Abstract
We report the prevalence of subjective cognitive impairment (SCI) and cognitive impairment no dementia (CIND), their socio-demographic profile, and the contribution of genetic background and shared familial environment to SCI and CIND. Subjects were 11,926 dementia-free twin individuals aged ≥ 65 from the Swedish Twin Registry. SCI was defined as subjective complaint of cognitive change without objective cognitive impairment and CIND was defined according to current criteria. Overall prevalence of SCI and CIND was 39% (95% CI 38-39) and 25% (95% CI 24-25). Among those with CIND, 57% had subjective cognitive complaints; 43% did not. In multivariate GEE models, both SCI and CIND were older compared with people without any cognitive impairment. CIND were also less educated, more likely to be unmarried and to have lower socioeconomic status (SES). SCI individuals differed from persons with CIND as they were older, more educated, more likely to be married, and to have higher SES. Co-twin control analysis, which corrects for common genetic and shared environmental background, confirmed that low education was still associated with CIND. Probandwise concordance for SCI and CIND was 63% and 52% in monozygotic twins, 63% and 50% in dizygotic same-sex twins, and 42% and 29% in dizygotic unlike-sex twins. Tetrachoric correlations showed no significant differences between monozygotic and dizygotic same-sex twins. We conclude that subjective and objective cognitive impairment are both highly prevalent among nondemented elderly yet have distinct sociodemographic profiles. Shared environmental influences rather than genetic background play a role in the occurrence of SCI and CIND.
Keywords: Cognitive impairment no dementia, concordance, population-based, prevalence, socio-demographic, subjective cognitive impairment, twin study
Introduction
Older adults frequently report the subjective feeling that their mental abilities have worsened. This phenomenon has been labeled subjective memory complaint or subjective memory impairment.1 Indeed, memory problems are frequently reported as one of the first losses in both normal and accelerated cognitive aging.2 Nonetheless, the cognitive problems observed in elderly people are not mainly and not only related to memory3 hence the term subjective cognitive impairment (SCI) has been proposed.4
Subjective cognitive complaints have been studied for a few decades and are currently included in the definition of mild cognitive impairment.5 The definition of cognitive impairment no dementia (CIND), on the other hand, does not require the presence of subjective cognitive complaints6, 7 and can rely solely on psychometrically detectable cognitive deficits in cases that do not fulfill a dementia diagnosis.8-10 Indeed, a number of studies have questioned SCI as a defining criterion for cognitive impairment11-13 and it has been suggested that cognitive complaints can be related to personality traits and to affective symptoms rather than to objective cognitive problems.14-16 Recently, however, two longitudinal studies have reported an increased risk of dementia in people with SCI and no objective cognitive impairment.17, 18 This finding suggests that SCI may precede the stage when the cognitive impairment is detectable rather than co-occurring with it.
In spite of the longstanding presence of the construct of SCI in dementia research and its frequent use as a case-finding tool, there have been only a few population-based reports on the prevalence of SCI and its distribution among different strata of the population.1, 4 In particular, there has been no population-based study exploring the prevalence of SCI without cognitive impairment.4 Also, available studies on subjective and objective cognitive impairment have generally been focused on their association rather than on the characterization of SCI and CIND in the population.1, 4 Additional insights on SCI and CIND can derive from twin studies, which are an ideal platform to investigate the role of genetic and environmental influences in age-related conditions.
In this framework, we aimed to compare SCI and CIND on the following aspects: i) their prevalence in a population-based twin study of nondemented elderly, ii) their association with socio-demographic factors, and iii) the contribution of genetic background and shared familial environment to the occurrence of SCI and CIND and to their association with socio-demographic factors.
Methods
Study Population
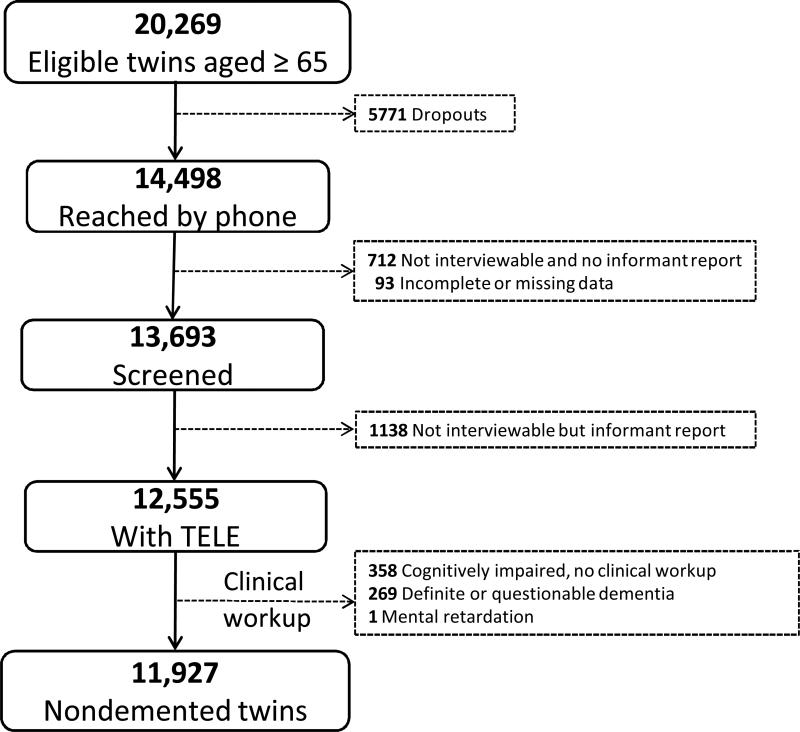
Participants were members of the nationwide Swedish Twin Registry. In 1988-2001, all living twins in the registry who were born in 1935 and earlier (aged 65 years or older) were invited to participate in HARMONY, a population-based twin study of aging and dementia.19 Of the 20,269 eligible twin individuals, 5,771 could not be contacted by phone and 712 were contacted but were unable to partake in the interview and there was no available informant. For a further 93 subjects the information was incomplete or missing. Of the remaining 13,693 twin individuals, 1,138 were not available for interview because of sickness or severe cognitive impairment but there was an interviewable next of kin. Out of the 12,555 participants with cognitive screening data we excluded people with: dementia (n=144), questionable dementia (n=125), cognitive impairment but missing clinical workup (n=358), and mental retardation (n=1). The final cohort consisted of 11,927 dementia-free twin individuals (Figure 1).
