Abstract
Aromatase inhibitors (AI), which are used to treat breast cancer, inhibit estrogen production in postmenopausal women. AI-associated musculoskeletal symptoms (AIMSS) occur in approximately half of treated women, and lead to treatment discontinuation in 20–30%. The etiology may be due in part to estrogen deprivation. In premenopausal women, lower estrogen levels have been associated with increased pain, as well as with impairment of descending pain inhibitory pathways, which may be a risk factor for developing chronic pain. We prospectively tested whether AI-induced estrogen deprivation alters pain sensitivity, thereby increasing the risk of developing AIMSS. Fifty postmenopausal breast cancer patients underwent pressure pain testing and conditioned pain modulation (CPM) assessment prior to AI initiation and after 3 and 6 months. At baseline, 26 of 40 (65%) assessed patients demonstrated impaired CPM, which was greater in those who had previously received chemotherapy (p=0.006). No statistically significant change in pressure pain threshold or CPM was identified following estrogen deprivation. In addition, there was no association with either measure of pain sensitivity and change in patient-reported pain with AI therapy. AIMSS are not likely due to decreased pain threshold or impaired CPM prior to treatment initiation, or to effects of estrogen depletion on pain sensitivity. Clinicaltrials.gov NCT01814397.
Perspective
This article presents our findings of the effect of estrogen deprivation on objective measures of pain sensitivity. In postmenopausal women, medication-induced estrogen depletion did not result in an identifiable change in pressure pain threshold or conditioned pain modulation. Impaired conditioned pain modulation may be associated with chemotherapy.
Keywords: pain threshold, conditioned pain modulation, breast cancer, estrogen deprivation, aromatase inhibitor
Introduction
Aromatase inhibitors (AI) inhibit the conversion of androgens to estrogens in postmenopausal women, thereby reducing circulating estrogen levels to approximately one-tenth of normal levels.8 This class of medication has been shown to significantly improve breast cancer outcomes in postmenopausal women with hormone receptor (HR)-positive breast cancer compared to the prior standard of care, tamoxifen.5 Treatment with an AI has therefore been recommended as part of the treatment regimen for all postmenopausal women with HR-positive early stage breast cancer.2
Despite positive oncological benefits, AIs can cause bothersome long-term side effects.4 These toxicities can negatively impact quality of life, which can lead to decreased adherence to AI therapy.19 In particular, AI-associated musculoskeletal symptoms (AIMSS) (e.g., pain and stiffness) can affect up to half of treated patients, and is the primary reason for discontinuation reported by about 75% of those who discontinue treatment early. Overall 20–30% of patients stop taking their initially prescribed AI medication because of toxicity.
The mechanism of AIMSS is poorly understood, but is thought to be related to the profound depletion of estrogen, either systemically or locally in joints.6, 21 A better understanding of the mechanism(s) that underlie AIMSS, and why some women develop them and others do not, may lead to interventions to prevent or treat them. Such interventions might result in higher levels of adherence and, in theory, even better outcomes for women with estrogen receptor (ER)-positive breast cancer.
Centrally-mediated descending control over pain is thought to utilize at least two pathways in humans: the norepinephrine-serotonin pathway and the opioidergic pathway. Estrogen may play a role in the modulation of the latter pathway. During a pain stressor, women with low levels of estrogen have been shown to have decreased activation of opioid neurotransmission, as assessed by PET imaging, resulting in hyperalgesic responses.35 In addition, a few studies in premenopausal women have reported an association between patient-reported pain severity and phase of the menstrual cycle, with lower levels of estrogen being associated with greater pain sensitivity.18, 23 Despite these few studies, the relationship between estrogen concentration and pain sensitivity remains complex and poorly understood.
Quantitative sensory testing (QST) permits the standardized assessment of pain sensitivity.15 Pressure pain threshold can be quantitated using an evoked pain stimulus at the thumbnail or other sites.9–11, 14, 26, 27 In addition, the magnitude of endogenous descending inhibition of neurons in response to noxious stimuli can be assessed using conditioned pain modulation (CPM) studies.39 CPM defines the physiologic response to repeated or chronic pain stimuli. Deficient or inefficient CPM is observed in patients with chronic pain syndromes of a variety of types. These QST methods, including both pressure pain threshold assessment and CPM studies, have been used successfully to demonstrate deficiencies in central pain processing and modulation in patients with many different chronic pain conditions compared to healthy controls. Such testing can also be used to differentiate underlying mechanisms within groups of individuals having nociceptive, neuropathic, or centralized forms of chronic pain.28, 37, 38 QST findings are highly correlated with changes in functional imaging activation patterns and clinical pain in patients with multiple different chronic pain conditions.7, 24, 25
Based on these considerations, we hypothesized that pre-existing low pain threshold and/or deficiencies in CPM would predispose women to AIMSS. We also hypothesized that AI-induced estrogen deprivation would decrease pain threshold and/or impair CPM over time, thereby increasing the risk of subsequently developing AIMSS. We therefore enrolled a cohort of women with early stage HR-positive breast cancer who were initiating AI therapy into a prospective study in which we applied QST methods to evaluate the effect of estrogen depletion on pain sensitivity and the predictive role of low pressure pain threshold and impaired CPM for development of AIMSS.
