over orthosteric modulation have been realized, allosteric modulation is not a panacea for drug discovery, and there are many pharmacological and chemical issues to consider when developing allosteric ligands. In terms of pharmacology, the sources of concern are the following: (a) The limited evolutionary pressure on allosteric sites can engender significant species differences; (b) the state dependence of allosteric modulators could be a liability in degenerative pathologies with progressive loss of endogenous orthosteric tone; (c) signal bias introduced by allosteric modulators could lead to unwanted or unpredicted physiological effects; and (d) allosteric modulators may be simultaneously activating homo- and heterodimers of the target receptor, which could unnecessarily complicate the physiological response (12, 15). Chemical complications center on very shallow allosteric ligand SAR, difficulty incorporating polar and solubilizing groups (lowering logP), and the presence of molecular switches (both through subtle chemical modification and via oxidative metabolism in vivo) that require more in-depth molecular pharmacological characterization (see Molecular Switches, below). Despite these challenges, two allosteric modulators of GPCRs have entered the market: a calcium-sensing receptor PAM named cinacalcet (5, Figure 2) (18) and a CCR5 NAM named maraviroc (6, Figure 2) (19). Moreover, numerous allosteric kinase inhibitors are in various stages of human clinical trials, and the benzodiazepines have been highly successful therapeutics that allosterically regulate ion channels.
THERAPEUTIC TARGETS FOR ALLOSTERIC MODULATORS
Allosteric ligands, as preclinical tool-compounds and probes, have been developed for numerous therapeutic targets, including ion channels (20, 21), caspases (22), kinases (15), phospholipases (23), and GPCRs (1-14). Delving into any one of these could consume this article, and extensive reviews, many very recent, have covered these targets and their allosteric ligands in great detail. Therefore, in this section, we focus on kinases and phospholipases, and in the next section, we focus on the emerging complexity of designing allosteric ligands for GPCRs.
Targeting Kinases with Allosteric Modulators
Protein phosphorylation is a key regulatory strategy for cellular signaling, occurring in nearly every known cellular signaling pathway (24), and defects in kinase activity give rise to a range of human diseases, including diabetes, cancer, and several neurologic disorders, making kinases attractive targets for therapeutic intervention (25, 26). Indeed, the growing number of FDA approvals for kinase inhibitors reveals the active interest of pharmaceutical companies in this area. However, despite some recent success, kinases remain difficult targets owing to significant conservation of structure across the human kinome. This conservation of structure is a consequence of the kinases’ shared catalytic activity—transfer of the γ-phosphate from a captured ATP to a protein substrate. The ATP binding pocket is highly conserved, but it represents the site of action for many of the kinase inhibitors that were developed initially and consequently shows little selectivity among the >500 known kinases (27). Because of the ongoing search for more selective compounds, much effort has been expended to find sites outside the ATP binding pocket that can be targeted by small molecules (28).
The ATP-competitive kinase inhibitors described above have been termed Type I inhibitors. Further studies have led to the development of three additional classes of allosteric ligands, which are differentiated by their sites of action. Type II inhibitors bind at the ATP site and extend into an adjacent allosteric pocket, whereas Type III inhibitors bind exclusively to allosteric pockets near the ATP site. Both of these types of inhibitors inactivate the kinase by locking it in an inactive conformation whereby a highly conserved activation loop is forced out of the hydrophobic pocket adjacent to the ATP site. In contrast, Type IV inhibitors bind to allosteric sites that are more remote from the ATP site (29). A broad range of modes of activity have been described for the known allosteric kinase inhibitors, from induction of structural reorganization, to prevention of active complex formation, to prevention of substrate recognition, to stabilization of inactive complexes, to induction of degradation, and, finally, to occupation of auto-inhibitory sites. However, there is evidence that many allosteric inhibitors select for a relatively small number of recurring inactive conformations among kinases. In particular, ligands acting at several known allosteric pockets—including the MEK pocket, the Akt1 pocket, the PIF pocket, and the ANS pocket—have been hypothesized to mediate inactivation through disruption of interactions with a nearby, well-conserved αC-helix (30, 31). Furthermore, both the MEK and PIF pockets have been found in several kinase families and have been shown to induce similar effects across members of the same family (31-33).
Owing to the therapeutic relevance of kinases, targeting their less conserved allosteric sites, which stabilize inactive conformations, is a leading strategy in kinase drug development. Allosteric kinase inhibitors that target Akt, Abl, PDK1, JNK1, CHK1, IGF-1R, CDK2, and mTOR have been developed, and they bear little or no structural resemblance to ATP-competitive inhibitors that target these kinases (15). The allosteric Akt kinase inhibitors represent a novel mechanism for kinase inhibition and a successful clinical development program that is currently in Phase II clinical trials (34). Akt, which consists of the three human isozymes Akt1, Akt2, and Akt3, exists in the cytoplasm in a closed PH-in conformation, with the pleckstrin homology (PH) domain blocking the ATP binding pocket and T308/S473. Upon recruitment to the plasma membrane, the kinase adopts a PH-out conformation, exposing the ATP binding pocket for phosphorylation (35-37). Researchers at Merck developed small-molecule, PH domain–dependent, isozyme-selective Akt inhibitors that were not competitive with ATP and did not inhibit the other members of the kinome (14, 36-40). Interestingly, the allosteric inhibitor 7 (Figure 3) inhibited not only the activity of Akt but also the phosphorylation of Akt. An extensive battery of biochemical, mutagenesis, and SAR studies suggested that allosteric inhibitor 7 and the later clinical candidate MK-2206 (8, Figure 3) stabilize the closed, PH-in conformation of Akt (14, 36-40). This was later confirmed by X-ray crystallography: Allosteric inhibitor 7 binds in a hydrophobic pocket formed by residues of both the PH and kinase domain interfaces, forming π-π stacking interactions with Trp80 and hydrogen bonds within both domains of the protein (41). Further in vivo fluorescence resonance energy transfer data showed that the presence of allosteric inhibitor 7 locked Akt into its PH-in conformation, prohibiting the phosphorylation of T308 and S473 (41). Further highlighting the advantages of allosteric inhibitors, ATP-competitive Akt inhibitors, such as 9 and 10 (Figure 3), induce hyperphosphorylation upon binding at the ATP site of Akt, imparting regulatory phosphorylation of their target (42). In contrast, allosteric Akt inhibitors, such as 7 and 8, inhibit both the physiological activation of Akt and the drug-induced Akt hyperphosphorylation, enhancing the therapeutic window and potentially contributing to the clinical efficacy and tolerability of MK-2206 (34-42) (Figure 3).
Figure 3.
The conformationally flexible Akt protein. (a) Akt in the cytoplasmic inactive conformation, also known as the PH-in conformation. Upon recruitment to the plasma membrane by PIP3, the kinase adopts an active, PH-out conformation, exposing T308 and S473 for phosphorylation. (b) Inhibition of Akt by allosteric inhibitors 7 and 8 leads to a PH-in conformation that inhibits both kinase activity and phosphorylation. ATP-competitive inhibitors bind to the catalytic site, leaving Akt in the PH-out conformation. Although inhibited, Akt can still be hyperphosphorylated owing to the conformation. (c) Structures of allosteric Akt inhibitors 7 and 8 and ATP-competitive Akt inhibitors 9 and 10. Abbreviations: ATP, adenosine triphosphate; PH, pleckstrin homology; PIP3, phosphatidylinositol (3,4,5)-triphosphate.
Progress in this field has been hindered by a lack of appropriate HTS technologies to identify allosteric kinase inhibitors and ligands that stabilize inactive conformations. Historically, slow and laborious ATP competition assays were used as secondary screens to identify noncompetitive, allosteric ligands. Recently, a next generation of binding assays has been shown to enable the detection of ligands that stabilize kinase conformations. Of these, FLiK (fluorescent labels in kinases) detects, without requiring kinase activity, conformational changes induced by ligand binding for p38α and Abl kinases (15). Despite this advance, new technologies and assays are required to more rapidly screen large compound collections for ligands that can modulate kinase activity and/or phosphorylation through more complex, allosteric mechanisms.
