Abstract
Mature naïve B cells possess a number of BCR coreceptors and other antigen receptors, including the MHC class I-like molecule CD1d, but little is known of the response of B cells to stimulation by the CD1d ligand, α-galactosylceramide (αGalCer). Previously, we showed that all-trans-retinoic acid (RA) increases the expression of CD1d and the magnitude of CD1d–mediated antibody production in vivo. Potential mechanisms could include changes in the expression of costimulatory molecules and transcription factors that regulate plasma cell formation. In the present study, we have used isolated purified B cells and in vivo studies to demonstrate that αGalCer and RA initiate a regulated expression of several genes essential for B cell activation and differentiation, such as Pax-5, Blimp-1, IRF-4 and activation-induced cytidine deaminase (Aid). Moreover, whereas αGalCer mainly increased the expression of Pax-5, CD40 and CD86 that are critical for B cell activation, RA predominantly increased CD138+ and Fas+-PNA+ B cells, which represent more advanced B cell differentiation. It is also noteworthy that αGalCer enriched a CD19hi subset of B cells, which represent B cells with more differentiated phenotype and higher potential for antibody production. In vivo, treatment with αGalCer enriched the CD19hi population, which, after sorting, produced more anti-TT IgG by ELISPOT assay. Together, our data demonstrate that RA and αGalCer can regulate B cell activation and differentiation at multiple levels in a complementary manner, facilitating the progress of B cells towards antibody secreting cells.
Keywords: CD19, CD40, CD86, CD138, Pax-5
Introduction
Vitamin A and its active metabolite all-trans-retinoic acid (RA) play essential roles in the regulation of innate and adaptive humoral and cell-mediated immunity (Allie et al., 2013; Chen et al., 2008; Engedal et al., 2004; Ertesvag et al., 2009; Iwata et al., 2003; Mora and von Andrian, 2009; Morikawa and Nonaka, 2005; Ross et al., 2011). Previous studies in tetanus toxoid (TT)-immunization animal models have shown that RA, alone or combined with other immune stimuli, can increase the antigen specific immunoglobulin (Ig) production and promote durable memory responses (Chen et al., 2011; Ma et al., 2005).
B cells, as a type of professional antigen-presenting cells, receive and transmit signals through a variety of receptors including the B cell receptor (BCR), MHC molecules and Toll-like receptors (Chen and Ross, 2005; Lang et al., 2001; Minguet et al., 2008). Moreover, several other cell-surface proteins, such as the costimulatory molecules CD40 and CD86, and the BCR co-receptor CD19, also regulate the B cell response to antigens (Baracho et al., 2011; Ertesvag et al., 2009; Sato et al., 1995). Recently, the MHC class I-like molecule CD1d has been shown to be expressed on B cells and capable of presenting glycolipid antigens to modulate B cell function and immune response (Barral et al., 2008; Chen et al., 2011; Devera et al., 2008; Dufour et al., 2008; Lang et al., 2008). It is interesting to note that in mouse spleen, B cells constitute a major cell population expressing CD1d (Chen et al., 2011). Although ample studies have recognized the role of CD1d in NKT cell activation, its role in B cell antigen-presentation and differentiation remains unknown.
CD1d recognizes a natural ligand, α-galactosylceramide (αGalCer), which serves as a prototype of a class of glycolipid antigens that activates iNKT cells when presented to the cell-surface CD1d molecule (Godfrey and Rossjohn, 2011; Mallevaey and Selvanantham, 2012; Matsuda et al., 2008). Previously, we have reported that RA is a potent regulator of CD1d gene expression in monocytic antigen presenting cells (Chen and Ross, 2007); and αGalCer markedly increases B cell proliferation in vitro, and synergizes with anti-μ, which, through ligation of the BCR, results in B cell activation (Chen et al., 2011). Moreover, αGalCer in vivo increases the antigen-specific antibody production in a TT-immunized mice, and treatment with RA enhances αGalCer–mediated response at both primary and secondary antibody response periods (Chen et al., 2011).
In the present study, we have used a previously defined in vitro B cell model as well as in vivo studies in intact mice to examine the mechanisms of RA in the regulation of CD1d-mediated B cell activation and differentiation. We hypothesized that one way in which RA may promote αGalCer–mediated B cell differentiation is through the expression of co-stimulatory molecules, and/or transcription factors that promote B cell activation and differentiation towards plasmacytic cells. We report here that RA and αGalCer are both potent regulators of B cell differentiation. Moreover, the level of CD19 expression helps to define a subset of B cells (CD19hi B cells) that are expanded in the presence of αGalCer and RA; these cells express a higher level of CD1d protein and are enriched in factors needed for B cell differentiation, as identified by CD138 and other markers of germinal center (GC) B cells. Thus, RA and αGalCer promote a B cell differentiation program that includes heightened expression of costimulatory molecules, BCR coreceptor CD19, and critical transcription factors, which are all necessary for improved B-cell antibody production.
Materials and Methods
Animals, preparation of splenocytes, B cell isolation and cell culture
Animal protocols were approved by the Institutional Animal Use and Care Committee of the Pennsylvania State University. Adult female BALB/c mice (approximately 8 weeks old, from Charles River Laboratories, Wilmington, MA) were used for animal experiment, and to obtain splenic B cells for in vitro and ex vivo study as described previously (Chen et al., 2011).
Briefly, mouse spleens were minced and a single cell suspension was incubated with an antibody cocktail that removes all other cells and retains B cells from the spleen, a negative selection process that enriches B cell without affecting B cell activity (Stemcell Technology, Vancouver, Canada). The purity of isolated B cells was approximately 95% affirmed by flow cytometry analysis based on CD19 staining. Cells were cultured in RPMI-1640 medium supplemented with 10% FBS and 5×10−5 M β-mercaptoethanol from Invitrogen (Grand Island, NY).
For the in vivo animal experiments, 8-week old female mice were immunized with tetanus toxoid (TT, 10 μg/mouse) subcutaneously in the lower flank, with or without αGalCer (2 μg/mouse). Some groups of mice were assigned to treatment with oral doses of RA (37.5 μg/mouse in 10 μl of canola oil) for 5 days. After 4 weeks, the mice received a second dose, 10 μg of TT, and the spleens were collected 7 days later for analysis. Half of the spleen tissue from each animal was used to isolate mononuclear cells for flow cytometry analysis or cell sorting, the other half of the spleen was embedded with OCT compound and stored at −80°C for immunofluorescence staining.
Antibodies and reagents
Antibodies used for flow cytometry analysis were: CD19-PEcy7, CD19-APC (clone 1D3), Fas-PE and CD138-PE (BD Biosciences, San Jose, CA), CD1d-FITC and IgDAlexa 647 (eBiosciences, San Diego, CA), IgG1-Alexa 488 (Invitrogen, Grand Island, NY), and CD19-FITC (clone MB19-1 from BioLegend, San Diego, CA). Peanut agglutinin (PNA)-fluorescein was from Vector laboratories Inc (Burlingame, CA). αGalCer (Alexis Biochemicals, San Diego, CA), and αGalCer (Sigma-Aldrich, St. Louis, MO), were both used at the concentration of 100 ng/ml. The anti-μ antibody was utilized in B cell proliferation (1 μg/ml; cat#115-006-075 from Jackson Laboratory, Bar Harbor, ME), Escherichia coli LPS (100 ng/ml) served as a pan-B cell stimulator (055:B5, from Sigma-Aldrich).
Flow cytometry analysis and sorting
For each assay, 105 cells were incubated with 0.1 μg of fluorescent-labeled antibody for one hour at room temperature. Cell proliferation activity was measured by CSFE labeling as described previously (Chen and Ross, 2005). Cell viability was tested by trypan blue, and propidium iodide was used to identify and gate live cells for flow cytometry analysis. Non-stained and isotype-control antibody-stained cells were used to determine the gates for analysis with the Accuri C6 software.