Figure 1.
Flow-chart of the study population.
Informed consent was required from all participants during the telephone interview and again in the clinical phase. The study was approved by the Regional Ethic Committee at Karolinska Institutet, Stockholm, Sweden; and by the Institutional Review Board of the University of Southern California, California, USA.
Cognitive assessment
The cognitive status of the participants was assessed following a three-step procedure. First, the cognitive screening, consisting of the administration of a validated telephone interview (TELE).20, 21 The TELE interview, which has been described in detail elsewhere,20 includes cognitive tasks that cover different cognitive areas, such as orientation, attention, episodic memory and reasoning. More specifically, the orientation task includes the 10 items of the MSQ;22 attention was measured by counting backwards in threes;23 reasoning was tapped with questions about similarities and differences between pairs of nouns;24 and episodic memory was evaluated using three-item recall.25 In case of failure to recall all three items, the subject was given the possibility to remember the items with a recognition task, in which he or she was required to identify the correct word or words within a list of distractors. TELE's cognitive performance is summarized in a total score that ranges from 0 (worst performance) to 19 (best performance).
TELE includes also a section investigating cognitive complaints, with a general question on subjective memory change “Have you noticed any change in your memory during the last three years?”, followed by more specific questions focusing on different cognitive problems such as forgetting errands, forgetting people's names, forgetting appointments, forgetting known places, and forgetting words. Further items addressed whether respondents were living independently, their employment status, their recent visits for medical care, their eyesight and hearing, assistance with practical tasks in daily life due to memory or cognitive problems, and their mood.
The second step consisted of an informant interview to next-of-kin of people who did not perform optimally or could not perform TELE. The informant interview included questions regarding the subject's health, functional status, activities of daily living, and employment status. If cognitive problems were indicated, follow-up questions were used to obtain a history of the impairment and the pattern of decline, as well as a description of any contacts with the health care system concerning the problems. The eleven items of the Blessed Dementia Rating Scale (BDRS) were also included in the informant interview. The BDRS assesses cognitive functioning in everyday activities and its score ranges from 0 to 17, with higher score indicating greater frequency of problems.26
The third and last step involved a complete dementia workup of people who were suspected of cognitive dysfunction according to TELE and BDRS combined. The dementia workup was similar to the protocol of the Consortium to Establish a Registry for Alzheimer's Disease and was performed by teams comprised of a physician and a nurse. It included a physical and neurological examination, a review of medical history, informant interview, and a neuropsychological assessment.
Definition of dementia
Clinical diagnoses of dementia followed DSM-IV criteria.27 Preliminary diagnoses made by the assessment team were reviewed by a diagnostic board consisting of a neurologist and a psychologist. When DSM-IV criteria were completely fulfilled, subjects were diagnosed as having “dementia” in contrast with a category of “questionable dementia” which was used for individuals who did not fulfill one of the first three DSM-IV diagnostic criteria but did exhibit either cognitive impairment or functional disability.19
Definition of SCI and CIND
SCI was defined as subjective cognitive change in the last three years in otherwise cognitively intact people. The operationalization criteria of SCI were as follows: 1) presence of subjective cognitive complaint was defined as self-reported cognitive change within the last three years asked to the subject as part of TELE; 2) absence of objective cognitive impairment defined as CIND, as described below; 3) absence of dementia defined on the basis of a clinical diagnosis following the DSM-IV criteria, as described above.
CIND was defined according to current criteria6-8, 10 as cognitive impairment in the absence of dementia and was operationalized as follows: 1) presence of cognitive impairment defined as a performance at least two standard deviations9 below the age- and education-specific mean on any of the four cognitive tasks of TELE; 2) absence of dementia defined on the basis of a clinical diagnosis following the DSM-IV criteria, as described above. The age- and education-specific means of TELE's cognitive tasks were based on the average performance of the dementia-free population classified in eight age and education specific groups.
Due to the operational definition adopted in this study, SCI and CIND were mutually exclusive as no one could be categorized as both SCI and CIND. However, the CIND group included both individuals with subjective cognitive complaints and individuals with no subjective cognitive complaints.
Covariates collection and definition
Information on age, sex and zygosity status was obtained from the Swedish Twin Registry. Information on education, marital status and occupation was gathered during the subject's telephone interview and verified by an informant when the subject was unable to be interviewed or did not perform well at TELE. The validity of ascertainment of zygosity based on self-reports of similarity has been tested against blood markers and found valid in 99% of the cases.28 There were four possible categories of zygosity: monozygotic, in case of identical twins; same-sex dizygotic (fraternal twins); unlike-sex dizygotic; and undetermined. This latter category included twin individuals for whom zygosity could not be ascertained.
Age in years was categorized in four groups of five years each, and a fifth group of people aged 85 and over. Education was categorized into three groups based on the years of attained formal education, ranging from low (0 to 7 years), to intermediate (8 to 10 years) and high (more than 10 years) education. Marital status was dichotomized into married versus non married, including in the married category also couples who were cohabiting without being married. Occupation was defined on the basis of an open-ended question about the main occupation in life. These were assigned occupational codes by Statistics Sweden and those codes were categorized as either high occupational SES, including all “white collar” or non-manual main occupations, or low occupational SES including all “blue collar” or manual main occupations.29
Data analysis
The prevalence rates (cases per 100 subjects) of SCI and CIND were calculated in five different age groups according to sex and education. Ninety-five percent confidence intervals (95% CI) were calculated based on the binomial distribution. Prevalence rates of CIND were based on the whole study population (N=11,927), while those of SCI were based on a subsample (N=11,419) with complete data on subjective cognitive complaints.
Groups according to cognitive status were compared with the rest of the sample using chi-square tests for categorical variables and one-way ANOVA or Mann-Whitney test for continuous variables. To investigate the association of SCI and CIND with sociodemographic factors SCI and CIND were compared to people without subjective or objective cognitive impairment (NCI). Further, to highlight similarities and differences in the socio-demographic profiles of SCI and CIND the two groups were also directly compared. We used the following two strategies: 1) Generalized Estimating Equation (GEE) models on the whole cohort; and 2) Conditional Logistic Regression on the twin pairs discordant for cognitive status. In more detail, GEE analysis is conceptually equivalent to logistic regression, but controls for the clustering of twins within a pair. On the other hand, Conditional Logistic Regression allows matching for unmeasured familial factors, such as genetic background and shared familial environment. If the association found with GEE models becomes attenuated in Conditional Logistic Regression models, familial factors are likely to play a role in the association. In contrast, if the association remains significant, the influence of genetic background and shared familial environment are likely to be marginal.28
Separate GEE models were run on the subsample with available occupational SES (N=9,828). Supplementary analyses were conducted on people with and without subjective cognitive complaints independent of CIND status and on people with CIND stratified by subjective cognitive complaints.