Targeting Phospholipases with Allosteric Modulators
Small-molecule modulators of enzyme activity fall into two broadly defined groups (Figure 4a): competitive inhibitors and allosteric inhibitors (43). A competitive inhibitor, hereafter termed substrate-based inhibitor, targets the enzyme’s or receptor’s binding site for its natural substrate or ligand, respectively. It is termed competitive because it often mimics the natural substrate or ligand of the receptor and competes for binding. An allosteric inhibitor binds to a distinct site on the surface of the enzyme or receptor that is independent of the substrate-binding domain. This allosteric binding mechanism can occur in one of two distinct ways: noncompetitive inhibition and uncompetitive inhibition. A noncompetitive inhibitor, as described in Figure 4a, binds to a distinct site on the enzyme before the substrate-binding event. In turn, the inhibitor distorts the active site so the enzyme’s natural substrate can no longer be bound. An uncompetitive inhibitor binds the enzyme-substrate (ES) complex and hinders product formation.
Figure 4.
Allosteric versus substrate-based inhibitors. (a) Small-molecule modulators of enzyme activity fall into two broadly defined groups: competitive inhibitors (also known as substrate-based inhibitors) and allosteric inhibitors. A substrate-based inhibitor (purple) binds to the active site of the enzyme, preventing the natural substrate from being consumed. An allosteric inhibitor (blue) binds to a distinct site on the surface of the enzyme to prevent either substrate binding (in a process termed noncompetitive inhibition) or product formation from the ESI complex (in a process termed uncompetitive inhibition). (b) These mechanisms are easily discerned through kinetic analysis. Substrate-based inhibitors raise the apparent KM of the enzyme for its substrate, whereas allosteric inhibitors alter both KM and Vmax values as allosteric inhibitors are outcompeted by increasing concentrations of substrate. Abbreviations: E, enzyme; ESI, enzyme-substrate-inhibitor complex; KM, affinity for substrate; Vmax, maximum velocity.
Allosteric and competitive binding mechanisms are easily discernible through the study of their kinetic profiles of enzyme inhibition (44). Substrate-based inhibitors typically mimic the enzymatic substrate, giving the inhibitor I an affinity for the active site of the enzyme E. Once the inhibitor is bound, the substrate cannot enter the active site, decreasing product formation. However, the enzyme’s natural substrate can saturate the active site and outcompete the inhibitor. As a result, the maximum velocity of the enzyme (Vmax) remains unchanged, whereas the apparent affinity of the enzyme for its substrate (KM) is right-shifted in the presence of the inhibitor (Figure 4b). Because allosteric inhibitors bind to an independent site on the enzyme, either before or after inhibitor binding, they cannot be outcompeted with increasing substrate. Thus, in the presence of an allosteric inhibitor, the KM and Vmax values of the target enzyme are altered—simultaneously right-shifted and lowered, respectively (45). Both competitive and allosteric inhibitors offer unique sets of advantages and disadvantages in drug design. However, allosteric binding mechanisms offer a distinct advantage in the design of small-molecule inhibitors for lipid-metabolizing enzymes.
As described in Figure 4, the ES complex must be formed before the product and is governed by changes in free energy (46). ES formation can thus be described by the equation ΔG = ΔH - TΔS, where ΔH is equivalent to the bond enthalpies before and after complex formation and ΔS is equivalent to the total entropic changes within the system (47). In protein-ligand interactions, desolvation energy is a prominent contributor to overall entropic changes in the formation of the ES complex (46). As the substrate diffuses into the active site, water molecules that once solvated the substrate become less ordered, with the caveat that more hydrophobic enzymes require a greater entropic cost for solvation. Thus, ΔS contributes less to substrate binding for water-soluble substrates and more to ES complex formation for more hydrophobic substrates (48). The same holds true for more hydrophobic substrate-based inhibitors in the formation of the EI complex versus the ES complex. Traditionally, effective inhibitor SAR rely on optimizing the ΔH component of the free energy equation for EI complex formation (49).
Lipid-metabolizing enzymes naturally bind hydrophobic substrates, meaning that S already plays a significant role in ES formation. Therefore, substrate-based inhibitors must rely on greater ΔS values for binding to overcome the entropic favorability of lipid substrate binding. In practice, this observation makes the identification of “real” SAR difficult for the medicinal chemist. Structural changes that increase the apparent Ki of an inhibitor for a lipid-metabolizing enzyme’s active site may be masked by inhibitor hydrophobicity, as the ΔS component of binding increases. These findings show that the design of substrate-based inhibitors for lipid-metabolizing enzymes must rely on overcoming large desolvation entropies associated with normal substrate diffusion to effectively compete with ES complex formation.
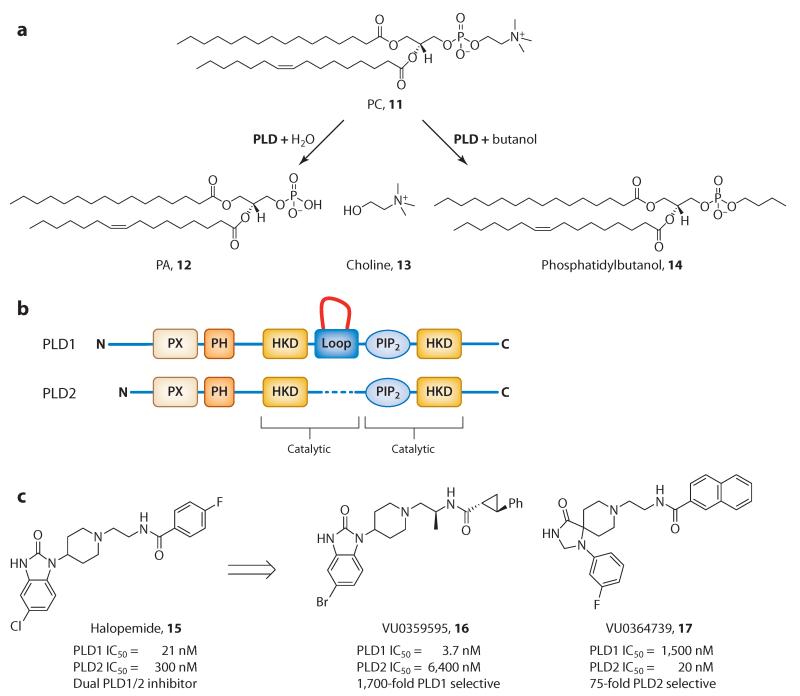
Given the rising prominence of lipid-signaling networks in disease states, there has never been a greater need for chemical tools that are capable of elucidating the roles of specific enzyme isoforms (or isozymes) in the production of signaling lipids. Recently, phospholipases (enzymes that hydrolyze phospholipids) have garnered attention as viable drug targets (50). Phospholipases are grouped into four major classes by the type of hydrolysis they catalyze: phospholipase A (subdivided into A1 and A2), phospholipase B, phospholipase C, and phospholipase D (PLD). PLD is a lipid-signaling enzyme that catalyzes the hydrolysis of phosphatidylcholine (11, Figure 5a) into phosphatidic acid (12, Figure 5a), an important lipid second messenger, and choline (13, Figure 5a) (23). Researchers have identified two mammalian isoforms of PLD, PLD1 and PLD2 (Figure 5b), which share 53% sequence identity and are functionally distinct. Both isoforms share a conserved histidine-lysine-aspartate amino acid domain that forms the catalytic site, as well as conserved phox homology (PX) and PH regulatory domains at the N terminus (23). Dysregulated PLD function has been implicated in cancer and central nervous system (CNS) disorders as well as in key stages of viral infection. However, the tools available to inhibit PLD activity have been limited to genetic and biochemical approaches, including the use of n-butanol, a ligand that competes for water in a transphosphatidylation exchange reaction (23).
Figure 5.