To sort B cells based on their CD19 expression, B cells were stained with anti-CD19-PEcy7 antibody and gated into CD19hi and CD19lo subgroups. Approximately 106 cells, phenotype hi or lo, were collected using BD Cytopeia Influx sorter for further analysis. In order to validate CD19hi/lo populations, two different anti-CD19 antibodies raised by different antigenic epitopes (clone ID3 from BD Biosciences, and MB19-1 from BioLegend) were used for detection of CD19, and yielded similar results.
Quantitative Real Time-PCR (qPCR)
B cell RNA was extracted using Qiagen mini kit and subjected to qPCR (Bio-Rad). The relative expression level was determined after normalizing to the expression of the housekeeping genes HPRT and tubulin-1. The PCR condition and the primer sequences for Pax-5, Aid (or Aicda), Blimp-1 and HPRT have been previously reported (Chen and Ross, 2005). IRF-4 primers were, forward: 5’-TGATCGACCAGATCGACAGC, reverse: 5’-GTTATGAACCTGCTGGGCTGG; Tubulin-1 primers were, forward: 5’-ATGGAGCCCTGAATGTTGAC, reverse: 5’-CTCAAAGCAAGCATTGGTGA.
Immunostaining
Spleen tissues were embedded in OCT compound and cryosections were prepared (8 μm). The sections were then fixed with ice-cold acetone for 10 min and subjected to staining with fluorescent conjugated antibodies to IgM and Ki-67 in PBS with 1% BSA for 2 hours at room temperature. Isotype control serum was used as negative control. The section was analyzed by digital microscopy using CellSens image software from Olympus.
ELISPOT assay
Spleen mononuclear cells were stained with CD19-PEcy7 antibody and subjected to flow sorting to collect CD19hi and CD19lo B cells. About 106 cells were used to perform the ELISPOT assay as described previously (Chen et al., 2011; Ma et al., 2005).
Statistical methods
Means, SE, and P values were determined using Prism software (GraphPad Software, Inc). P values were calculated by t-test or ANOVA followed by Tukey's post-hoc analysis unless specified in the legend. P < 0.05 was considered significant.
Results
Retinoic acid and αGalCer differentially regulate the expression of genes required for B cell proliferation and differentiation
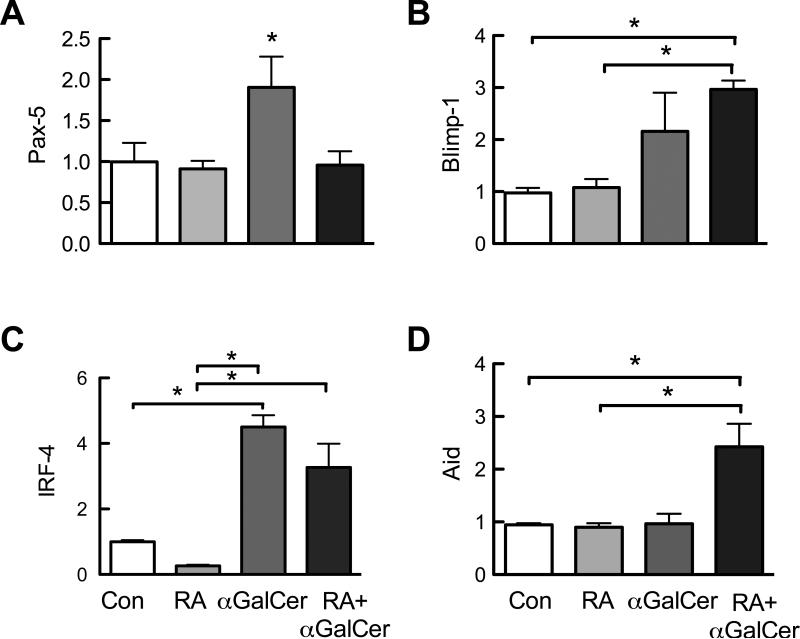
To study the role of RA and αGalCer in B cell activation process, we evaluated several key factors involved in B cell activation and the course of B cell differentiation. Isolated splenic B cells were treated for 2 days with RA (10 nM) and αGalCer (100 ng/ml) then analyzed by qRT-PCR to determine gene expression levels. As shown in Figure 1, αGalCer increased the expression of the transcription factors Pax-5 (Fig. 1A), Blimp-1 (Fig. 1B), and IRF-4 (Fig. 1C), that regulate B cell expansion and the differentiation of antibody-secreting B cells, respectively (Schebesta et al., 2002; Wuerffel et al., 2007). RA alone did not alter these factors, however, RA exerted a differential regulatory effects on stimulated B cells. RA decreased αGalCer–stimulated Pax-5 and IRF-4 expression, while increasing αGalCer–stimulated Blimp-1 expression. Moreover, RA and αGalCer in combination increased expression of Aid, although neither was effective alone (Fig. 1D); this gene encodes the activation-induced cytidine deaminase required for class switch recombination. As these genes are known to be critical for controlling B cell proliferation (Pax-5) and the differentiation of antibody-secreting plasma cells (IRF-4, Blimp-1 and Aid), these results indicate that RA and αGalCer play differential yet complementary roles in controlling B cell proliferation, class switching, and differentiation.
Fig. 1.
RA and αGalCer differentially regulate the expression of genes that control B cell proliferation and differentiation. Spleen B cells were isolated and cultured in 24-well plates (106 cells/1 ml medium) in the presence and absence of RA (20 nM) and/or αGalCer (100 ng/ml) for 2 days. Cells were then subjected to real-time qPCR analysis to determine the expression Pax-5 (A), Blimp-1 (B), IRF-4 (C) and Aid (D) genes. The graphs represent two independent experiments each in duplicate. *, P < 0.05.
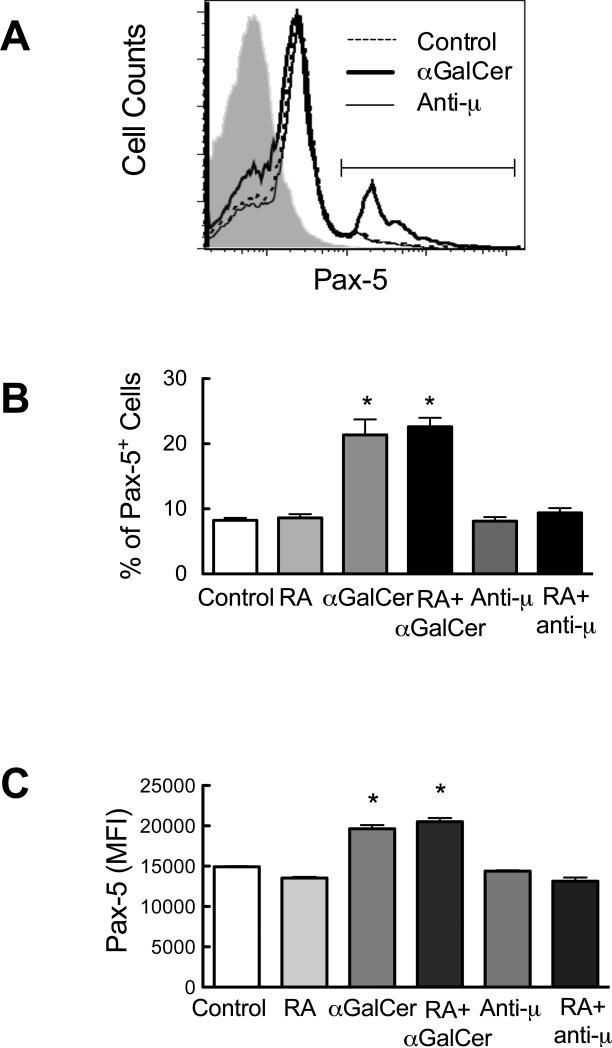
Because Pax-5 is central to the regulation of B cell activation, we also tested Pax-5 expression at the protein level using intracellular staining and flow cytometry analysis. Isolated B cells were cultured in the presence and/or absence of RA and αGalCer for 4 days. We also used anti-μ antibody in this experiment to stimulate B cell directly via BCR ligation. As shown in Figure 2A, αGalCer increased the Pax-5 expression on B cells, represented by the increase of both percent of positive cells defined by the gate (Fig. 2B) and the mean fluorescence intensity (MFI) of Pax-5 staining (Fig. 2C). In contrast, anti-μ did not alter the Pax-5 expression of B cells. It is noteworthy that while RA decreased Pax-5 mRNA after αGalCer stimulation, RA did not affect the Pax-5 protein expression under the same conditions. These data further support that αGalCer is a potent stimulus that affects B cell activity, while also suggesting that αGalCer may regulate B cell activation in a different way compared to stimulation via the BCR as observed in anti-μ treated B cells.