Probandwise Concordance was calculated according to the formula of 2C/(2C+D), in which C is the number of twin pairs concordant for the studied outcome and D is the number of discordant twin pairs.30 For SCI, the pairs were considered concordant when both twins had SCI and discordant when one twin had SCI and the other was NCI. For CIND, the pairs were considered concordant when both twins had CIND and discordant when one twin had CIND and the other was NCI. To further investigate the conditional probability of a twin to be affected by SCI or CIND, given that the co-twin is affected, we ran Tetrachoric Correlations with 95% confidence intervals. Tetrachoric correlations represent the correlation of liability between relatives and are analogous to intra-class correlation based on continuous data.30 Both probandwise concordance and tetrachoric correlations were performed for monozygotic, dizygotic same-sex and dizygotic unlike-sex twin pairs separately. Concordance rates and tetrachoric correlations calculated including discordant pairs where one had SCI and the other had CIND (n=592) are reported in the supplementary analyses.
Missing values were imputed with Multiple Imputation, based on information available for other covariates.31
The significance level was set at p<0.05. All statistical analyses were performed using the statistical software packages PASW 18.0 and STATA 10.0.
Results
We detected 4,602 persons with SCI without objective cognitive impairment and 2,927 cases of CIND. In all, 3890 persons had no subjective or objective cognitive impairment (NCI). Table 1 shows the characteristics of the total population and of the different groups according to cognitive status.
Table 1.
Subjects’ characteristics in the whole study population and in persons with subjective cognitive impairment (SCI), cognitive impairment no dementia (CIND), and no cognitive impairment (NCI). Number of subjects; percent in brackets.
| Study population n=11,927 | SCI n=4,602 | CIND n=2,927 | NCI n=3,890 | |
|---|---|---|---|---|
| Age groups | ||||
| 65-69 | 4,426 (37) | 1,524 (33) | 1,035 (35) | 1,676 (43) |
| 70-74 | 3,439 (29) | 1,382 (30) | 853 (29) | 1,048 (27) |
| 75-79 | 2,348 (20) | 995 (22) | 565 (19) | 687 (18) |
| 80-84 | 1,194 (10) | 500 (11) | 301 (10) | 344 (9) |
| 85+ | 520 (4) | 202 (4) | 173 (6) | 135 (3) |
| Gender | ||||
| Women | 6,240 (55) | 2,076 (45) | 1,680 (57) | 2,133 (55) |
| Men | 5,037 (45) | 2,526 (55) | 1,247 (43) | 1,757 (45) |
| Education | ||||
| Low | 5,982 (50) | 2,275 (49) | 1,579 (54) | 1,852 (48) |
| Intermediate | 3,660 (31) | 1,372 (30) | 997 (34) | 1,166 (30) |
| High | 2,285 (19) | 955 (21) | 351 (12) | 872 (22) |
| Marital Status | ||||
| Married | 6,983 (62) | 2,883 (63) | 1,677 (58) | 2,485 (64) |
| Unmarried | 4,292 (38) | 1,718 (37) | 1,210 (42) | 1,405 (36) |
| Occupational SES | ||||
| Low | 4,854 (49) | 1,830 (47) | 1,406 (60) | 1,476 (38) |
| High | 4,974 (51) | 2,055 (53) | 956 (40) | 2,485 (64) |
| Zygosity | ||||
| Monozygotic | 2,815 (24) | 1,116 (24) | 658 (23) | 925 (24) |
| Same-sex dizygotic | 4,880 (41) | 1,885 (41) | 1,196 (41) | 1,577 (41) |
| Unlike-sex dizygotic | 4,050 (34) | 1,532 (33) | 1,021 (35) | 1,332 (34) |
| Indetermined | 182 (1) | 69 (2) | 52 (2) | 56 (1) |
As described in Table 2, the majority of people with SCI (58%) reported more than two complaints, with the most common complaint being “forgetting people's names” (85%), followed by “forgetting words” (49%). Most CIND cases were mild (78%), having only one cognitive domain impaired.
Table 2.
Severity and type of subjective cognitive impairment (SCI) and cognitive impairment no dementia (CIND). Mean TELE* scores with standard deviations as well as number and percentages within SCI and CIND are shown.
| No (%) | TELE score mean (SD) | ||
|---|---|---|---|
| SCI | |||
| Severity | Complaint in 1 domain | 1,958 (42) | 16.7 (1.5) |
| Complaints in 2+ domains | 2,644 (58) | 16.3 (1.4) | |
| Type | Forgets errands | 1,370 (30) | 16.2 (1.4) |
| Forgets people's name | 3,915 (85) | 16.5 (1.5) | |
| Forgets appointments | 74 (2) | 16.0 (1.4) | |
| Forget known places | 158 (3) | 15.6 (1.5) | |
| Forgets words | 2,263 (49) | 16.4 (1.4) | |
| Other | 326 (7) | 16.2 (1.5) | |
| CIND | |||
| Severity | 1 test impaired | 2,282 (78) | 14.0 (1.4) |
| 2+ tests impaired | 645 (22) | 11.2 (1.9) | |
| Type | Orientation | 1,129 (39) | 13.3 (2.2) |
| Reasoning | 840 (29) | 12.7 (2.2) | |
| Calculation | 914 (31) | 12.3 (2.2) | |
| Episodic memory | 841 (29) | 12.8 (2.3) |
Telephone cognitive screening test: scores range from 0 (worse) to 19 (best).