(a) Biochemistry of PLD. PLD catalyzes the hydrolysis of PC (11) into PA (12) and choline (14). In the presence of a primary alcohol, such as n-butanol, PLD catalyzes a competitive transphosphatidylation reaction that yields phosphatidylbutanol (15). (b) PLD1 and PLD2 structures. Sequence of PLD1 and PLD2 highlighting the PX and PH domains, the two HKD motifs, the two catalytic sites, and a loop in PLD1 that is absent in PLD2. Overall, homology between the two PLD isoforms is only 53%. (c) Structures and PLD inhibitory activity of the dual PLD1/2 inhibitor halopemide (16), the PLD1-selective inhibitor VU0359595 (17), and the PLD2-selective inhibitor VU0364739 (18), all of which allosterically regulate PLD, leading to the observed isozyme selectivity. Abbreviations: HKD, histidine-lysine-aspartate; PA, phosphatidic acid; PC, phosphatidylcholine; PH, pleckstrin homology; PIP2, phosphatidylinositol (4,5)-bisphosphate; PLD, phospholipase D; PX, phox homology.
The identification of halopemide (15, Figure 5c), a 1980s-era antipsychotic agent, as a PLD inhibitor in 2007 represented a major advance (51). Halopemide, a dopamine antagonist (D2 pIC50 = 7), also potently inhibits both PLD1 (IC50 = 21 nM) and PLD2 (IC50 = 300 nM) (52); however, like most atypical antipsychotics, it possesses several off-target effects. In clinical trials with halopemide that achieved exposures whereby both PLD isozymes were inhibited, no adverse events were noted and all biochemistry was normal, suggesting that inhibition of PLD in humans is well tolerated and safe (53). On the basis of the conformational flexibility of the PLD enzymes, the presence of a PH domain, and the piperidine benzimidazolone moiety, halopemide (15) was reminiscent of the chemotype 7 (Figure 3c) that engendered allosteric Akt kinase inhibition (39). Thus, halopemide (15) represented an attractive lead from which to assess modes of inhibition to develop isozyme-selective PLD inhibitors (52). A diversity-oriented synthesis approach generated ~260 analogs, from which VU0359595 (16, Figure 5c) emerged as a 1,700-fold PLD1-selective inhibitor (54). The benzimidazolone core favored PLD1 inhibition, and a chiral (S)-methyl group on the ethylene diamine linkers further enhanced PLD1 inhibition. Preferential PLD2 inhibition could be achieved with a bioisoteric triazaspirone scaffold, leading to the compound VU0364739 (17, Figure 5c), which displays a 75-fold preference for inhibition of PLD2 over PLD1 (55, 56). Both VU0359595 and VU0364739 (16 and 17, Figure 5c), as well as more advanced PLD inhibitors in these series (57), displayed significantly improved drug metabolism and pharmacokinetic properties while eliminating biogenic amine activity. Interestingly, incorporation of the chiral (S)-methyl group into the PLD2-preferring triazaspirone scaffold led to a >150-fold increase in PLD1 activity, providing potent dual PLD1/2 inhibitors. This incorporation represents the first example of a molecular switch (see Molecular Switches, below) among phospholipase ligands (57). Studies in PLD mutants lacking the N terminus (near the PX and PH domains) found a diminution in activity. These data, coupled with data from other biochemical studies, have confirmed that VU0359595 and VU0364739 (16 and 17, Figure 5c) bind with high affinity to a site within the PH domain and induce a conformational change that results in a second binding site elsewhere in the enzyme. These binding characteristics, reminiscent of the allosteric Akt inhibition mechanism (39), lead to unprecedented levels of PLD isozyme selectivity (23, 52). Tools such as VU0359595 and VU0364739 have been invaluable in dissecting the role of the individual PLD isozymes in multiple systems. As with allosteric modulators of kinases, those of phospholipases are not easily identifiable through current screening methods and technologies.
THE EMERGING COMPLEXITY OF DESIGNING ALLOSTERIC LIGANDS FOR GPCRs
Several recent reviews (1-14) extensively discuss caveats to the pharmacology and development of allosteric GPCR ligands and provide comprehensive lists of known GPCR allosteric modulators. Of all the allosteric ligands, those modulating GPCR functional activity and downstream signaling are the most mature, and the kinetic, functional assays (calcium and/or thallium flux assays) are well developed for both HTS and routine screening (e.g., triple-add protocols) (1-14). As mentioned above, SAR are notoriously shallow for GPCR allosteric modulators, and subtle electronic or steric perturbation to a ligand often leads to a complete loss of activity, significantly complicating chemical and drug metabolism/pharmacokinetic optimization toward clinical candidates. Here, we discuss new data and concerns about molecular switches, ago-PAMs, allosteric agonists (more properly termed bitopic ligands), and ligand-biased signaling.
Molecular Switches
During the development of allosteric theory, it was recognized that in a ternary complex model, the affinity and cooperativity of any allosteric compound are the two major factors that determine activity; the operational model of agonist action further revealed that these factors can lead to unique efficacies for the bound receptor (58). That is, an allosteric ligand, either on its own or in the presence of another ligand, can exhibit high affinity for a target without engendering any apparent effects on the signaling properties of this overall complex. When that observation is combined with the fact that small conformational changes in a protein can have significant effects on its signaling properties, it uncovers the possibility that a series of structurally similar compounds might engage a target equally well, yet the individual members could engender entirely different modes of activity (59). Indeed, this possibility has been borne out repeatedly across several different chemical series and a wide variety of protein targets.
The propensity of a given chemical series to produce agonists, PAMs, NAMs, or SAMs from structurally similar compounds has been termed mode switching, and the underlying subtle structural changes that cause these divergent outcomes are often referred to as molecular switches (60). Such molecular switches have been frequently reported in the metabotropic glutamate receptor 5 (mGlu5) literature, by several different groups, across a range of chemical scaffolds that interact with the well-characterized 2-methyl-6-(phenylethynyl)pyridine (MPEP) allosteric binding site (Figure 6a) (61-67). Such switches are by no means limited to mGlu5; significant examples have been reported for the group II and III metabotropic glutamate receptors as well (68, 69). Indeed, the phenomenon of mode switching appears to be relatively widespread across a diverse set of targets for allosteric ligands, including muscarinic receptors, chemoattractant receptors, and voltage-gated potassium channels (70-73). This research has generated a large body of SAR data, but a unified vision of the underlying ligand-receptor interactions that cause mode switching has yet to be elucidated for these targets.
Figure 6.
(a) A classic example of molecular switches within a series of MPEP-site mGlu5 allosteric ligands. A potent mGlu5 PAM (18) is converted into an mGlu5 SAM (19) via replacement of the cyclopentyl amide with a methyl amide. Conversely, incorporation of a nitrogen atom into the southern phenyl ring of the PAM (18) leads to a pyridyl congener (20) that acts as an mGlu5 NAM. (b) An mGlu5 PAM, VU0403602 (21), is metabolized to an active metabolite (22) with mGlu5 allosteric agonist activity, leading to toxicity. Abbreviations: mGlu5, metabotropic glutamate receptor 5; MPEP, 2-methyl-6-(phenylethynyl)pyridine; NAM, negative allosteric modulator; PAM, positive allosteric modulator; SAM, silent allosteric modulator.
Recognition of the utility of SAMs, also known as neutral allosteric ligands, as tools to understand the nature of these allosteric binding sites has led to some progress in understanding the molecular determinants of affinity versus cooperativity, especially at mGlus (66, 68, 74). These SAMs have high affinity for allosteric receptor sites, as measured by radioligand binding studies, but no measurable efficacy in functional assays; thus, they are useful tools for competition analysis against known PAMs or NAMs at the same receptor. However, a compound that appears to be a SAM in one functional assay might have efficacy in a separate functional assay, owing to the compound exhibiting ligand bias (1, 75). Additionally, given the frequency with which structurally related compounds exhibit differential activation among related receptor subtypes, many subtype-selective compounds may act as SAMs at a related receptor (76, 77). These complications highlight the importance of understanding the effects of an allosteric modulator in an array of functional assays, as well as its binding profile at related receptors, in order to gather the most accurate picture of its activity.
In addition to the use of SAMs, the use of directed mutagenesis studies has been helpful in uncovering the key contacts that need to be made with a target receptor in order to elicit positive versus negative cooperativity (78). This approach was recently taken with mGlu5, and it uncovered key residues that, when mutated, caused PAMs to signal as NAMs and vice versa (79). This type of approach was also successfully applied to the thyrotropin receptor, providing a possible basis for the rational design of allosteric ligands at hormone receptors and yielding a general proof of concept for the inclusion of mutagenesis studies to help elucidate structural determinants of mode of action (80).