Fig. 2.
αGalCer increases Pax-5 protein in spleen B cells. Purified B cells (5 × 105 cells/0.5 ml in 48-well plates) were cultured for 4 days in the presence and absence of RA (20 nM) and/or αGalCer (100 ng/ml) or anti-μ (1 μg/ml), and subjected to flow cytometric analysis to detect CD19 and Pax-5 molecules. Live B cells were gated on CD19+ cells to evaluate the Pax-5 levels. A. Representative histograms showing the expression level of Pax-5 in B cells. The horizontal line indicates the gate for Pax-5+ cells. B and C, αGalCer increased Pax-5 expression levels as shown by increased percent of positive cells and MFI (Mean fluorescence intensity). *, P < 0.05 compared to control. The data shown are representative of at least three independent experiments each performed in triplicate.
αGalCer increases expression of the co-stimulatory molecule CD40 and CD86 on B cells
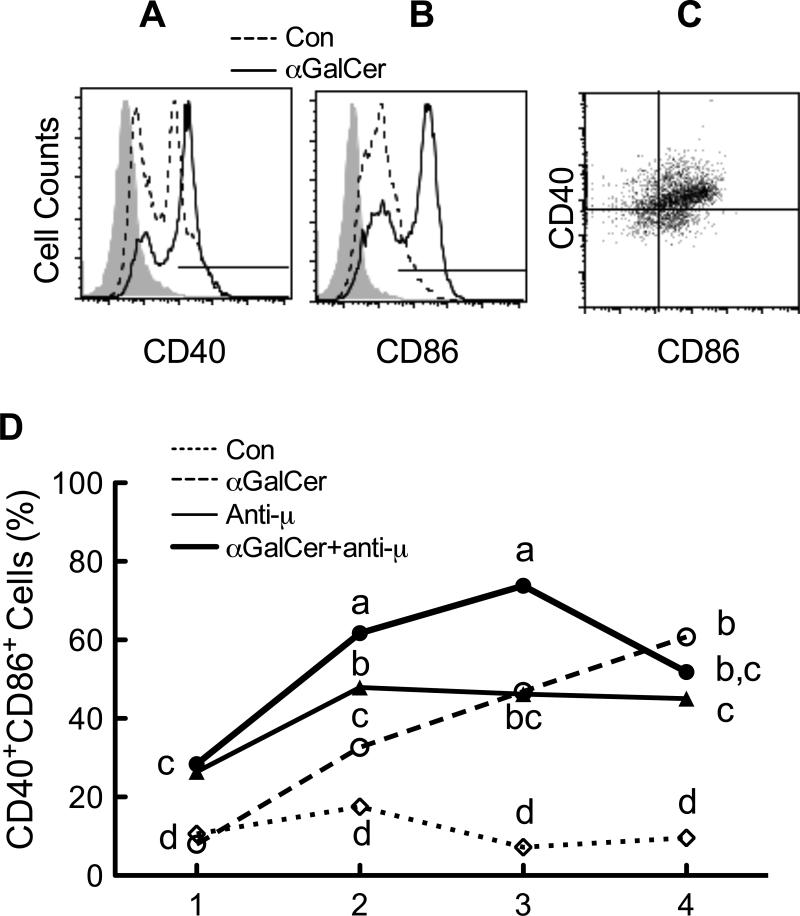
To further characterize the effect of αGalCer on B cell function, we evaluated the level of co-stimulatory receptors, CD40 and CD86, on B cells during in vitro culture and treatment. Splenocytes were stimulated for 4 days with αGalCer and/or anti-μ in the presence and absence of RA, and CD40 and CD86 expression were determined each day on CD19+ and IgD+ cells. CD40 and CD86 double-positive cells, shown in the upper right quadrant of Figure 3C, were quantified daily throughout the 4-day study. As shown in Fig. 3D, the percentage of CD40+-CD86+ cells was steadily enriched in the presence of αGalCer, starting on day 2 and continuing throughout the 4-day experiment. This outcome was different from the kinetics in response to BCR stimulation with anti-μ, as the latter treatment quickly increased the number of CD40+-CD86+ cells (detected on day 1), and then the level remained steady to day 4. Stimulation of B cells with the combination of αGalCer and anti-μ resulted in a higher percentage of CD40+-CD86+ cells, compared to either individual treatment alone, on days 2 and 3. RA did not affect the expression of costimulatory receptors (data not shown). These results imply that both αGalCer and anti-μ activate B cells to express major costimulatory receptors that promote B cell activation and differentiation; however, αGalCer may use a different pathway compared to anti-μ, which is suggested by the difference in kinetics after each stimulus and by their increased effect on B cell costimulatory molecule expression when both were added together.
Fig. 3.
αGalCer and anti-μ increased the co-stimulatory molecules CD40 and CD86 on B cells. Splenocytes were cultured in the presence and absence of RA (20 nM), anti-μ (1 μg/ml) and/or αGalCer (100 ng/ml) at 5 × 105 cells/0.5 ml in 48-well plates for a total of 4 days. Cells were harvested each day and subjected to flow cytometric analysis. Cells were first gated on CD19 and IgD positive cells, and then analyzed for CD40 and CD86 positive cells. A and B, Representative histograms showing the CD40 and CD86 expression on CD19+-IgD+ B cells after 2 days of culture and treatment. The negative control is shown in filled grey from cells stained with isotype control antibody, cell treated with medium only by the dashed line, and cells treated with αGalCer by the solid line. C. CD40 and CD86 double positive B cells are shown in the upper right quadrant, a representative graph from cells treated with αGalCer and anti-μ for 2 days. D. CD40 and CD86 double positive cells were increased after stimulation. The data shown are representative of three independent experiments, each performed in triplicate. Data were analyzed using a two-way ANOVA followed by Bonferroni post hoc test. Groups with different letters differed significantly, a>b>c>d, P < 0.05.