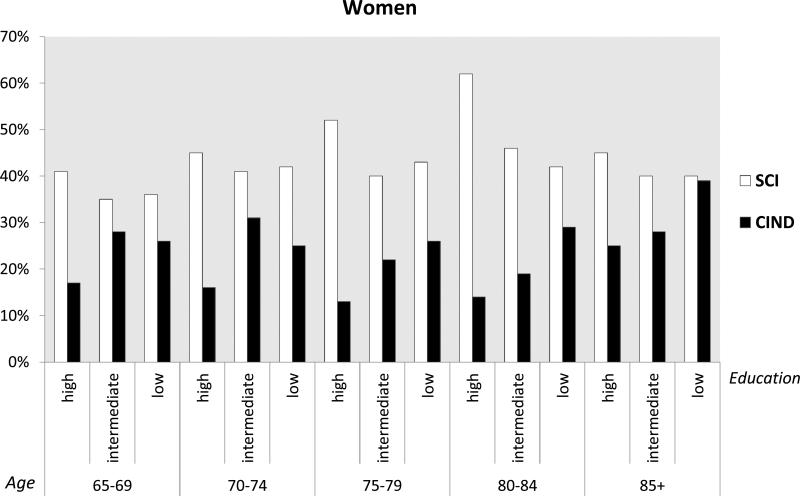
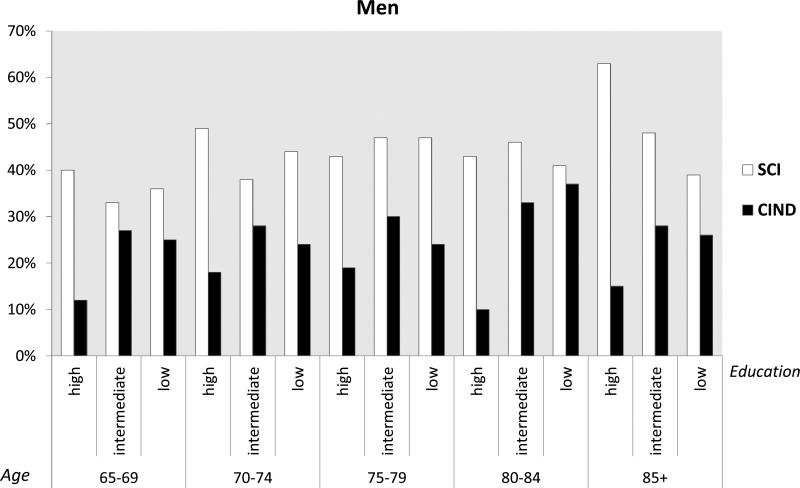
The overall prevalence of SCI was 39% (95% CI 38-39) and that of CIND was 25% (95% CI 24-25). The prevalence of SCI was significantly higher than that of CIND, as shown by the non-overlapping confidence intervals. Rates of SCI were significantly higher in people with high compared to low occupational SES (43 vs. 39 per cent). Increased odds of SCI were observed in all older age groups compared to the youngest old (65-69 years old), although there was not a linear increase with increasing age (prevalence per cent: 65-69 yrs: 37; 70-74 yrs: 43; 75-79 yrs: 45; 80-84 yrs: 44; 85+ yrs: 40). A reverse demographic profile was observed in CIND compared to SCI. Rates of CIND were significantly higher in people with low compared to high occupational SES (29 vs. 19 per cent) and also in people with low/medium compared to high educational level (27 vs. 15 per cent), and in unmarried compared to married people (27 vs. 23 per cent). In CIND only the oldest old (85+ years old) had increased odds compared to the youngest old (prevalence per cent: 65-69 yrs: 23; 70-74 yrs: 25; 75-79 yrs: 24; 80-84 yrs: 25; 85+ yrs: 33). In age-, gender- and education-specific figures, SCI was more prevalent in higher educated men and women regardless of the age group (Figures 2 and 3). On the other hand, CIND was consistently more prevalent among people with low/medium education, regardless of both age and gender (Figures 2 and 3).
Figure 2.
Prevalence per 100 and 95% confidence intervals of subjective cognitive impairment (SCI) and cognitive impairment no dementia (CIND) by age and education in women.
Figure 3.
Prevalence per 100 and 95% confidence intervals of subjective cognitive impairment (SCI) and cognitive impairment no dementia (CIND) by age and education in men.
In multivariate GEE models comparing SCI and CIND respectively with NCI (Table 3), older age was the only socio-demographic aspect associated with SCI. CIND was also associated with older age, although there was not a clear trend with increasing age. In addition, CIND was associated with lower educational level, unmarried status, and in the model including SES, with lower occupational SES and female gender. When directly comparing SCI with CIND (Table 3), people with SCI were older, more likely to have higher education, to be married, and to have higher occupational SES, while there were no significant differences according to gender.
Table 3.
Association of subjective cognitive impairment (SCI) and cognitive impairment no dementia (CIND) with sociodemographic variables. Odds ratios with 95% confidence intervals from unmatched Generalized Estimating Equation (GEE) are shown.
| SCI^ versus NCI | CIND^ versus NCI | CIND versus SCI^^ | ||||
|---|---|---|---|---|---|---|
| Model 1a | Model 2b | Model 1a | Model 2b | Model 1a | Model 2b | |
| Age groups | ||||||
| 65-68 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| 70-74 | 1.4 (1.3-1.6)** | 1.4 (1.3-1.6)** | 1.3 (1.1-1.4)** | 1.3 (1.1-1.5)** | 1.1 (1.0-1.3)* | 1.1 (1.0-1.3) |
| 75-79 | 1.6 (1.4-1.8)** | 1.6 (1.4-1.8)** | 1.2 (1.1-1.4)** | 1.2 (1.0-1.4)* | 1.3 (1.1-1.5)** | 1.3 (1.1-1.5)** |
| 80-84 | 1.6 (1.4-1.9)** | 1.7 (1.4-2.0)** | 1.3 (1.1-1.6)** | 1.3 (1.0-1.6)* | 1.2 (1.0-1.5)* | 1.3 (1.1-1.6)** |
| 85+ | 1.7 (1.3-2.1)** | 1.7 (1.3-2.3)** | 1.8 (1.4-2.3)** | 1.8 (1.4-2.4)** | 0.9 (0.7-1.1) | 0.9 (0.7-1.1) |
| Gender | ||||||
| Man | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Woman | 1.0 (0.9-1.1) | 1.0 (0.9-1.1) | 1.0 (0.9-1.1) | 1.1 (1.0-1.3)* | 1.0 (0.9-1.1) | 0.9 (0.8-1.0) |
| Education | ||||||
| Low | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Intermediate | 1.0 (0.9-1.1) | 1.0 (0.9-1.1) | 1.0 (0.9-1.1) | 1.2 (1.0-1.3)* | 1.0 (0.9-1.1) | 0.9 (0.9-1.0) |
| High | 0.9 (0.8-1.1) | 1.0 (1.0-1.2) | 0.5 (0.4-0.6)** | 0.7 (0.6-0.8)** | 1.9 (1.6-2.2)** | 1.4 (1.2-1.7)** |
| Married | ||||||
| No | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Yes | 1.1 (0.9-1.2) | 1.0 (0.9-1.1) | 0.9 (0.8-0.9)* | 0.8 (0.7-0.9)** | 1.2 (1.1-1.3)** | 1.3 (1.1-1.4)** |
| SES | ||||||
| Low | 1.0 | 1.0 | 1.0 | |||
| High | 0.9 (0.8-1.0) | 0.6 (0.5-0.7)** | 1.5 (1.3-1.7)** | |||
SCI and CIND are compared with twins with no subjective nor objective cognitive impairment (NCI)
CIND compared with SCI
Adjusted for age, gender, education and marital status
Adjusted for age, gender, education, marital status and SES
Significant with p<0.05
Significant with p>0.01.