Another consideration when discussing mode switching is the potential influence of metabolism on the pharmacological profile of an allosteric modulator. Because compounds with extremely similar structures exhibit differing modes of activity, a metabolite of an allosteric ligand potentially could, through introduction of a molecular switch, oppose the action of the parent compound. Recently, the first example of a cytochrome P450 (CYP)-mediated molecular switch was reported (Figure 6b) (81). In this instance, an mGlu5 PAM, VU0403602 (21, Figure 6b), was devoid of agonist activity in vitro, yet it elicited epileptiform activity characteristic of an mGlu5 agonist in vivo. The epileptiform activity elicited by VU0403602 (21) in vivo could be blocked by MPEP (suggesting mGlu5-mediated activity), but it was also blocked by ABT (1-aminobenzotriazole), a pan-CYP inhibitor. Extensive metabolic profiling led to the discovery that M1 (22, Figure 6b), an oxidative metabolite of VU0403602 (21), was a potent allosteric mGlu5 agonist that elicited the epileptiform activity—not the parent drug. In the context of a drug discovery program, the possibility of generating a metabolite with a pharmacological profile that is entirely disparate from the parent, and potentially toxic, represents a serious risk to clinical development and necessitates that more attention be given to the pharmacological profiles of major metabolites of allosteric ligands (22, Figure 6b).
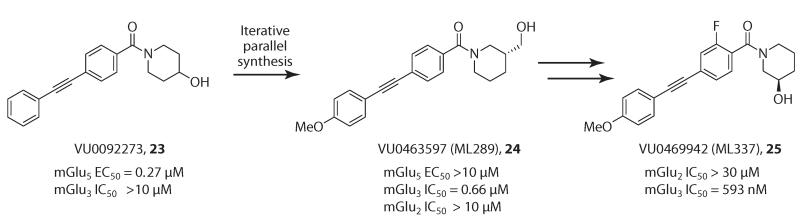
Finally, in addition to modulating the mode of pharmacology, molecular switches can also modulate receptor subtype selectivity. In some instances, doing so can be detrimental, whereas in other cases, this phenomenon allows access to ligands for a particular subtype within a receptor family that was previously inaccessible (22, Figure 6b). A recent example exploiting this attribute was reported, enabling the development of the first highly selective mGlu3 NAMs (77). In this instance, a potent mGlu5 PAM, VU0092273 (23, Figure 7) (EC50 = 270 nM), displayed weak activity (IC50 > 10 μM) at mGlu3, and an iterative parallel synthesis effort ensued to identify a molecular switch that would eliminate mGlu5 activity while enhancing mGlu3 activity. A p-OMe moiety on the southern phenyl ring served this role, eliminating mGlu5 activity and affording a submicromolar mGlu3 NAM, VU0463597 (24, Figure 7), which is >15-fold more potent at mGlu3 than mGlu2. Further optimization led to CNS penetrant VU0469942 (25, Figure 7), which has an IC50 that is >50-fold lower at mGlu3 than at mGlu2 (82). In addition to this unique find, the study also addressed the issue of shallow SAR. The mGlu3 NAM VU0469942 (25) was a highly cleared compound in rat, and the major route of metabolism was CYP-mediated dealkylation of the critical p-OMe moiety that engendered the mGlu3 activity and selectivity. All attempts to electronically or sterically shunt this metabolic pathway failed. Ultimately, exchanging the hydrogen atoms for isotopic deuterium in the p-OMe group reduced both in vitro and in vivo clearance by ~50% while maintaining mGlu3 NAM activity, thus representing a new strategy to overcome metabolic liability while working within shallow allosteric SAR.
Figure 7.
Taking advantage of molecular switches to gain access to novel subtype-selective allosteric modulators. An mGlu5 PAM, VU0092273 (23), is optimized into a highly selective and potent mGlu3 NAM, VU0469942 (25).
Ago-Allosteric Modulators
Ago-allosteric modulators are defined as allosterically binding ligands that mediate a receptor response in the absence of an orthosteric ligand while also producing a potentiating effect in the presence of an orthosteric ligand (83, 84). Identifying such ligands has been a convoluted process, highlighting the necessity of utilizing radioligand binding studies in tandem with a wide range of functional assays. In many cases, an allosteric ligand has been identified, classified, and subsequently reclassified (and often reclassified once again) on the basis of further study and access to constantly evolving screening assays. For instance, one of the earliest reported ago-allosteric modulators was the 2-aminothiophene PD81,723. It was initially characterized as a pure PAM for the adenosine A1 receptor when it was shown to increase [3H]-N6-cyclohexyladenosine binding and to enhance the dromotropic effect on activated A1 receptor in guinea pig heart tissue (85, 86). However, later studies demonstrated that PD81,723 also stabilizes G protein binding to the receptor, suggesting alternative modes of action (87). Ultimately, PD81,723 was reclassified as a putative ago-allosteric modulator when it was shown to independently decrease forskolin-induced cAMP formation in a concentration-dependent manner (88, 89).
In spite of the convoluted nature of identifying and characterizing ago-allosteric modulators, interest in identifying further compounds and mechanisms continues to increase. Noetzel and colleagues (90) recently examined the functional relevance of mGlu5 ago-PAMs VU0360172 and VU0092273 versus pure mGlu5 PAMs in the CNS. Rat in vivo experiments revealed that both ago-PAMs and pure PAMs demonstrate equal efficacy in reversing amphetamine-induced hyperlocomotion—the reversal of which is used as a predictor of potential antipsychotic activity. Additionally, their studies examined the in vitro effects of VU0360172 and VU0092273 in mGlu5-expressing cell lines with differential receptor expression profiles. In the cell lines expressing higher receptor loads, the compounds behaved as ago-PAMs, but in the cell lines with lower expression, the compounds behaved as pure PAMs. These experiments suggest that receptor expression levels need to be carefully considered in in vitro assays of ago-allosteric modulators; moreover, they suggest that, in certain cases, current assay capabilities may not reflect an unswerving picture of the division between pure PAMs and ago-PAMs when it comes to efficacy in native systems in which receptor reserve may vary by brain region. Allosteric modulators have long been recognized to hold potential as novel therapeutics because of their high receptor-subtype selectivity and decreased propensity to induce receptor desensitization/internalization, but they are dependent on the presence of endogenous ligand. Thus, the built-in agonist signaling profiles of ago-allosteric modulators may represent an attractive alternative therapy for degenerative diseases in which endogenous ligand tone becomes attenuated over the course of the disease as well as for diseases in which endogenous tone at a particular synapse is low. However, in other cases, such as mGlu5 PAMs, a pure PAM is desired because an ago-PAM may lead to epileptiform activity (see above).
Allosteric Agonists
The issue raised above in the context of receptor reserve-dependent pharmacology with PAMs versus ago-PAMs is relevant to the discussion on allosteric agonists, allosteric ligands that bind at a site distinct from the orthosteric site to elicit activation in the absence of the endogenous ligand. The GPCR research community increased its interest in this strategy when Portoghese (91) applied such ligands to the study of opioid receptor dimerization and demonstrated that such ligands have the potential to exhibit increased binding affinity and subtype selectivity relative to their monovalent counterparts.
More recently, the development of bitopic ligands has capitalized on these potential advantages and further utilized the rich allosteric pharmacology of GPCRs. Bitopic ligands are similar to heterobivalent ligands in that they possess two distinct pharmacophores covalently linked by a suitably long and flexible linker region; however, a bitopic ligand carries one pharmacophore that acts orthosterically and one that acts allosterically, enabling it to target both the orthosteric and allosteric sites of a single receptor (92). Owing to this unique allosteric/orthosteric composition, bitopic ligands are expected to possess complex binding properties that might lie somewhere between an allosteric ternary complex model and an orthosteric competitive binding model. Indeed, the current proposed modes of bitopic binding reflect (a) how a bitopic ligand could occupy the orthosteric and allosteric sites simultaneously, (b) how a bitopic ligand could occupy only the orthosteric site or the allosteric site (the so-called flip-flop mode of binding), or (c) how two ligands may cooperatively occupy both sites (93, 94).