RA and αGalCer regulate B cell-mediated response in vivo
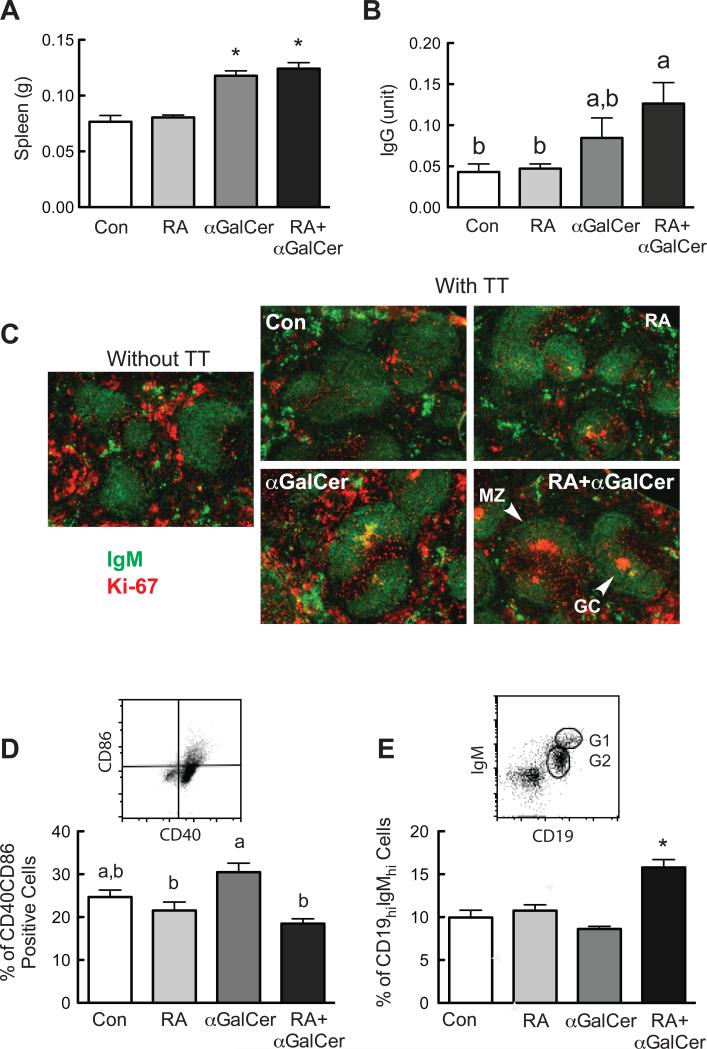
We then tested the role of RA and αGalCer in the regulation of the B cell mediated response in vivo, using TT-immunized mice as a validated model of a T-cell dependent humoral immune response (DeCicco et al., 2001) in which αGalCer was previously shown to increase primary and secondary antibody production (Chen et al., 2011). As shown in Figure 4A, spleen weight was increased in mice treated with αGalCer, independent of RA treatment, as compared to control mice, although analysis by flow cytometry did not show a marked change of cell populations after treatment (data not shown). As anticipated, RA and αGalCer treatment increased TT-specific IgG in the plasma (Fig. 4B), which is consistent with our previous observations (Chen et al., 2011). Immunostaining of spleen sections was conducted using anti-IgM to detect B cells and anti-Ki-67, a nuclear antigen and proliferation marker to detect cells that have recently undergone proliferation, such as germinal center (GC) B cells (Tierens et al., 1999). While no positive Ki-67 staining was observed in the follicle of the control group after immunization, similar as in the spleen of non-immunized mice, small GCs were occasionally observed in the group treated with RA only. In contrast, distinct GCs were observed within the splenic follicles from αGalCer and RA+αGalCer treated groups (Fig. 4C). These results further support that RA and αGalCer treatments interact in vivo enhance the TT-specific GC response.
Fig. 4.
RA and αGalCer regulate B cell differentiation in vivo. Spleens from the immunized animals with and without RA and/or αGalCer treatment (refer to Material and Methods) were collected and subjected flow cytometry analysis and immunofluorescent staining. A. Spleen weight is increased after αGalCer treatment. B. Serum TT-specific IgG levels determined by ELISA. C. Immunofluorescent staining of mouse spleens showing the spleen follicle with marginal zone (MZ) and germinal center (GC) identified by IgM (Green) and Ki-67 (Red). D. Comparison of CD40-CD86 positive cells after treatments. Splenocytes were first gated on CD19+ cells, and then analyzed for their CD40-CD86 staining. E. RA and αGalCer increased CD19hi-IgMhi positive B cells. The in vivo experiment was performed more than three times with n=3 to 5 each time.
Mouse splenocytes from this animal study were also subjected to flow cytometry analysis to examine the expression of costimulatory receptors. As shown in Fig. 4D, mice treated with αGalCer had more cells that were CD40+-CD86+, indicative of increased costimulatory molecule expression. The increase is not as dramatic as that in the in vitro experiment as shown in Fig. 3D, most likely due to the complex regulation of other cells and timing of the analysis. Examination of B cells with CD19 and IgM staining showed that a group of CD19+-IgM+ B cells were more intensely stained, termed CD19hi-IgMhi cells, in gate G1 (Fig. 4E). This cell population was increased significantly in mice that received the combined treatment with RA and αGalCer, whereas the cell population in gate G2 (IgMlo-CD19lo) did not change after treatment (data not shown). Furthermore, by comparing the level of expression of Pax-5 and costimulatory factors within these two groups of B cells, it can be seen that the CD19hi-IgMhi population had a higher level of Pax-5, CD40 and CD86 (supplemental Figure S1), indicative of a more activated cell phenotype.
Retinoic acid and αGalCer regulate B cell CD19 expression, which is associated with B cell differentiation status
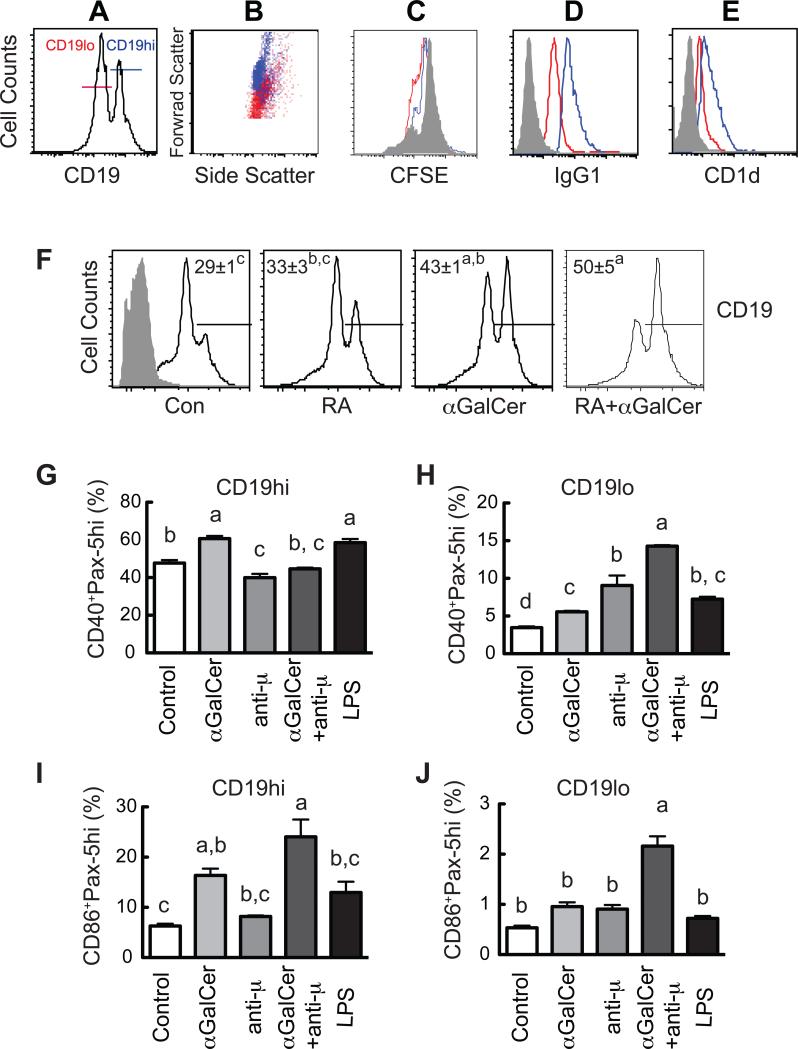
It is known that CD19 is an essential B cell surface receptor that closely related to the function of BCR (Aiba et al., 2008; Sato et al., 1995; Tedder et al., 1997). To further characterize role of CD19 expression in B cell activation, in vitro experiments were performed with our established B cell model. B cell purified from mouse spleen were stimulated by RA and αGalCer, and subjected to flow cytometry to evaluate the phenotype of the cells. We observed that cultured murine B cells developed during culture into two discrete populations, which could be separated based on their level of CD19 expression into CD19hi and CD19lo B cells (Figure 5A). CD19hi B cells were larger in size (increased forward scatter) compared with CD19lo B cells (Fig. 5B), and underwent less CFSE dye dilution (Fig. 5C), indicative of reduced cell proliferation. These cells also expressed a higher level of surface (s)IgG1 (Fig. 5D), indicative of a class-switched and more mature phenotype. Additionally and of note, the CD19hi B cells also expressed more CD1d, the receptor for αGalCer, than the CD19lo B cells (Fig. 5E).
Fig. 5.