In matched co-twin control models (Table 4), the association of CIND with education was unchanged while the association of SCI with education and the associations of SCI and CIND with SES and marital status were attenuated.
Table 4.
Association of subjective cognitive impairment (SCI) and cognitive impairment no dementia (CIND) with sociodemographic variables. Adjusted odds ratios with 95% confidence intervals from co-twin control Conditional Regression analysis are shown.
| SCI versus NCI | CIND versus NCI | CIND versus SCI | |
|---|---|---|---|
| Education | |||
| Low | 1.0 | 1.0 | 1.0 |
| Intermediate | 1.0 (0.8-1.4) | 0.9 (0.6-1.3) | 1.3 (0.9-1.8) |
| High | 1.0 (0.7-1.5) | 0.5 (0.3-0.8)** | 0.7 (0.5-1.1) |
| Married | |||
| No | 1.0 | 1.0 | 1.0 |
| Yes | 1.2 (1.0-1.5) | 0.9 (0.7-1.2) | 0.9 (0.7-1.1) |
| SES | |||
| Low | 1.0 | 1.0 | 1.0 |
| High | 1.0 (0.8-1.4) | 0.7 (0.4-1.0) | 0.9 (0.7-1.1) |
*Significant with p<0.05
Significant with p>0.01.
Concordance rates for SCI and CIND were similar in monozygotic and same-sex dizygotic twin pairs (Table 5). These patterns were confirmed by tetrachoric correlation coefficients, which did not significantly differ between monozygotic and same-sex dizygotic twins (Table 5). In unlike-sex dizygotic twins both concordance rates and correlation coefficients were lower compared to both monozygotic and same-sex dizygotic twins for SCI but not for CIND.
Table 5.
Similarity of monozygotic, dizygotic and unlike sex twins for SCI and CIND.
| Monozygotic | Dizygotic | Unlike sex | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N Pairs | Probadwise Concordance Rates | Tetrachoric Correlations (95% CI) | N Pairs | Probadwise Concordance Rates | Tetrachoric Correlations (95% CI) | N Pairs | Probadwise Concordance Rates | Tetrachoric Correlations (95% CI) | |
| SCI | 554 | 63 | 0.31 (0.23 -0.38) | 778 | 63 | 0.31 (0.23-0.36) | 1,153 | 43 | 0.13 (0.06-0.22) |
| CIND | 308 | 52 | 0.40 (0.30 -0.49) | 467 | 50 | 0.29 (0.21 -0.37) | 276 | 29 | 0.23 (0.14-0.32) |
Supplementary analyses
Cognitive complaints regardless of objective cognitive impairment had a prevalence of 52%. Complainers were significantly older when compared with people without cognitive complaints regardless of objective cognitive impairment, while the two groups were similar in terms of other socio-demographic variables.
Within people with CIND, 57% had subjective cognitive complaints and 43% had no subjective cognitive complaints. These two groups generally had similar demographic profiles, with the exception that CIND with cognitive complaints were significantly older than CIND without cognitive complaints.
When including SCI/CIND discordant pairs (N=592), results were similar to those reported for SCI/NCI and CIND/NCI discordant pairs, showing a basic overlapping between monozygotic and same-sex dizygotic twin pairs in both concordance rates and tetrachoric correlations.
Discussion
In this population-based twin study of nondemented elderly the overall prevalence rates of SCI and CIND were 39% and 25% respectively. These figures are within the 25-57% and 5-32% ranges previously reported for cognitive complaints1, 32-35 and CIND.9, 36-41 Notably our estimates of CIND were similar to those reported by the Canadian Study of Health and Aging,42 which used operational criteria close to those adopted in the present study.
SCI was more prevalent in older compared to younger age groups. This finding was confirmed by multivariate models and suggests that cognitive complaints may be part of the aging process, in agreement with the great majority of previous studies.1, 14, 32-34 Similarly, CIND was more prevalent in older compared to younger old but there was not a clear trend with age. When directly compared with SCI, people with CIND tended to be younger. Previous studies on CIND have reported contradictory results regarding an association with age among those already aged 65 or older.37, 43, 44,7, 39, 45 It is likely that the adjustment for age commonly used in the operationalization of CIND may affect trends with age in the studied samples.
There were not significant differences by gender in the prevalence of SCI or CIND and this was confirmed by multivariate models. Association with female gender was observed only when comparing CIND with NCI and adjusting for SES. Our findings are generally in line with previous prevalence studies of CIND that reported no association or association with female gender,36 whereas they are in contrast with most literature on cognitive complaints which points toward a positive association with female gender.33-35, 46 However, most previous studies included people with CIND in the SCI group.
When directly compared with CIND, SCI was more prevalent among people with higher education and occupational SES and in married individuals. When using NCI as the reference, CIND was still associated with lower education, unmarried status, and lower SES while SCI was no longer associated with these factors. Most previous studies on subjective cognitive complaints reported association with lower education.33, 35, 47-49 Discrepancies can again be explained with the inclusion of people with CIND in the SCI group. On the other hand, there is general agreement on the association of CIND with lower education.39, 43-45 Only a few studies have investigated SES in relation to CIND50, 51 and, to our knowledge, no population-based study reported data on the distribution of SCI according to SES. Recently, increased risk of incident CIND was reported in people with lower occupational SES, although the association with prevalent CIND did not reach significance.52 Similarly to SES, marital status has not been included in most prevalence reports of SCI or CIND. Available evidence supports our finding of an association of CIND with unmarried status.39, 53
The different distributions of SCI and CIND by education, SES, and marital status may have alternative explanations. One explanation is in terms of cognitive and brain reserve.54 Indeed, older people with higher education, higher SES, and who are married may be protected against overt cognitive impairment even in the face of initial loss of brain integrity because of improved use of cognitive strategies (high vs. lower education), improved lifestyle (high vs. lower SES) and richer social network (married vs. unmarried). In these people cognitive complaints may be among the first signs of underlying neurodegeneration.55-57 An alternative hypothesis would be that people with higher education, higher SES, and who are married may take a greater interest in their health and be more aware of even minimal cognitive changes. This is in agreement with findings which showed that people with richer social networks have higher odds of SCI32 and that people with higher education had a lower chance of anosognosia of their cognitive deficits.58 However, the direct comparison of SCI with NCI showed no difference according to education, SES or marital status.