This idea of combining pharmacophores to create a synergistic effect has its roots in the message-address concept for bivalent ligands introduced by Schwyzer in the late 1970s (95). This concept describes a bivalent ligand that consists of two distinct pieces: (a) a pharmacophore that acts as the message by promoting transduction of the signal to the effector and (b) a pharmacophore that acts as an address by possessing characteristics that increase the specificity of the ligand-receptor interaction. In the case of bitopic ligands, the address piece enables the ability to generate subtype-selective ligands for GPCR families with highly conserved orthosteric sites, such as muscarinic acetylcholine receptors (mAChRs) or mGlus. In these cases, the allosteric pharmacophore acts as the subtype-specific address to guide the more promiscuous orthosteric pharmacophore message. Furthermore, the inherent presence of the orthosteric message has made bitopic ligands particularly attractive for therapies targeting neurodegenerative diseases in which endogenous ligand tone has been attenuated.
The complexity of bitopic ligands is limited not only to their rational design but also to their characterization. Several compounds that once were considered to be monovalent have since been suspected to be bitopic. A great deal of work by multiple labs has recently demonstrated that most reported allosteric agonists, e.g., those for M1, are actually bitopic ligands (93, 96-98). These ligands bind to a distinct allosteric site that engenders functional M1 selective activation, but at higher concentrations, the ligands also bind at the orthosteric site and typically act as orthosteric antagonists of M2-5; moreover, there are molecular switches that abolish binding at the allosteric site, leading to ligands that are pan-orthosteric mAChR antagonists. A recent study by Conn and coworkers (17) adds additional complexity to the development of M1 allosteric (bitopic) agonists. Here, two related M1 allosteric (bitopic) agonists displayed receptor reserve–dependent pharmacology (range of partial agonism) and induced brain region–specific responses (due to M1 expression levels and receptor reserve) that correspond with behavioral effects in animal models. Moreover, these bitopic ligands displayed ligand-biased signaling, wherein they had equivalent responses in a standard calcium mobilization assay yet different effects on β-arrestin recruitment and ERK1/2 phosphorylation. Together, these data reveal that M1 allosteric agonists can differentially regulate coupling of M1 to different signaling pathways, and this regulation can dramatically alter the actions of these compounds on specific brain circuits important for pharmacodynamic readouts; therefore, M1 PAMs, which potentiate endogenous ACh, may represent a less complicated approach for selective M1 activation.
With the increasing amount of research being devoted to allosterism in GPCRs, bitopic ligands are an exciting and necessary subcategory of study. Not only do the ligands appear to offer a source for novel molecular probes and possible disease therapies, but their very nature holds the potential to reveal more about the spatial and thermodynamic interactions between the orthosteric and allosteric sites of a GPCR. Nevertheless, a wholly unambiguous method of identifying a bitopic ligand is needed, although it may remain elusive until the advent of crystal structures and/or radioligands for the GPCR targets of interest.
SUMMARY AND CONCLUSIONS
Our understanding of—and the tremendous advantages of—modulating a diverse array of molecular targets via allosteric modulation has increased dramatically in the past decade. Allosteric ligands targeting GPCRs have entered the market, and, along with allosteric kinase inhibitors, are in various stages of clinical development for a wide range of CNS disorders and oncology applications. In the early stages of discovery, allosteric ligands, by offering unprecedented selectivity and improved physiochemical properties, are allowing researchers to dissect the roles and therapeutic potential of discrete members of highly conserved families of receptors. Despite these advantages, some concerns still need to be addressed: shallow SAR, chemical and metabolic molecular switches, the in vivo ramifications of ligand-biased signaling, and the potential for differences in activity between species. However, the past five years have witnessed the emergence of key principles and strategies for the design and development of ligands/drugs for allosteric sites, and this approach offers new opportunities for the development of highly selective therapeutic agents spanning a broad spectrum of human disease.
ACKNOWLEDGMENTS
The authors thank Janssen Pharmaceuticals (Johnson & Johnson), Bristol-Myers Squibb, AstraZeneca, the National Institutes of Health, the National Institute of Mental Health, and the National Institute on Drug Abuse for grant support, and C.W.L. thanks the Warren Foundation for establishing the William K. Warren Jr. Chair in Medicine.
Footnotes
DISCLOSURE STATEMENT
C.W.L. has served as a consultant for GlaxoSmithKline, Amgen, AbbVie, and PureTech. He receives research support that includes salaries from Janssen Pharmaceuticals (Johnson & Johnson), Bristol-Myers Squibb, and AstraZeneca for the development of allosteric modulators of GPCRs.
LITERATURE CITED
- 1.Kenakin T, Miller LJ. Seven transmembrane receptors as shapeshifting proteins: the impact of allosteric modulation and functional selectivity on new drug discovery. Pharmacol. Rev. 2010;62:265–304. doi: 10.1124/pr.108.000992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Umbarger HE. Evidence for a negative-feedback mechanism in the biosynthesis of isoleucine. Science. 1956;123:848. doi: 10.1126/science.123.3202.848. [DOI] [PubMed] [Google Scholar]
- 3.Fenton AW. Allostery: an illustrated definition for the ‘second secret of life’. Trends Biochem. Sci. 2008;33:420–25. doi: 10.1016/j.tibs.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 2009;8:41–54. doi: 10.1038/nrd2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bridges TM, Lindsley CW. G-protein-coupled receptors: from classical modes of modulation to allosteric mechanisms. ACS Chem. Biol. 2008;3:530–42. doi: 10.1021/cb800116f. [DOI] [PubMed] [Google Scholar]
- 6.Christopoulos A, Kenakin T. G protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 2002;54:323–74. doi: 10.1124/pr.54.2.323. [DOI] [PubMed] [Google Scholar]
- 7.Christopoulos A. Allosteric binding sites on cell surface receptors: novel targets for drug discovery. Nat. Rev. Drug Discov. 2002;1:198–210. doi: 10.1038/nrd746. [DOI] [PubMed] [Google Scholar]
- 8.Kenakin TP. 7TM receptor allostery: putting numbers to shapeshifting proteins. Trends Pharmacol. Sci. 2009;30:460–69. doi: 10.1016/j.tips.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Lane RJ, Abdul-Ridha A, Canals M. Regulation of G protein-coupled receptors by allosteric ligands. ACS Chem. Neurosci. 2013;4:527–34. doi: 10.1021/cn400005t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monod J, Wyman J, Changeux J-P. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 11.Mohler HF, Fritschy JM, Rudolph U. A new benzodiazepine pharmacology. J. Pharmacol. Exp. Ther. 2002;300:2–8. doi: 10.1124/jpet.300.1.2. [DOI] [PubMed] [Google Scholar]