Characteristics of the CD19+ B cells and the correlation of CD40, CD86 and Pax-5 expression in CD19+ B cell subsets. Spleen B cells were cultured in the presence and absence of RA and/or αGalCer for 4 days. B cells were stained with CD19, CD1d, IgG1, or IgD, respectively. A group of cells were also labeled with CFSE on day 0 to monitor the B cell division. From A to E, Differential characteristics of CD19lo (red) and CD19hi (blue) B cells. A sample of B cells cultured for 4 days in the presence of RA (20 nM) was presented. A. Gating of CD19lo and CD19hi cells. B. Cell distribution on forward and side scatter. C. CFSE dye dilution on day 4 (B cells of day 0 are in gray). sIgG1 (D) and CD1d (E) expressions were shown, filled gray showed negative staining using the isotype control antibodies. F. αGalCer and RA increase CD19hi expression. The A gate was set to analyze the CD19hi B cell population. G and H. αGalCer and anti-μ differentially regulated CD40+Pax-5hi, and CD86+Pax-5hi cells in CD19hi and CD19lo cells. B cells cultured for 24 hours were analyzed for their CD40, CD86, Pax-5 and CD19 expression. Live cells were first gated on CD19hi and CD19lo groups and then analyzed for CD40 and Pax-5 or CD86 and Pax-5. Data are representative of three or more independent experiments, n=3 per experiment. Groups with different letters differed significantly, a>b>c>d, P < 0.05.
We then characterized the effect of B cell stimulation on the CD19hi population, comparing control, RA, αGalCer, and RA+αGalCer-treated cells, the anomer αGalCer was used as control for αGalCer. αGalCer alone, but not the αGalCer, significantly enriched the CD19hi B cell subset (43±1% vs. 29±1%, P<0.05) (Fig. 5F). In the presence of RA there was a trend towards an increase in this population, although it missed being statistically significant. However, the largest increase was observed with the combined treatment in which half of the cells were CD19hi (50±5%), which was significantly greater than for either the control or RA-treated cells.
To determine if αGalCer promotes B cell maturation, we also measured the surface level of IgD on B cells, which often serves as a marker of mature B cells in splenic follicles (Cariappa et al., 2007). sIgD was also increased by αGalCer (Supplemental Fig. S2; note especially a group of cells with higher expression level of IgD, IgDhi, shown in the gate). Co-staining of CD19 and IgD showed that essentially all of the IgDhi B cells were also CD19hi. αGalCer significantly increased the percentage of CD19hi-IgDhi B cells, and the addition of RA tended to further increase the proportion of CD19hi-IgDhi B cells (Supplemental Fig. S3).
The stimulated B cells were then analyzed for their coexpression of the costimulatory receptors, CD40 and CD86, and Pax-5 protein based on CD19 levels. As shown in supplemental Fig. S4, most of the CD40+ B cells were Pax-5hi and resided in the CD19hi B cell population, while the Pax-5lo B cells were mainly CD40– and within the CD19lo B cell population. Similarly, most of the B cells staining for CD86 were Pax-5hi and CD19hi B cells, although a few of the CD86+ cells resided in the Pax-5lo-CD19lo population.
Next, the CD40+-Pax-5hi and CD86+-Pax-5hi cell populations were analyzed after stimulation with αGalCer. Treatment with αGalCer increased the CD40+-Pax-5hi B cells in both the CD19hi (Fig. 5G) and CD19lo (Fig. 5H) subsets, while anti-μ greatly increased CD40+Pax-5hi cells only in CD19lo B cells. Moreover, αGalCer significantly increased the percentage of CD86+-Pax-5hi B cells in the CD19hi B cell population (Fig. 5I), whereas the combination of αGalCer and anti-μ tripled the percentage of CD86+Pax5 hi B cells, within the CD19hi and CD19lo populations (Fig. 5H and J). In this experiment, we also included a treatment with LPS due to its broad spectrum of B cell stimulation, and it is interesting to note that LPS-stimulated B cells were similar to that of αGalCer in regard to CD40 and CD86 costimulatory molecule expression, together with Pax-5hi expression, on both CD19hi and CD19lo B cells (Fig. 5G, H, I and J).
Retinoic acid and αGalCer regulate B cell differentiation into plasmacytic cells
Next, we asked whether RA and αGalCer regulate B cell differentiation into plasma cells and examined the relationship of B cell differentiation with CD19 expression. CD138 (syndecan-1) was chosen to identify the plasmacytic B cells (Jourdan et al., 2011). B cells were cultured in the presence and absence of RA, αGalCer, and/or anti-μ for 4 days and then were analyzed for their CD138 expression in B cells that were gated based on their level of CD19 expression (Figure 6).
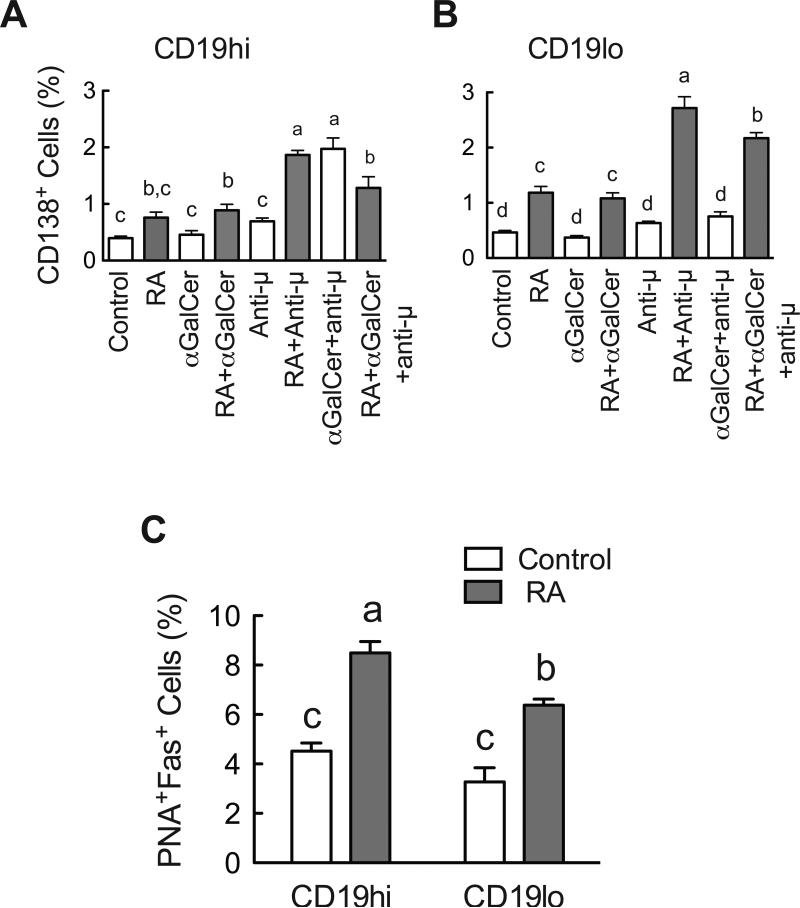
Fig. 6.
RA and αGalCer differentially regulate plasmacyte differentiation. B cells were cultured in the presence and absence of RA, αGalCer and/or anti-μ for 4 days and costained with antibodies. Cells were gated on their CD19 expression and then analyzed for CD138 or Fas-PNA staining. A. RA increased CD138+ B cells. B. CD19hi population contained a higher percentage of Fas+-PNA+ B cells, and RA increased Fas+-PNA+ on both CD19hi and CD19lo populations. Groups with different letters differed significantly, a>b>c>d, P < 0.05.
Neither αGalCer nor anti-μ alone was a potent stimulus for inducing CD138 expression (Fig. 6A). However, the combination of both significantly increased the percentage of CD138+ cells in the CD19hi B cell population. Moreover, RA increased the CD138 cell population in both the CD19hi and CD19lo populations, acting additively with anti-μ. Thus, regardless of stimulus (αGalCer, anti-μ, or both combined), RA increased the percentage of B cells expressing CD138.
Based on the result that RA increased CD138 expression on B cells, the effect of RA was further confirmed by staining cultured B cells with the GC B cell markers Fas and PNA (Hao et al., 2008; Luzina et al., 2001) (Fig. 6C). RA significantly increased the Fas+-PNA+ B cell percentages in both the CD19hi and CD19lo B cell populations (Fig. 6C). In comparison, our previous work had shown that neither αGalCer nor anti-μ affected this population (Chen et al., 2011). Together these data further enforce that RA, αGalCer and anti-μ differentially promote B cell differentiation. Whereas RA promoted B cell differentiation as shown by several criteria, αGalCer and anti-μ may need an extra signal, such as RA, to regulate the further differentiation of B cells.