Matched analysis on twin pairs showed confounding by familial factors in the association of SCI with education and SCI and CIND with marital status and SES. On the other hand, the association of CIND with education was unchanged. This confirms previous evidence from the HARMONY Study which showed the independence of the relationship between education and dementia from genetic background.59
Probandwise concordance showed the absence of a substantial difference between monozygotic and dizygotic twins, pointing towards a limited role of genetic background. Tetrachoric correlations confirmed these findings, suggesting that in nondemented elderly twins shared familial environment may play a role in determining SCI and, even more so, CIND. Our results do not confirm previous evidence from the HARMONY Study that showed a moderate association of genetic background with cognitive dysfunction.60 Discrepancies can be explained by the different operationalization of cognitive impairment and by subjects’ selection. Indeed, while in our study cases were generally mild, the prior study included a more heterogeneous sample spanning from mild to severe cognitive impairment and dementia.60
Our study has many strengths, which include: a) the nation-wide population-based design; b) the broad age range; c) the detection of the prevalence of two major non-dementia syndromes, SCI and CIND, in the same population; d) the stratification of prevalence by age, gender, and education and the inclusion of other, less studied, sociodemographic factors; e) the direct comparison of SCI and CIND with NCI and with each other; and e) the possibility to evaluate the contribution of genetic background and shared familial environment to SCI and CIND and to their association with sociodemographic factors.
There are also some limitations to be considered. First, SCI and CIND were assessed with a relatively brief, although highly validated, telephone interview. Consequently, the risk of misclassification cannot be ruled out. Yet the cognitive screening represented only the first of a three-step assessment of cognitive functioning which involved reports from close informants and direct extensive clinical examination of subjects with suspected cognitive impairment. Therefore, we feel confident about the exclusion of dementia cases. Secondly, the definition of SCI was based on a single question, accompanied by other questions aimed at characterizing the type of cognitive complains reported by the subjects. Indeed, although a few questionnaires on cognitive complaints are available, none of them has been extensively validated and there is still considerable variability in the assessment of cognitive complaints.61 Thirdly, the twin-study design may have limited the generalizability of our prevalence estimates and relative sociodemographic profiles. However, other studies have found the Swedish Twin Registry sample to be comparable to the general population of the country.19, 28, 62 Moreover, we statistically controlled for the clustering of twins within a pair when investigating association with sociodemographic factors.
In conclusion, both SCI and CIND are highly prevalent in the elderly population yet have distinct demographic profiles, suggesting that SCI and CIND may be distinct entities and not simply different stages of the same process. Genetic background has a limited role in determining SCI and CIND, pointing towards a more prominent role of environment factors in SCI and CIND. This implies that these conditions can be prevented and that their prevalence in the population can be reduced.
Acknowledgments
This study was supported by grants from the Swedish Council for Working Life and Social Research, Regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet, the Swedish Brain Power Initiative, and NIH Grant No. R01AG08724. Private funding from Stiftelsen Gamla Tjänarinnor and Gun and Bertil Stohnes Foundation has also been provided.
References
- 1.Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psychiatry. 2000;15:983–991. doi: 10.1002/1099-1166(200011)15:11<983::aid-gps238>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 2.Lister JP, Barnes CA. Neurobiological changes in the hippocampus during normative aging. Arch Neurol. 2009;66:829–833. doi: 10.1001/archneurol.2009.125. [DOI] [PubMed] [Google Scholar]
- 3.Backman L, Jones S, Berger AK, Laukka EJ, Small BJ. Multiple cognitive deficits during the transition to Alzheimer's disease. J Intern Med. 2004;256:195–204. doi: 10.1111/j.1365-2796.2004.01386.x. [DOI] [PubMed] [Google Scholar]
- 4.Reisberg B, Prichep L, Mosconi L, et al. The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer's disease. Alzheimers Dement. 2008;4:S98–S108. doi: 10.1016/j.jalz.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 6.Ebly EM, Parhad IM, Hogan DB, Fung TS. Prevalence and types of dementia in the very old: results from the Canadian Study of Health and Aging. Neurology. 1994;44:1593–1600. doi: 10.1212/wnl.44.9.1593. [DOI] [PubMed] [Google Scholar]
- 7.Graham JE, Rockwood K, Beattie BL, et al. Prevalence and severity of cognitive impairment with and without dementia in an elderly population. Lancet. 1997;349:1793–1796. doi: 10.1016/S0140-6736(97)01007-6. [DOI] [PubMed] [Google Scholar]
- 8.Caracciolo B, Palmer K, Monastero R, Winblad B, Backman L, Fratiglioni L. Occurrence of cognitive impairment and dementia in the community: a 9-year-long prospective study. Neurology. 2008;70:1778–1785. doi: 10.1212/01.wnl.0000288180.21984.cb. [DOI] [PubMed] [Google Scholar]
- 9.De Ronchi D, Faranca I, Berardi D, et al. Risk factors for cognitive impairment in HIV-1-infected persons with different risk behaviors. Archives of neurology. 2002;59:812–818. doi: 10.1001/archneur.59.5.812. [DOI] [PubMed] [Google Scholar]