- 12.Melancon BJ, Hopkins CR, Wood MR, Emmitte KA, Niswender CM, et al. Allosteric modulation of seven transmembrane spanning receptors: theory, practice, and opportunities for central nervous system drug discovery. J. Med. Chem. 2012;55:1445–64. doi: 10.1021/jm201139r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomson Reuters. Search term: allosteric receptor modulators. Web of Knowledge, New York: 2013. updated July 2013, retrieved January 19, 2013. http://www.webofknowledge.com/ [Google Scholar]
- 14.Lindsley CW. The Akt/PKB family of protein kinases: a review of small molecule inhibitors and progress towards target validation: a 2009 update. Curr. Top. Med. Chem. 2010;10:458–77. doi: 10.2174/156802610790980602. [DOI] [PubMed] [Google Scholar]
- 15.Fang Z, Grütter C, Rauh D. Strategies for the selective regulation of kinases with allosteric modulators: exploiting exclusive structural features. ACS Chem. Biol. 2013;8:58–70. doi: 10.1021/cb300663j. [DOI] [PubMed] [Google Scholar]
- 16.Digby GJ, Conn PJ, Lindsley CW. Orthosteric- and allosteric-induced ligand-directed trafficking at GPCRs. Curr. Opin. Drug Discov. Dev. 2010;13:577–86. [PMC free article] [PubMed] [Google Scholar]
- 17.Digby GJ, Noetzel MJ, Bubser M, Utley TJ, Walker AG, et al. Novel allosteric agonists of the M1 muscarinic acetylcholine receptor induce brain region-specific responses and correspond with behavioral effects in animal models. J. Neurosci. 2012;32:8532–44. doi: 10.1523/JNEUROSCI.0337-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nemeth EF, Heaton WH, Miller M, Fox J, Balandrin MF, et al. Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J. Pharmacol. Exp. Ther. 2004;308:627–35. doi: 10.1124/jpet.103.057273. [DOI] [PubMed] [Google Scholar]
- 19.Meanwell NA, Kadow JF. Maraviroc, a chemokine CCR5 receptor antagonist for the treatment of HIV infection and AIDS. Curr. Opin. Investig. Drugs. 2007;8:669–81. [PubMed] [Google Scholar]
- 20.Monaghan DT, Irvine MW, Costa BM, Fang G, Jane DE. Pharmacological modulation of NMDA receptor activity and the advent of negative and positive allosteric modulators. Neurochem. Int. 2012;61:581–92. doi: 10.1016/j.neuint.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taly A, Corringer P-J, Guedin D, Lestage P, Changeux J-P. Nicotinic receptors: allosteric transitions and therapeutic targets in the nervous system. Nat. Rev. Drug Discov. 2009;8:733–50. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- 22.Häcker HG, Sisay MT, Gütschow M. Allosteric modulation of caspases. Pharmacol. Ther. 2011;132:180–95. doi: 10.1016/j.pharmthera.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Selvy PE, Lavieri RR, Lindsley CW, Brown HA. Phospholipase D: enzymology, signaling, and chemical modulation. Chem. Rev. 2011;111:6064–119. doi: 10.1021/cr200296t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson LN, Lewis RJ. Structural basis for control by phosphorylation. Chem. Rev. 2001;101:2209–42. doi: 10.1021/cr000225s. [DOI] [PubMed] [Google Scholar]
- 25.Pawson T, Scott JD. Protein phosphorylation in signaling—50 years and counting. Trends Biochem. Sci. 2005;30:286–90. doi: 10.1016/j.tibs.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Cohen P. Protein kinases—the major drug targets of the twenty-first century? Nat. Rev. Drug Discov. 2002;1:309–15. doi: 10.1038/nrd773. [DOI] [PubMed] [Google Scholar]
- 27.Zheng J, Knighton DR, Ten Eyck LF, Karlsson R, Xuong N, et al. Crystal structure of the catalytic subunit of cAMP-dependent protein kinase complexed with MgATP and peptide inhibitor. Biochemistry. 1993;32:2154–61. doi: 10.1021/bi00060a005. [DOI] [PubMed] [Google Scholar]
- 28.Hantschel O. Structure, regulation, signaling, and targeting of Abl kinases in cancer. Genes Cancer. 2012;3:436–46. doi: 10.1177/1947601912458584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palmieri L, Rastelli G. αC helix displacement as a general approach for allosteric modulation of protein kinases. Drug Discov. Today. 2013;18:407–14. doi: 10.1016/j.drudis.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Betzi S, Alam R, Martin M, Lubbers DJ, Han H, et al. Discovery of a potential allosteric ligand binding site in CDK2. ACS Chem. Biol. 2011;6:492–501. doi: 10.1021/cb100410m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busschots K, Lopez-Garcia LA, Lammi C, Stroba A, Zeuzem S, et al. Substrate-selective inhibition of protein kinase PDK1 by small compounds that bind to the PIF-pocket allosteric docking site. Chem. Biol. 2012;19:1152–63. doi: 10.1016/j.chembiol.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Garcia LA, Schulze JO, Fröhner W, Zhang H, Süss E, et al. Allosteric regulation of protein kinase PKCζby the N-terminal C1 domain and small compounds to the PIF-pocket. Chem. Biol. 2011;18:1463–73. doi: 10.1016/j.chembiol.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Fischmann TO, Smith CK, Mayhood TW, Myers JE, Reichert P, et al. Crystal structures of MEK1 binary and ternary complexes with nucleotides and inhibitors. Biochemistry. 2009;48:2661–74. doi: 10.1021/bi801898e. [DOI] [PubMed] [Google Scholar]
- 34.Hirai H, Sootome H, Nakatsuru Y, Miyama K, Taguchi S, et al. MK-2206, an allosteric Akt inhibitor, enhances tumor efficacy by standard chemotherapeutic agents or molecular targeted drugs in vitro and in vivo. Mol. Cancer Ther. 2010;9:1956–67. doi: 10.1158/1535-7163.MCT-09-1012. [DOI] [PubMed] [Google Scholar]
- 35.Kumar CC, Madison V. AKT crystal structure and AKT-specific inhibitors. Oncogene. 2005;24:7493–501. doi: 10.1038/sj.onc.1209087. [DOI] [PubMed] [Google Scholar]
- 36.Cheng JQ, Lindsley CW, Cheng GZ, Yang H, Nicosia SV. The Akt/PKB pathway: molecular target for cancer drug discovery. Oncogene. 2005;24:7482–92. doi: 10.1038/sj.onc.1209088. [DOI] [PubMed] [Google Scholar]
- 37.Kim D, Cheng GZ, Lindsley CW, Yang H, Cheng JQ. Targeting phosphatidylinositol-3 kinase/Akt pathway for cancer therapy. Curr. Opin. Investig. Drugs. 2005;6:1250–58. [PubMed] [Google Scholar]
- 38.Barnett SF, Bilodeau MT, Lindsley CW. The Akt/PKB family of protein kinases: a review of small molecule inhibitors and progress towards target validation. Curr. Top. Med. Chem. 2005;5:109–25. doi: 10.2174/1568026053507714. [DOI] [PubMed] [Google Scholar]
- 39.Lindsley CW, Zhao Z, Duggan ME, Barnett SF, Defeo-Jones D, et al. Allosteric Akt (PKB) kinase inhibitors: discovery and SAR of isozyme selective inhibitors. Bioorg. Med. Chem. Lett. 2005;15:761–64. doi: 10.1016/j.bmcl.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 40.DeFeo-Jones D, Barnett SF, Fu S, Hancock PJ, Haskell KM, et al. Tumor cell sensitization to apoptotic stimuli by selective inhibition of specific Akt/PKB family members. Mol. Cancer Ther. 2005;4:271–79. [PubMed] [Google Scholar]
- 41.Calleja V, Laguerre M, Parker PJ, Larijani B. Role of a novel PH-kinase domain interface in PKB/Akt regulation: structural mechanism for allosteric inhibition. PLoS Biol. 2009;7:e17. doi: 10.1371/journal.pbio.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okuzumi T, Fielder D, Zhang C, Gray DC, Aizenstein B, et al. Inhibitor hijacking of Akt activation. Nat. Chem. Biol. 2009;5:484–93. doi: 10.1038/nchembio.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Copeland RA. Evaluation of Enzyme Inhibitors in Drug Discovery: A Guide for Medicinal Chemists and Pharmacologists. 1st ed. Wiley-Intersci; New York: 2005. [PubMed] [Google Scholar]
- 44.Copeland RA. Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis. 2nd ed Wiley-VCH; New York: 2000. [Google Scholar]
- 45.Voet D, Voet JG, Pratt CW. 2nd ed Wiley; New York: 2005. Fundamentals of Biochemistry: Life at the Molecular Level. [Google Scholar]