αGalCer promote the differentiation of antibody secreting cells with CD19hi phenotype
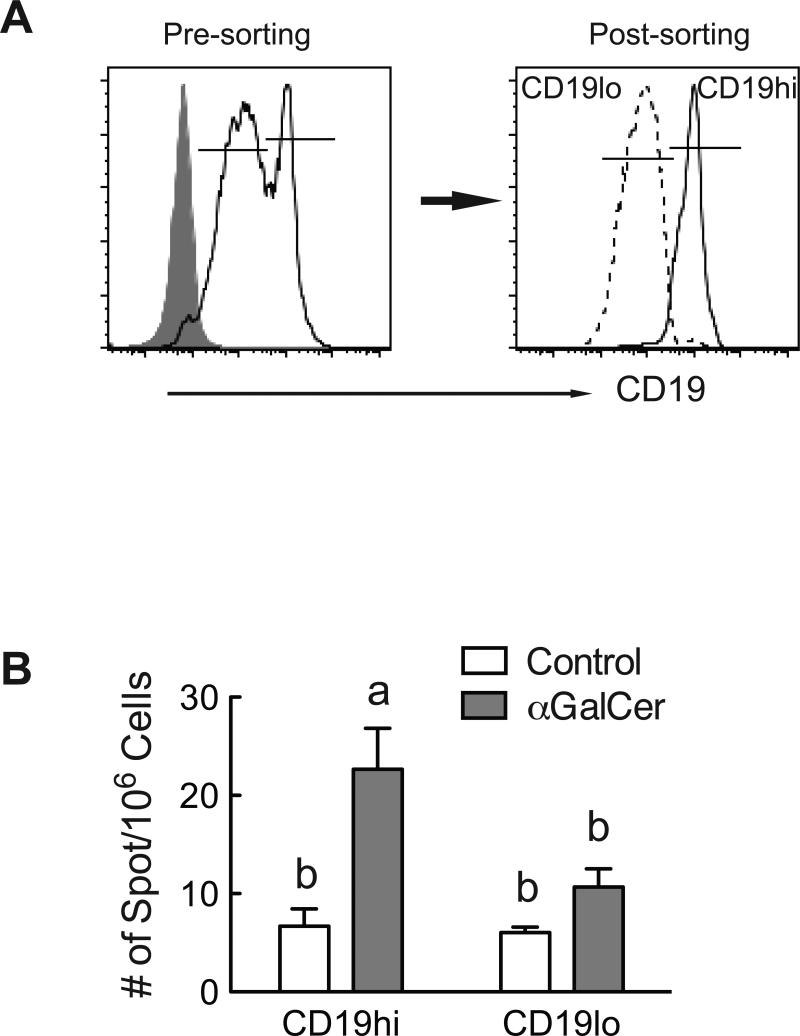
To test the ability of αGalCer augment the functional activity of CD19+ B cells in vivo, we again used the TT-immunization model in which αGalCer was previously shown to increase primary and secondary antibody production (Chen et al., 2011). Mice were treated with or without αGalCer (2 μg) at the time of immunization. We did not include a group treated with RA since αGalCer was the major factor that increased the CD19hi B cell population, as shown in Figs. 4 and 5. Seven days after boosting with TT (peak of secondary response), we isolated fresh splenic cells from control and αGalCer-treated mice and sorted them into CD19hi and CD19lo B cells (Figure 7A). The sorted cells were then subjected to ELISPOT assay to assess the number of anti-TT IgG secreting cells. As predicted by the results of our in vitro studies, αGalCer treatment in vivo increased the number of TT-specific antibody secreting cells, and this difference was significant for the population of CD19hi B cells (Fig. 7B). These results further demonstrate that αGalCer can enrich antigen-specific CD19hi B cells that differentiate into antibody-secreting cells after a T-cell dependent protein immunization.
Fig. 7.
αGalCer increases the anti-TT IgG secreting B cells in CD19hi B cells. Splenocytes from TT-immunized mice with and without αGalCer were collected and stained for CD19, then sorted into CD19hi and CD19lo B cells for ELISPOT assay. A. Representative histograms showing the CD19+ cell population before and after sorting. B. ELISPOT assay showing that CD19hi B cells in αGalCer treated mice had higher number of antibody-secreting cells. Data were analyzed by two-way ANOVA followed by Bonferroni post hoc test, n=3. Groups with different letters differed significantly, a>b, P < 0.05.
Discussion
B cell activation and differentiation is a complex process that is intensively regulated at multiple stages. The controlled expression and coordination of surface receptors and intracellular transcription factors are required to ensure the appropriate development of naïve resting B cells into mature, secretory B cells producing an effective humoral response. Previous studies showed that the glycolipid antigen αGalCer can synergize with anti-μ to induce B cell proliferation and increase the antibody response, and both of these phenomena are enhanced by RA (Chen et al., 2011). In the current study, we have examined the gene expression and phenotype of B cells along the B cell activation process using an in vitro culture model in conjunction with in vivo animal experiments. Both in vitro and in vivo studies demonstrated that αGalCer and RA are potent B cell regulators along with BCR stimulation. The expression levels of important intracellular molecules, such as Pax-5, Blimp-1, IRF-4 and Aid, and cell surface receptors, such as the costimulatory receptors CD40 and CD86, and the plasmacyte marker CD138, were significantly increased after αGalCer and RA treatment. In the intact animal, αGalCer and RA promoted GC formation and increased antibody secreting cells after antigen stimulation.
The differential effects of αGalCer and RA on B cells differentiation may be important for the development of a potent immune response. As shown in these experiments, αGalCer is a potent stimulus of Pax-5, IRF-4, CD40 and CD86 expression. Pax-5 is known as a transcription factor essential for B cell expansion and early differentiation (Holmes et al., 2008). The elevated Pax-5 gene and protein expression of αGalCer-treated B cells is consistent with our previous observation that αGalCer stimulates B cell proliferation, an important early step for humoral immune response. IRF-4 is recognized as a critical factor regulating B cell maturation and differentiation commitment by activating the transcription of Blimp-1 gene (Nutt and Tarlinton, 2011). Its expression is up-regulated after mitogen stimulation (Grumont and Gerondakis, 2000), and lacking of IRF-4 results in reduced responsiveness of B cells to LPS and BCR ligation, leading to aberrant antibody production (Mittrucker et al., 1997; Sciammas et al., 2006). In addition to these transcription factors, the activation of cell surface CD40 and CD86, which act as co-stimulatory receptors with the BCR, provide indispensable signals for B cell activation and CSR. These data together suggest that αGalCer promotes B cell expansion and induces the early differentiation of B cells by increasing the expression of these essential factors. On the other hand, RA not only increased the expression of CD138 and the percentage of PNA+-Fas+ cells, a phenotype indicative of plasma cell differentiation, but RA also increased the gene expression of Aid, which is a requisite enzyme for CSR. Moreover, RA suppressed αGalCer-mediated Pax-5 gene expression and, at same time, it increased the αGalCer-stimulated Blimp-1 gene expression. It is well established that the suppression of Pax-5 gene expression by Blimp-1 is a required step for B cell plasmacytic differentiation (Lin et al., 2002). Overall, these data further support our previous study which showed that RA acts mainly in favor of cell differentiation that promotes B cells towards antibody secreting cells, while αGalCer may need extra assistance to promote the later stage of differentiation (Chen et al., 2011; Chen and Ross, 2005). However, as shown in our in vivo experiment (Fig. 4B,C), it is often the combined effect from both RA and αGalCer produces the marked response in terms of antibody production.