- 10.Narasimhalu K, Ang S, De Silva DA, et al. Severity of CIND and MCI predict incidence of dementia in an ischemic stroke cohort. Neurology. 2009;73:1866–1872. doi: 10.1212/WNL.0b013e3181c3fcb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Purser JL, Fillenbaum GG, Wallace RB. Memory complaint is not necessary for diagnosis of mild cognitive impairment and does not predict 10-year trajectories of functional disability, word recall, or short portable mental status questionnaire limitations. J Am Geriatr Soc. 2006;54:335–338. doi: 10.1111/j.1532-5415.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- 12.Jungwirth S, Fischer P, Weissgram S, Kirchmeyr W, Bauer P, Tragl KH. Subjective memory complaints and objective memory impairment in the Vienna-Transdanube aging community. J Am Geriatr Soc. 2004;52:263–268. doi: 10.1111/j.1532-5415.2004.52066.x. [DOI] [PubMed] [Google Scholar]
- 13.Riedel-Heller SG, Matschinger H, Schork A, Angermeyer MC. Do memory complaints indicate the presence of cognitive impairment? Results of a field study. Eur Arch Psychiatry Clin Neurosci. 1999;249:197–204. doi: 10.1007/s004060050087. [DOI] [PubMed] [Google Scholar]
- 14.Reid LM, Maclullich AM. Subjective memory complaints and cognitive impairment in older people. Dement Geriatr Cogn Disord. 2006;22:471–485. doi: 10.1159/000096295. [DOI] [PubMed] [Google Scholar]
- 15.Jorm AF, Butterworth P, Anstey KJ, et al. Memory complaints in a community sample aged 60-64 years: associations with cognitive functioning, psychiatric symptoms, medical conditions, APOE genotype, hippocampus and amygdala volumes, and white-matter hyperintensities. Psychol Med. 2004;34:1495–1506. doi: 10.1017/s0033291704003162. [DOI] [PubMed] [Google Scholar]
- 16.Hanninen T, Reinikainen KJ, Helkala EL, et al. Subjective memory complaints and personality traits in normal elderly subjects. J Am Geriatr Soc. 1994;42:1–4. doi: 10.1111/j.1532-5415.1994.tb06064.x. [DOI] [PubMed] [Google Scholar]
- 17.Jessen F, Wiese B, Bachmann C, et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67:414–422. doi: 10.1001/archgenpsychiatry.2010.30. [DOI] [PubMed] [Google Scholar]
- 18.Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 2010;6:11–24. doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gatz M, Fratiglioni L, Johansson B, et al. Complete ascertainment of dementia in the Swedish Twin Registry: the HARMONY study. Neurobiol Aging. 2005;26:439–447. doi: 10.1016/j.neurobiolaging.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Gatz M, Reynolds C, Nikolic J, Lowe B, Karel M, Pedersen N. An empirical test of telephone screening to identify potential dementia cases. Int Psychogeriatr. 1995;7:429–438. doi: 10.1017/s1041610295002171. [DOI] [PubMed] [Google Scholar]
- 21.Gatz M, Reynolds CA, John R, Johansson B, Mortimer JA, Pedersen NL. Telephone screening to identify potential dementia cases in a population-based sample of older adults. Int Psychogeriatr. 2002;14:273–289. doi: 10.1017/s1041610202008475. [DOI] [PubMed] [Google Scholar]
- 22.Kahn RL, Goldfarb AI, Pollack M, Peck A. Brief objective measures for the determination of mental status in the aged. Am J Psychiatry. 1960;117:326–328. doi: 10.1176/ajp.117.4.326. [DOI] [PubMed] [Google Scholar]
- 23.Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 24.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 26.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 27.Association AP, editor. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 28.Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med. 2002;252:184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- 29.Andel R, Crowe M, Pedersen NL, et al. Complexity of work and risk of Alzheimer's disease: a population-based study of Swedish twins. J Gerontol B Psychol Sci Soc Sci. 2005;60:P251–258. doi: 10.1093/geronb/60.5.p251. [DOI] [PubMed] [Google Scholar]
- 30.Gatz M, Pedersen NL, Berg S, et al. Heritability for Alzheimer's disease: the study of dementia in Swedish twins. J Gerontol A Biol Sci Med Sci. 1997;52:M117–125. doi: 10.1093/gerona/52a.2.m117. [DOI] [PubMed] [Google Scholar]
- 31.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley; New York: 1987. [Google Scholar]
- 32.Trouton A, Stewart R, Prince M. Does social activity influence the accuracy of subjective memory deficit? Findings from a British community survey. J Am Geriatr Soc. 2006;54:1108–1113. doi: 10.1111/j.1532-5415.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 33.Montejo P, Montenegro M, Fernandez MA, Maestu F. Subjective memory complaints in the elderly: Prevalence and influence of temporal orientation, depression and quality of life in a population-based study in the city of Madrid. Aging Ment Health. 2011;15:85–96. doi: 10.1080/13607863.2010.501062. [DOI] [PubMed] [Google Scholar]
- 34.Park MH, Min JY, Min HY, Lee HJ, Lee DH, Song MS. Subjective memory complaints and clinical characteristics in elderly Koreans: a questionnaire survey. Int J Nurs Stud. 2007;44:1400–1405. doi: 10.1016/j.ijnurstu.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Westoby CJ, Mallen CD, Thomas E. Cognitive complaints in a general population of older adults: prevalence, association with pain and the influence of concurrent affective disorders. Eur J Pain. 2009;13:970–976. doi: 10.1016/j.ejpain.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Panza F, D'Introno A, Colacicco AM, et al. Current epidemiology of mild cognitive impairment and other predementia syndromes. Am J Geriatr Psychiatry. 2005;13:633–644. doi: 10.1176/appi.ajgp.13.8.633. [DOI] [PubMed] [Google Scholar]
- 37.Gavrila D, Antunez C, Tormo MJ, et al. Prevalence of dementia and cognitive impairment in Southeastern Spain: the Ariadna study. Acta Neurol Scand. 2009;120:300–307. doi: 10.1111/j.1600-0404.2009.01283.x. [DOI] [PubMed] [Google Scholar]
- 38.Nunes B, Silva RD, Cruz VT, Roriz JM, Pais J, Silva MC. Prevalence and pattern of cognitive impairment in rural and urban populations from Northern Portugal. BMC Neurol. 2010;10:42. doi: 10.1186/1471-2377-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fei M, Qu YC, Wang T, Yin J, Bai JX, Ding QH. Prevalence and distribution of cognitive impairment no dementia (CIND) among the aged population and the analysis of socio-demographic characteristics: the community-based cross-sectional study. Alzheimer Dis Assoc Disord. 2009;23:130–138. doi: 10.1097/WAD.0b013e318190a59d. [DOI] [PubMed] [Google Scholar]