- 46.Stein RL. Kinetics of Enzyme Action: Essential Principles for Drug Hunters. Wiley; New York: 2011. [Google Scholar]
- 47.Carey FA, Sundberg RJ. Advanced Organic Chemistry, Part A: Structure and Mechanisms. 5th ed. Springer; New York: 2008. [Google Scholar]
- 48.Villá J, Štrajbl M, Glennon TM, Sham YY, Chu ZT, Warshel A. How important are entropic contributions to enzyme catalysis? Proc. Natl. Acad. Sci. USA. 2000;97:11899–904. doi: 10.1073/pnas.97.22.11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silverman RB. The Organic Chemistry of Drug Design and Drug Action. 2nd ed Elsevier; Burlington, MA: 2004. [Google Scholar]
- 50.Athenstaedt K, Daum G. Phosphatidic acid, a key intermediate in lipid metabolism. Eur. J. Biochem. 1999;266:1–16. doi: 10.1046/j.1432-1327.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- 51.Monovich L, Mugrage B, Quadros E, Toscano K, Tommasi R, et al. Optimization of halopemide for phospholipase D2 inhibition. Bioorg. Med. Chem. Lett. 2007;17:2310–11. doi: 10.1016/j.bmcl.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 52.Scott SA, Selvy PE, Buck JR, Cho HP, Criswell TL, et al. Design of isoform-selective phospholipase D inhibitors that modulate cancer cell invasiveness. Nat. Chem. Biol. 2009;5:108–17. doi: 10.1038/nchembio.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Cuyper H, van Praag HM, Verstraeten D. The clinical significance of halopemide, a dopamine-blocker related to the butyrophenones. Neuropsychobiology. 1984;12:211–16. doi: 10.1159/000118141. [DOI] [PubMed] [Google Scholar]
- 54.Lewis JA, Scott SA, Lavieri RR, Buck JR, Selvy PE, et al. Design and synthesis of isoform-selective phospholipase D (PLD) inhibitors. Part I: Impact of alternative halogenated privileged structures on PLD1 specificity. Bioorg. Med. Chem. Lett. 2009;19:1916–19. doi: 10.1016/j.bmcl.2009.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lavieri RR, Scott SA, Lewis JA, Selvy PE, Armstrong MD, et al. Design and synthesis of isoform-selective phospholipase D (PLD) inhibitors. Part II: Identification of the 1,3,8-triazaspiro[4,5]decan-4-one privileged structure that engenders PLD2 selectivity. Bioorg. Med. Chem. Lett. 2009;19:2240–43. doi: 10.1016/j.bmcl.2009.02.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lavieri RR, Scott SA, Selvy PE, Kim K, Jadhav S, et al. Design, synthesis, and biological evaluation of halogenated N-(2-(4-oxo-1-phenyl-1,3,8-triazaspiro[4.5]decan-8-yl)ethyl)benzamides: discovery of an isoform-selective small molecule phospholipase D2 inhibitor. J. Med. Chem. 2010;53:6706–19. doi: 10.1021/jm100814g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Reilly MC, Scott SA, Brown KA, Oquin TH, III, Thomas PG, et al. Development of dual PLD1/2 and PLD2 selective inhibitors from a common 1,3,8-triazaspiro[4.5]decane core: discovery of ML298 and ML299 that decrease invasive migration in U87-MG glioblastoma cells. J. Med. Chem. 2013;56:2695–99. doi: 10.1021/jm301782e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Black JW, Leff P. Operational models of pharmacological agonism. Proc. R. Soc. B. 1983;220:141–62. doi: 10.1098/rspb.1983.0093. [DOI] [PubMed] [Google Scholar]
- 59.May LT, Leach K, Sexton PM, Christopoulos A. Allosteric modulation of G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2007;47:1–51. doi: 10.1146/annurev.pharmtox.47.120505.105159. [DOI] [PubMed] [Google Scholar]
- 60.Wood MR, Hopkins CR, Brogan JT, Conn PJ, Lindsley CW. “Molecular switches” on mGluR allosteric ligands that modulate modes of pharmacology. Biochemistry. 2011;50:2403–10. doi: 10.1021/bi200129s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lamb JP, Engers DW, Niswender CM, Rodriguez AL, Venable DF, et al. Discovery of molecular switches within the ADX-47273 mGlu5 PAM scaffold that modulate modes of pharmacology to afford potent mGlu5 NAMs, PAMs and partial antagonists. Bioorg. Med. Chem. Lett. 2011;21:2711–14. doi: 10.1016/j.bmcl.2010.11.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sams AG, Mikkelsen GK, Brodbeck RM, Pu X, Ritzén A. Efficacy switching SAR of mGluR5 allosteric modulators: highly potent positive and negative modulators from one chemotype. Bioorg. Med. Chem. Lett. 2011;21:3407–10. doi: 10.1016/j.bmcl.2011.03.103. [DOI] [PubMed] [Google Scholar]
- 63.Sharma S, Rodriguez AL, Conn PJ, Lindsley CW. Synthesis and SAR of a mGluR5 allosteric partial antagonist lead: unexpected modulation of pharmacology with slight structural modifications to a 5-(phenylethynyl)pyrimidine scaffold. Bioorg. Med. Chem. Lett. 2008;18:4098–101. doi: 10.1016/j.bmcl.2008.05.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sharma S, Kedrowski J, Rook JM, Smith RL, Jones CK, et al. Discovery of molecular switches that modulate modes of metabotropic glutamate receptor subtype 5 (mGlu5) pharmacology in vitro and in vivo within a series of functionalized, regioisomeric 2- and 5-(phenylethynyl)pyrimidines. J. Med. Chem. 2009;52:4103–6. doi: 10.1021/jm900654c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Packiarajan M, Mazza Ferreira CG, Hong SP, White AD, Chandrasena G, et al. N-Aryl pyrrolidinonyl oxadiazoles as potent mGluR5 positive allosteric modulators. Bioorg. Med. Chem. Lett. 2012;22:5658–62. doi: 10.1016/j.bmcl.2012.06.094. [DOI] [PubMed] [Google Scholar]
- 66.Rodriguez AL, Nong Y, Sekaran NK, Alagille D, Tamagnan GD, Conn PJ. A close structural analog of 2-methyl-6-(phenylethynyl)-pyridine acts as a neutral allosteric site ligand on metabotropic glutamate receptor subtype 5 and blocks the effects of multiple allosteric modulators. Mol. Pharmacol. 2005;68:1793–802. doi: 10.1124/mol.105.016139. [DOI] [PubMed] [Google Scholar]
- 67.Zou MF, Cao J, Rodriguez AL, Conn PJ, Newman AH. Design and synthesis of substituted N-(1,3-diphenyl-1H-pyrazol-5-yl)benzamides as positive allosteric modulators of the metabotropic glutamate receptor subtype 5. Bioorg. Med. Chem. Lett. 2011;21:2650–54. doi: 10.1016/j.bmcl.2010.12.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schann S, Mayer S, Franchet C, Frauli M, Steinberg E, et al. Chemical switch of a metabotropic glutamate receptor 2 silent allosteric modulator into dual metabotropic glutamate receptor 2/3 negative/positive allosteric modulators. J. Med. Chem. 2010;53:8775–79. doi: 10.1021/jm101069m. [DOI] [PubMed] [Google Scholar]
- 69.Utley T, Haddenham D, Salovich JM, Zamorano R, Vinson PN, et al. Synthesis and SAR of a novel metabotropic glutamate receptor 4 (mGlu4) antagonist: unexpected ‘molecular switch’ from a closely related mGlu4 positive allosteric modulator. Bioorg. Med. Chem. Lett. 2011;21:6955–59. doi: 10.1016/j.bmcl.2011.09.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Digby GJ, Utley TJ, Lamsal A, Sevel C, Sheffler DJ, et al. Chemical modification of the M1 agonist VU0364572 reveals molecular switches in pharmacology and a bitopic binding mode. ACS Chem. Neurosci. 2012;3:1025–36. doi: 10.1021/cn300103e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gharagozloo P, Lazareno S, Miyauchi M, Popham A, Birdsall NJ. Substituted pentacyclic carbazolones as novel muscarinic allosteric agents: synthesis and structure-affinity and cooperativity relationships. J. Med. Chem. 2002;45:1259–74. doi: 10.1021/jm010946z. [DOI] [PubMed] [Google Scholar]
- 72.Luker T, Bonnert R, Schmidt J, Sargent C, Paine SW, et al. Switching between agonists and antagonists at CRTh2 in a series of highly potent and selective biaryl phenoxyacetic acids. Bioorg. Med. Chem. Lett. 2011;21:3616–21. doi: 10.1016/j.bmcl.2011.04.101. [DOI] [PubMed] [Google Scholar]