While αGalCer and anti-μ both promoted B cell activation, their effects on specific factors were often non-identical. Whereas αGalCer enriched the CD19hi B cell population, and increased Pax-5, CD40 and CD86 expression, anti-μ mainly increased CD40 and CD86 expression, which occurred in a more rapid fashion as compared to that caused by αGalCer stimulation, and a heightened response was observed when αGalCer and anti-μ were combined to treat B cells. As both BCR and CD1d are expressed on the B cell surface, it will be very interesting to determine in the future if their signaling pathways may not only complement one another, but also crosstalk to amplify the cell's response to both protein and glycolipid antigen stimulation.
The level of B cell activation via BCR stimulation is intimately related to the expression of the coreceptor CD19, which is also required for CD1d+ B cell development (Bouaziz et al., 2008; Yanaba et al., 2008). It is interesting to learn that the CD19 expression directly correlated with B cell differentiation activities, representing distinctive differentiation and functional B cell populations as determined by the size, proliferation potential and gene expression patterns of the cells. We identified that B cells cultured in vitro developed into CD19hi and CD19lo B cell subsets. Compared to the CD19lo B cell subset, the CD19hi group of B cells expressed higher levels of sIgG and sIgD, higher levels of factors involved in B cell proliferation and differentiation (Pax-5, CD40 and CD86), and had more CD138+ and Fas+-PNA+ B cells, further differentiated B cells. It is particularly interesting to note that the CD1d molecule was expressed at a higher level in the CD19hi B cell population as well. As Pax-5, CD40 and CD86 are well known to be critical factors required for B cell development and functional activation (Cerutti et al., 2011; Jain et al.; Johnson and Calame, 2003; Suvas et al., 2002), the colocalization of Pax-5, CD40, and CD86 in CD19hi B cells suggests a higher immune competence of this group of cells compared to CD19lo cells, which was confirmed by the ELISPOT analysis of the CD19+ cells from TT-immunized mice, in which the number of antibody-secreting cells was nearly 3 times higher in CD19hi B cells from αGalCer-treated TT-immunized mice as compared to control-treated immunized mice. Since αGalCer stimulation combined with RA treatment expanded the CD19hi B cell subset, and Pax5 is known to increase CD19 expression (Pridans et al., 2008), these results suggest that RA and αGalCer may promote B cell activation and differentiation through elevated expression of CD19, which in turn may regulate the signaling of CD1d and the BCR, in ways that control B cell responsiveness. The recognition that CD5+CD19hiCD1d+ B10 B-cells are important in the inflammatory response and tumor immunity, and that CD19-deficient mice fail to develop B10 B-cells, further supports the importance of the association of CD19 and CD1d molecules in B cell maturation (DiLillo et al., 2010; Yanaba et al., 2008).
Animal studies and clinical trials using RA and αGalCer independently have shown that these agents are potent therapeutic regimens in the treatment of cancer and immune disorders, as well as for vaccine development (Corgnac et al., 2013; Huang et al., 2013; Iclozan et al., 2013; Ronchi and Falcone, 2008). Previously we have shown that combined treatment of RA and αGalCer decreased breast cancer metastasis growth in a mouse model (Chen and Ross, 2012). Our present data from studies of normal B cells may provide guidance for future studies of B cell pathologies in which reduced growth and increased cell differentiation could be of benefit, and/or for improving the humoral immune response to various classes of antigens under conditions relevant to human vaccination.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health [DK 41479].
Abbreviations
- αGalCer
α-galactosylceramide
- AID
activation induced deaminase
- GC
germinal center
- IRF
interferon regulatory factor
- RA
all-trans-retinoic acid
- TT
tetanus toxoid
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
References
- Aiba Y, Kameyama M, Yamazaki T, Tedder TF, Kurosaki T. Regulation of B-cell development by BCAP and CD19 through their binding to phosphoinositide 3-kinase. Blood. 2008;111:1497–1503. doi: 10.1182/blood-2007-08-109769. [DOI] [PubMed] [Google Scholar]
- Allie SR, Zhang W, Tsai CY, Noelle RJ, Usherwood EJ. Critical Role for All-trans Retinoic Acid for Optimal Effector and Effector Memory CD8 T Cell Differentiation. J Immunol. 2013;190:2178–2187. doi: 10.4049/jimmunol.1201945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracho GV, Miletic AV, Omori SA, Cato MH, Rickert RC. Emergence of the PI3-kinase pathway as a central modulator of normal and aberrant B cell differentiation. Curr Opin Immunol. 2011;23:178–183. doi: 10.1016/j.coi.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral P, Eckl-Dorna J, Harwood NE, De Santo C, Salio M, Illarionov P, Besra GS, Cerundolo V, Batista FD. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc Natl Acad Sci U S A. 2008;105:8345–8350. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- Cariappa A, Chase C, Liu H, Russell P, Pillai S. Naive recirculating B cells mature simultaneously in the spleen and bone marrow. Blood. 2007;109:2339–2345. doi: 10.1182/blood-2006-05-021089. [DOI] [PubMed] [Google Scholar]
- Cerutti A, Puga I, Cols M. Innate control of B cell responses. Trends Immunol. 2011;32:202–211. doi: 10.1016/j.it.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Mosovsky KL, Ross AC. Retinoic Acid and α-Galactosylceramide Differentially Regulate B Cell Activation In Vitro and Augment Antibody Production In Vivo. Clin Vaccine Immunol. 2011;18:1015–1020. doi: 10.1128/CVI.00004-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Ross AC. Vitamin A and immune function: retinoic acid modulates population dynamics in antigen receptor and CD38-stimulated splenic B cells. Proc Natl Acad Sci U S A. 2005;102:14142–14149. doi: 10.1073/pnas.0505018102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Ross AC. Retinoic acid regulates CD1d gene expression at the transcriptional level in human and rodent monocytic cells. Exp Biol Med (Maywood) 2007;232:488–494. [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Ross AC. All-trans-retinoic acid and the glycolipid alpha-galactosylceramide combined reduce breast tumor growth and lung metastasis in a 4T1 murine breast tumor model. Nutrition and cancer. 2012;64:1219–1227. doi: 10.1080/01635581.2012.718404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Esplin BL, Garrett KP, Welner RS, Webb CF, Kincade PW. Retinoids accelerate B lineage lymphoid differentiation. J Immunol. 2008;180:138–145. doi: 10.4049/jimmunol.180.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corgnac S, Perret R, Derre L, Zhang L, Stirnemann K, Zauderer M, Speiser DE, Mach JP, Romero P, Donda A. CD1d-antibody fusion proteins target iNKT cells to the tumor and trigger long-term therapeutic responses. Cancer immunology, immunotherapy : CII. 2013;62:747–760. doi: 10.1007/s00262-012-1381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCicco KL, Youngdahl JD, Ross AC. All-trans-retinoic acid and polyriboinosinic : polyribocytidylic acid in combination potentiate specific antibody production and cell-mediated immunity. Immunology. 2001;104:341–348. doi: 10.1046/j.1365-2567.2001.01317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devera TS, Shah HB, Lang GA, Lang ML. Glycolipid-activated NKT cells support the induction of persistent plasma cell responses and antibody titers. Eur J Immunol. 2008;38:1001–1011. doi: 10.1002/eji.200738000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLillo DJ, Matsushita T, Tedder TF. B10 cells and regulatory B cells balance immune responses during inflammation, autoimmunity, and cancer. Ann N Y Acad Sci. 2010;1183:38–57. doi: 10.1111/j.1749-6632.2009.05137.x. [DOI] [PubMed] [Google Scholar]