- 40.Choo IH, Lee DY, Lee JH, et al. The prevalence of cognitive impairment with no dementia in older people: the Seoul study. Int J Geriatr Psychiatry. 2009;24:306–312. doi: 10.1002/gps.2107. [DOI] [PubMed] [Google Scholar]
- 41.Di Carlo A, Lamassa M, Baldereschi M, et al. CIND and MCI in the Italian elderly: frequency, vascular risk factors, progression to dementia. Neurology. 2007;68:1909–1916. doi: 10.1212/01.wnl.0000263132.99055.0d. [DOI] [PubMed] [Google Scholar]
- 42.Ebly EM, Hogan DB, Parhad IM. Cognitive impairment in the nondemented elderly. Results from the Canadian Study of Health and Aging. Arch Neurol. 1995;52:612–619. doi: 10.1001/archneur.1995.00540300086018. [DOI] [PubMed] [Google Scholar]
- 43.De Ronchi D, Berardi D, Menchetti M, et al. Occurrence of cognitive impairment and dementia after the age of 60: a population-based study from Northern Italy. Dement Geriatr Cogn Disord. 2005;19:97–105. doi: 10.1159/000082660. [DOI] [PubMed] [Google Scholar]
- 44.Unverzagt FW, Gao S, Baiyewu O, et al. Prevalence of cognitive impairment: data from the Indianapolis Study of Health and Aging. Neurology. 2001;57:1655–1662. doi: 10.1212/wnl.57.9.1655. [DOI] [PubMed] [Google Scholar]
- 45.Di Carlo A, Baldereschi M, Amaducci L, et al. Cognitive impairment without dementia in older people: prevalence, vascular risk factors, impact on disability. The Italian Longitudinal Study on Aging. J Am Geriatr Soc. 2000;48:775–782. doi: 10.1111/j.1532-5415.2000.tb04752.x. [DOI] [PubMed] [Google Scholar]
- 46.Blazer DG, Hays JC, Fillenbaum GG, Gold DT. Memory complaint as a predictor of cognitive decline: a comparison of African American and White elders. J Aging Health. 1997;9:171–184. doi: 10.1177/089826439700900202. [DOI] [PubMed] [Google Scholar]
- 47.Bassett SS, Folstein MF. Memory complaint, memory performance, and psychiatric diagnosis: a community study. J Geriatr Psychiatry Neurol. 1993;6:105–111. doi: 10.1177/089198879300600207. [DOI] [PubMed] [Google Scholar]
- 48.Gagnon M, Dartigues JF, Mazaux JM, et al. Self-reported memory complaints and memory performance in elderly French community residents: results of the PAQUID Research Program. Neuroepidemiology. 1994;13:145–154. doi: 10.1159/000110373. [DOI] [PubMed] [Google Scholar]
- 49.Schofield PW, Jacobs D, Marder K, Sano M, Stern Y. The validity of new memory complaints in the elderly. Arch Neurol. 1997;54:756–759. doi: 10.1001/archneur.1997.00550180064014. [DOI] [PubMed] [Google Scholar]
- 50.Kim KW, Park JH, Kim MH, et al. A nationwide survey on the prevalence of dementia and mild cognitive impairment in South Korea. J Alzheimers Dis. 2011;23:281–291. doi: 10.3233/JAD-2010-101221. [DOI] [PubMed] [Google Scholar]
- 51.Atti AR, Forlani C, De Ronchi D, et al. Cognitive Impairment after Age 60: Clinical and Social Correlates in the “Faenza Project”. J Alzheimers Dis. 2010;21:1325–1334. doi: 10.3233/jad-2010-091618. [DOI] [PubMed] [Google Scholar]
- 52.Marengoni A, Fratiglioni L, Bandinelli S, Ferrucci L. Socioeconomic Status During Lifetime and Cognitive Impairment No-Dementia in Late Life: The Population-Based Aging in the Chianti Area (InCHIANTI) Study. J Alzheimers Dis. 2011 doi: 10.3233/JAD-2011-101863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atti AR, Forlani C, De Ronchi D, et al. Cognitive Impairment after Age 60: Clinical and Social Correlates in the “Faenza Project”. J Alzheimers Dis. 2010 doi: 10.3233/jad-2010-091618. [DOI] [PubMed] [Google Scholar]
- 54.Fratiglioni L, Wang HX. Brain reserve hypothesis in dementia. J Alzheimers Dis. 2007;12:11–22. doi: 10.3233/jad-2007-12103. [DOI] [PubMed] [Google Scholar]
- 55.van Norden AG, Fick WF, de Laat KF, et al. Subjective cognitive failures and hippocampal volume in elderly with white matter lesions. Neurology. 2008;71:1152–1159. doi: 10.1212/01.wnl.0000327564.44819.49. [DOI] [PubMed] [Google Scholar]
- 56.Visser PJ, Verhey F, Knol DL, et al. Prevalence and prognostic value of CSF markers of Alzheimer's disease pathology in patients with subjective cognitive impairment or mild cognitive impairment in the DESCRIPA study: a prospective cohort study. Lancet Neurol. 2009;8:619–627. doi: 10.1016/S1474-4422(09)70139-5. [DOI] [PubMed] [Google Scholar]
- 57.Barnes LL, Schneider JA, Boyle PA, Bienias JL, Bennett DA. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology. 2006;67:1581–1585. doi: 10.1212/01.wnl.0000242734.16663.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spitznagel MB, Tremont G. Cognitive reserve and anosognosia in questionable and mild dementia. Arch Clin Neuropsychol. 2005;20:505–515. doi: 10.1016/j.acn.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 59.Gatz M, Mortimer JA, Fratiglioni L, et al. Accounting for the relationship between low education and dementia: a twin study. Physiol Behav. 2007;92:232–237. doi: 10.1016/j.physbeh.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reynolds CA, Fiske A, Fratiglioni L, Pedersen NL, Gatz M. Heritability of an age-dependent categorical phenotype: cognitive dysfunction. Twin Res Hum Genet. 2006;9:17–23. doi: 10.1375/183242706776403055. [DOI] [PubMed] [Google Scholar]
- 61.Abdulrab K, Heun R. Subjective Memory Impairment. A review of its definitions indicates the need for a comprehensive set of standardised and validated criteria. Eur Psychiatry. 2008;23:321–330. doi: 10.1016/j.eurpsy.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 62.Simmons SF, Johansson B, Zarit SH, Ljungquist B, Plomin R, McClearn GE. Selection bias in samples of older twins? A comparison between octogenarian twins and singletons in Sweden. J Aging Health. 1997;9:553–567. doi: 10.1177/089826439700900407. [DOI] [PubMed] [Google Scholar]