- 73.Cheung YY, Yu H, Xu K, Zou B, Wu M, et al. Discovery of a series of 2-phenyl-N-(2-(pyrrolidin-1-yl)phenyl)acetamides as novel molecular switches that modulate modes of Kv7.2 (KCNQ2) channel pharmacology: identification of (S)-2-phenyl-N-(2-(pyrrolidin-1-yl)phenyl)butanamide (ML252) as a potent, brain penetrant Kv7.2 channel inhibitor. J. Med. Chem. 2012;55:6975–79. doi: 10.1021/jm300700v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gregory KJ, Noetzel MJ, Rook JM, Vinson PN, Stauffer SR, et al. Investigating metabotropic glutamate receptor 5 allosteric modulator cooperativity, affinity, and agonism: enriching structure-function studies and structure-activity relationships. Mol. Pharmacol. 2012;82:860–75. doi: 10.1124/mol.112.080531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noetzel MJ, Gregory KJ, Vinson PN, Manka JT, Stauffer SR, et al. A novel metabotropic glutamate receptor 5 positive allosteric modulator acts at a unique site and confers stimulus bias to mGlu5 signaling. Mol. Pharamacol. 2013;83:835–47. doi: 10.1124/mol.112.082891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bridges TM, Kennedy JP, Noetzel MJ, Breininger ML, Gentry PR, et al. Chemical lead optimization of a pan Gq mAChR M1, M3, M5 positive allosteric modulator (PAM) lead. Part II: Development of a potent and highly selective M1 PAM. Bioorg. Med. Chem. Lett. 2010;20:1972–75. doi: 10.1016/j.bmcl.2010.01.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sheffler DJ, Wenthur CJ, Bruner JA, Carrington SJ, Vinson PN, et al. Development of a novel, CNS-penetrant, metabotropic glutamate receptor 3 (mGlu3) NAM probe (ML289) derived from a closely related mGlu5 PAM. Bioorg. Med. Chem. Lett. 2012;22:3921–25. doi: 10.1016/j.bmcl.2012.04.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mølck C, Harpsøe K, Gloriam DE, Clausen RP, Madsen U, et al. Pharmacological characterization and modeling of the binding sites of novel 1,3-bis(pyridinylethynyl)benzenes as metabotropic glutamate receptor 5-selective negative allosteric modulators. Mol. Pharmacol. 2012;82:929–37. doi: 10.1124/mol.112.078808. [DOI] [PubMed] [Google Scholar]
- 79.Gregory KJ, Nguyen ED, Reiff SD, Squire EF, Stauffer SR, et al. Probing the metabotropic glutamate receptor 5 (mGlu5) positive allosteric modulator (PAM) binding pocket: discovery of point mutations that engender a “molecular switch” in PAM pharmacology. Mol. Pharmacol. 2013;83:991–1006. doi: 10.1124/mol.112.083949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoyer I, Haas AK, Kreuchwig A, Schülein R, Krause G. Molecular sampling of the allosteric binding pocket of the TSH receptor provides discriminative pharmacophores for antagonist and agonists. Biochem. Soc. Trans. 2013;4:213–17. doi: 10.1042/BST20120319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rook JM, Noetzel MJ, Pouliot WA, Bridges TM, Vinson PN, et al. Unique signaling profiles of positive allosteric modulators of metabotropic glutamate receptor subtype 5 determine differences in in vivo activity. Biol. Psychiatry. 2013;73:501–9. doi: 10.1016/j.biopsych.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wenthur CJ, Morrison R, Felts AS, Smith KA, Engers JL, et al. Discovery of (R)-(2-fluoro-4-((-4-methoxyphenyl)ethynyl)phenyl) (3-hydroxypiperidin-1-yl)methanone (ML337), an mGlu3 selective and CNS penetrant negative allosteric modulator (NAM) J. Med. Chem. 2013;56:5208–12. doi: 10.1021/jm400439t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schwartz TW, Holst B. Ago-allosteric modulation and other types of allostery in dimeric 7TM receptors. J. Recept. Signal Transduct. Res. 2006;26:107–28. doi: 10.1080/10799890600567570. [DOI] [PubMed] [Google Scholar]
- 84.Schwartz TW, Holst B. Allosteric enhancers, allosteric agonists and ago-allosteric modulators: Where do they bind and how do they act? Trends Pharmacol. Sci. 2007;28:366–73. doi: 10.1016/j.tips.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 85.Bruns RF, Fergus JH. Allosteric enhancement of adenosine A1 receptor binding and function by 2-amino-3-benzoylthiophenes. Mol. Pharmacol. 1990;38:939–49. [PubMed] [Google Scholar]
- 86.Amoah-Apraku B, Xu J, Lu JY, Pelleg A, Bruns RF, Belardinelli L. Selective potentiation by an A1 adenosine receptor enhancer of the negative dromotropic action of adenosine in the guinea pig heart. J. Pharmacol. Exp. Ther. 1993;266:611–17. [PubMed] [Google Scholar]
- 87.Bhattacharya S, Linden J. The allosteric enhancer, PD 81,723, stabilizes human A1 adenosine receptor coupling to G proteins. Biochim. Biophys. Acta. 1995;1265:15–21. doi: 10.1016/0167-4889(94)00204-r. [DOI] [PubMed] [Google Scholar]
- 88.Kollias-Baker CA, Ruble J, Jacobson M, Harrison JK, Ozeck M, et al. Agonist-independent effect of an allosteric enhancer of the A1 adenosine receptor in CHO cells stably expressing the recombinant human A1 receptor. J. Pharmacol. Exp. Ther. 1997;281:761–68. [PubMed] [Google Scholar]
- 89.Figler H, Olsson RA, Linden J. Allosteric enhancers of A1 adenosine receptors increase receptor-G protein coupling and counteract guanine nucleotide effects on agonist binding. Mol. Pharmacol. 2003;64:1557–64. doi: 10.1124/mol.64.6.1557. [DOI] [PubMed] [Google Scholar]
- 90.Noetzel MJ, Rook JM, Vinson PN, Cho HP, Days E, et al. Functional impact of allosteric agonist activity of selective positive allosteric modulators of metabotropic glutamate receptor subtype 5 in regulating central nervous system function. Mol. Pharmacol. 2012;81:120–33. doi: 10.1124/mol.111.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Portoghese PS. Bivalent ligands and the message-address concept in the design of selective opioid receptor antagonists. Trends Pharmacol. Sci. 1989;10:230–35. doi: 10.1016/0165-6147(89)90267-8. [DOI] [PubMed] [Google Scholar]
- 92.Mohr K, Tränkle C, Kostenis E, Barocelli E, De Amici M, Holzgrabe U. Rational design of dualsteric GPCR ligands: quests and promise. Br. J. Pharmacol. 2010;159:997–1008. doi: 10.1111/j.1476-5381.2009.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Valant C, Gregory KJ, Hall NE, Scammells PJ, Lew MJ, et al. A novel mechanism of G protein-coupled receptor functional selectivity: muscarinic partial agonist McN-A-343 as a bitopic orthosteric/allosteric ligand. J. Biol. Chem. 2008;283:29312–21. doi: 10.1074/jbc.M803801200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Valant C, Lane JR, Sexton PM, Christopoulos A. The best of both worlds? Bitopic orthosteric/allosteric ligands of G protein-coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2012;52:153–78. doi: 10.1146/annurev-pharmtox-010611-134514. [DOI] [PubMed] [Google Scholar]
- 95.Schwyzer R. ACTH: a short introductory review. Ann. N. Y. Acad. Sci. 1977;297:3–26. doi: 10.1111/j.1749-6632.1977.tb41843.x. [DOI] [PubMed] [Google Scholar]
- 96.Roszkowski AP. An unusual type of sympathetic ganglionic stimulant. J. Pharmacol. Exp. Ther. 1961;132:156–70. [PubMed] [Google Scholar]
- 97.May LT, Avlani VA, Langmead CJ, Herdon HJ, Wood MD, et al. Structure-function studies of allosteric agonism at M2 muscarinic acetylcholine receptors. Mol. Pharmacol. 2007;72:463–76. doi: 10.1124/mol.107.037630. [DOI] [PubMed] [Google Scholar]
- 98.Mitchelson FJ. The pharmacology of McN-A-343. Pharmacol. Ther. 2012;135:216–45. doi: 10.1016/j.pharmthera.2012.05.008. [DOI] [PubMed] [Google Scholar]