- Dufour FD, Silveira PA, Baxter AG. Interactions between B-lymphocytes and type 1 NKT cells in autoimmune diabetes. J Immunotoxicol. 2008;5:249–257. doi: 10.1080/15476910802131543. [DOI] [PubMed] [Google Scholar]
- Engedal N, Ertesvag A, Blomhoff HK. Survival of activated human T lymphocytes is promoted by retinoic acid via induction of IL-2. Int Immunol. 2004;16:443–453. doi: 10.1093/intimm/dxh048. [DOI] [PubMed] [Google Scholar]
- Ertesvag A, Naderi S, Blomhoff HK. Regulation of B cell proliferation and differentiation by retinoic acid. Semin Immunol. 2009;21:36–41. doi: 10.1016/j.smim.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Rossjohn J. New ways to turn on NKT cells. J Exp Med. 2011;208:1121–1125. doi: 10.1084/jem.20110983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumont RJ, Gerondakis S. Rel induces interferon regulatory factor 4 (IRF-4) expression in lymphocytes: modulation of interferon-regulated gene expression by rel/nuclear factor kappaB. J Exp Med. 2000;191:1281–1292. doi: 10.1084/jem.191.8.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Duncan GS, Seagal J, Su YW, Hong C, Haight J, Chen NJ, Elia A, Wakeham A, Li WY, et al. Fas receptor expression in germinal-center B cells is essential for T and B lymphocyte homeostasis. Immunity. 2008;29:615–627. doi: 10.1016/j.immuni.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes ML, Pridans C, Nutt SL. The regulation of the B-cell gene expression programme by Pax5. Immunol Cell Biol. 2008;86:47–53. doi: 10.1038/sj.icb.7100134. [DOI] [PubMed] [Google Scholar]
- Huang YL, Hung JT, Cheung SK, Lee HY, Chu KC, Li ST, Lin YC, Ren CT, Cheng TJ, Hsu TL, et al. Carbohydrate-based vaccines with a glycolipid adjuvant for breast cancer. Proc Natl Acad Sci U S A. 2013;110:2517–2522. doi: 10.1073/pnas.1222649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer immunology, immunotherapy : CII. 2013;62:909–918. doi: 10.1007/s00262-013-1396-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. International immunology. 2003;15:1017–1025. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- Jain S, Chodisetti SB, Agrewala JN. CD40 signaling synergizes with TLR-2 in the BCR independent activation of resting B cells. PLoS One. 2011;6:e20651. doi: 10.1371/journal.pone.0020651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K, Calame K. Transcription factors controlling the beginning and end of B-cell differentiation. Curr Opin Genet Dev. 2003;13:522–528. doi: 10.1016/j.gde.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Jourdan M, Caraux A, Caron G, Robert N, Fiol G, Reme T, Bollore K, Vendrell JP, Le Gallou S, Mourcin F, et al. Characterization of a transitional preplasmablast population in the process of human B cell to plasma cell differentiation. J Immunol. 2011;187:3931–3941. doi: 10.4049/jimmunol.1101230. [DOI] [PubMed] [Google Scholar]
- Lang GA, Devera TS, Lang ML. Requirement for CD1d expression by B cells to stimulate NKT cell-enhanced antibody production. Blood. 2008;111:2158–2162. doi: 10.1182/blood-2007-10-117309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P, Stolpa JC, Freiberg BA, Crawford F, Kappler J, Kupfer A, Cambier JC. TCR-induced transmembrane signaling by peptide/MHC class II via associated Ig-alpha/beta dimers. Science. 2001;291:1537–1540. doi: 10.1126/science.291.5508.1537. [DOI] [PubMed] [Google Scholar]
- Lin KI, Angelin-Duclos C, Kuo TC, Calame K. Blimp-1-dependent repression of Pax-5 is required for differentiation of B cells to immunoglobulin M-secreting plasma cells. Molecular and cellular biology. 2002;22:4771–4780. doi: 10.1128/MCB.22.13.4771-4780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzina IG, Atamas SP, Storrer CE, daSilva LC, Kelsoe G, Papadimitriou JC, Handwerger BS. Spontaneous formation of germinal centers in autoimmune mice. J Leukoc Biol. 2001;70:578–584. [PubMed] [Google Scholar]
- Ma Y, Chen Q, Ross AC. Retinoic acid and polyriboinosinic:polyribocytidylic acid stimulate robust anti-tetanus antibody production while differentially regulating type 1/type 2 cytokines and lymphocyte populations. J Immunol. 2005;174:7961–7969. doi: 10.4049/jimmunol.174.12.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallevaey T, Selvanantham T. Strategy of lipid recognition by invariant natural killer T cells: ‘one for all and all for one’. Immunology. 2012;136:273–282. doi: 10.1111/j.1365-2567.2012.03580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the ‘Swiss-Army knife’ of the immune system. Curr Opin Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minguet S, Dopfer EP, Pollmer C, Freudenberg MA, Galanos C, Reth M, Huber M, Schamel WW. Enhanced B-cell activation mediated by TLR4 and BCR crosstalk. Eur J Immunol. 2008;38:2475–2487. doi: 10.1002/eji.200738094. [DOI] [PubMed] [Google Scholar]
- Mittrucker HW, Matsuyama T, Grossman A, Kundig TM, Potter J, Shahinian A, Wakeham A, Patterson B, Ohashi PS, Mak TW. Requirement for the transcription factor LSIRF/IRF4 for mature B and T lymphocyte function. Science. 1997;275:540–543. doi: 10.1126/science.275.5299.540. [DOI] [PubMed] [Google Scholar]
- Mora JR, von Andrian UH. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Semin Immunol. 2009;21:28–35. doi: 10.1016/j.smim.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa K, Nonaka M. All-trans-retinoic acid accelerates the differentiation of human B lymphocytes maturing into plasma cells. Int Immunopharmacol. 2005;5:1830–1838. doi: 10.1016/j.intimp.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Nutt SL, Tarlinton DM. Germinal center B and follicular helper T cells: siblings, cousins or just good friends? Nature immunology. 2011;12:472–477. doi: 10.1038/ni.2019. [DOI] [PubMed] [Google Scholar]
- Pridans C, Holmes ML, Polli M, Wettenhall JM, Dakic A, Corcoran LM, Smyth GK, Nutt SL. Identification of Pax5 target genes in early B cell differentiation. J Immunol. 2008;180:1719–1728. doi: 10.4049/jimmunol.180.3.1719. [DOI] [PubMed] [Google Scholar]
- Ronchi F, Falcone M. Immune regulation by invariant NKT cells in autoimmunity. Frontiers in bioscience : a journal and virtual library. 2008;13:4827–4837. doi: 10.2741/3042. [DOI] [PubMed] [Google Scholar]
- Ross AC, Chen Q, Ma Y. Vitamin A and retinoic acid in the regulation of B-cell development and antibody production. Vitam Horm. 2011;86:103–126. doi: 10.1016/B978-0-12-386960-9.00005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato S, Steeber DA, Tedder TF. The CD19 signal transduction molecule is a response regulator of B-lymphocyte differentiation. Proc Natl Acad Sci U S A. 1995;92:11558–11562. doi: 10.1073/pnas.92.25.11558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schebesta M, Heavey B, Busslinger M. Transcriptional control of B-cell development. Curr Opin Immunol. 2002;14:216–223. doi: 10.1016/s0952-7915(02)00324-2. [DOI] [PubMed] [Google Scholar]
- Sciammas R, Shaffer AL, Schatz JH, Zhao H, Staudt LM, Singh H. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity. 2006;25:225–236. doi: 10.1016/j.immuni.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Suvas S, Singh V, Sahdev S, Vohra H, Agrewala JN. Distinct role of CD80 and CD86 in the regulation of the activation of B cell and B cell lymphoma. J Biol Chem. 2002;277:7766–7775. doi: 10.1074/jbc.M105902200. [DOI] [PubMed] [Google Scholar]
- Tedder TF, Inaoki M, Sato S. The CD19-CD21 complex regulates signal transduction thresholds governing humoral immunity and autoimmunity. Immunity. 1997;6:107–118. doi: 10.1016/s1074-7613(00)80418-5. [DOI] [PubMed] [Google Scholar]
- Tierens A, Delabie J, Michiels L, Vandenberghe P, De Wolf-Peeters C. Marginal-zone B cells in the human lymph node and spleen show somatic hypermutations and display clonal expansion. Blood. 1999;93:226–234. [PubMed] [Google Scholar]
- Wuerffel R, Wang L, Grigera F, Manis J, Selsing E, Perlot T, Alt FW, Cogne M, Pinaud E, Kenter AL. S-S synapsis during class switch recombination is promoted by distantly located transcriptional elements and activation-induced deaminase. Immunity. 2007;27:711–722. doi: 10.1016/j.immuni.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.