Materials and Methods
Patients
Eligible patients were recruited to this prospective clinical study from June 2009 until January 2012 at a single institution (Clinicaltrials.gov NCT01814397). Patients were eligible if they were postmenopausal women with stage 0–III hormone receptor positive breast cancer who were planning to receive a standard dose of aromatase inhibitor therapy (i.e., anastrozole 1 milligram orally daily, exemestane 25 milligrams orally daily, or letrozole 2.5 milligrams orally daily). Surgical resection, chemotherapy, and radiation therapy, as indicated, were completed prior to study enrollment. Patients were ineligible if they had received prior AI therapy, had pre-existing grade 2 or higher sensory neuropathy, chemotherapy-induced fingernail changes thought to interfere with QST, a history of schizophrenia, or a history of suicidal ideation or attempt within the 2 years prior to enrollment. Patients who reported an average pain of at least 8 out of 10 at baseline were excluded. The protocol was approved by the University of Michigan Institutional Review Board, and all enrolled patients provided written informed consent.
Study Design
Patients completed all evaluations in the University of Michigan Chronic Pain and Fatigue Research Center (CPFRC). Prior to initiation of AI therapy, patients underwent phlebotomy and QST and completed self-report questionnaires, as described below. Following the baseline visit, patients initiated treatment with one of the three AI medications as directed by their treating medical oncologist. After 3 months of AI therapy, patients again underwent phlebotomy and QST, and after 6 months they underwent QST.
Quantitative Sensory Testing
QST was performed using a standardized protocol.10, 12, 13, 16 In brief, discrete pressure stimuli were delivered by a custom-built apparatus that eliminated direct patient contact by the examiner (Supplemental Figure 1). This device employed a hydraulic system to apply pressure to the thumbnail bed via a 1-cm2 hard rubber circular probe. The probe was positioned over the center of the patient’s non-dominant thumbnail by a hand-held plastic housing, and the hydraulic system was activated by placing calibrated weights on a moveable platform and adjusting valves to control stimulus timing. The probe was lowered to apply pressure consistent with the weight on the moveable platform. The combination of valves and calibrated weights produced controlled and repeatable stimulation. All patients underwent device familiarization and training prior to testing.
To assess pressure pain threshold, the testing sequence consisted of a series of ascending pressure stimuli delivered at 25 second intervals beginning at 0.5 kg/cm2 and increasing in 0.5 kg/m2 increments until tolerance or to a maximum of 10 kg/cm2 (Supplemental Figure 2A). The duration of each pressure was 5 seconds. Patients rated the intensity of each pressure sensation using a 0–100 numerical rating scale (NRS; 0 = no pain, 100 = worst pain imaginable). These pain ratings were used to interpolate via a regressed function each individual’s Pain50. Pain50, defined as the amount of applied pressure in kg/cm2 that evoked a pain intensity rating of 50 out of 100, served as a measure of suprathreshold pressure pain sensitivity.
Endogenous pain modulation was then evaluated using the pressure delivery apparatus described above and following a standardized CPM paradigm (Supplemental Figure 2B).39 CPM procedures use a conditioning stimulus (a noxious stimulus that activates pain modulatory systems) and a test stimulus (a noxious stimulus used to evaluate the analgesic response to the test stimulus). Pressure equivalent to Pain50 for the individual patient was applied via a probe to the non-dominant thumbnail for 30 seconds as a test stimulus, and the patient rated the intensity of the pressure at 10 second intervals. Ten minutes later a pressure conditioning stimulus was then continuously applied to the dominant thumbnail for 60 seconds at the same Pain50 intensity as the test stimulus. After 30 seconds of conditioning stimulation, the test stimulus was again applied to the non-dominant thumbnail for 30 seconds and the patient rated the intensity of the pressure every 10 seconds. CPM magnitude was calculated as the difference (second minus first) in the mean of the 3 pain ratings to the test stimulus applied prior to and during the conditioning stimulus. Higher CPM values indicate less efficient CPM.
Patient-reported Outcomes
Patients completed self-report questionnaires at each time point. Pain, the primary symptom of interest, was assessed using an 11-point Likert scale. Each patient recorded her average pain daily for 7 consecutive days prior to each time point, and values were averaged to obtain an average pain value for each time point. Additional patient-reported outcomes that were assessed at baseline included: fatigue (Multidimensional Fatigue Index (MFI)),33 sleep problems (Medical Outcomes Study (MOS)-Sleep questionnaire),17, 36 cognitive dysfunction (Multiple Abilities Self-Report Questionnaire (MASQ)),31 and depression (Center for Epidemiologic Studies Depression Scale (CESD)).29
Estradiol Assessment
Serum was collected prior to AI initiation and after 3 months of therapy. Serum estradiol quantitation was performed using an ultrasensitive gas chromatography tandem mass spectroscopy-based assay as previously described (inVentiv Health Clinical).30 Lower limit of quantitation was 2 pg/ml.
Statistical Methods
The primary endpoint of the study was change in pain threshold (Pain50) and CPM between the baseline and 3 month assessments. Change over time in patient-reported pain, Pain50 and CPM were assessed using repeated measures mixed models using the square root of pain, the natural log of Pain50, and CPM to evaluate for normality of outcomes, without covariates. For assessment of change in pain50, with 50 patients we had 80% power to detect a change of 0.6–0.73 over 3 months, assuming a standard deviation of 1.5–1.8. For CPM, we had 80% power to detect a change of 7.3 assuming a standard deviation of 18.
Associations between patient-reported symptoms and pain sensitivity scores were assessed using Spearman rank correlation due to the non-normal distribution of the symptom scores. Associations between baseline patient-reported symptoms and treatment discontinuation were assessed using a two sample Wilcoxon Mann-Whitney test. Linear regression models were used to assess associations between baseline estradiol levels and the baseline QST measures. Linear regression models were also used to assess associations between baseline QST measures with the clinical factors of prior chemotherapy use, prior tamoxifen use, and body mass index (BMI), as well as treatment discontinuation. For all statistical tests, p-values ≤0.05 were considered statistically significant.
Results
Baseline characteristics
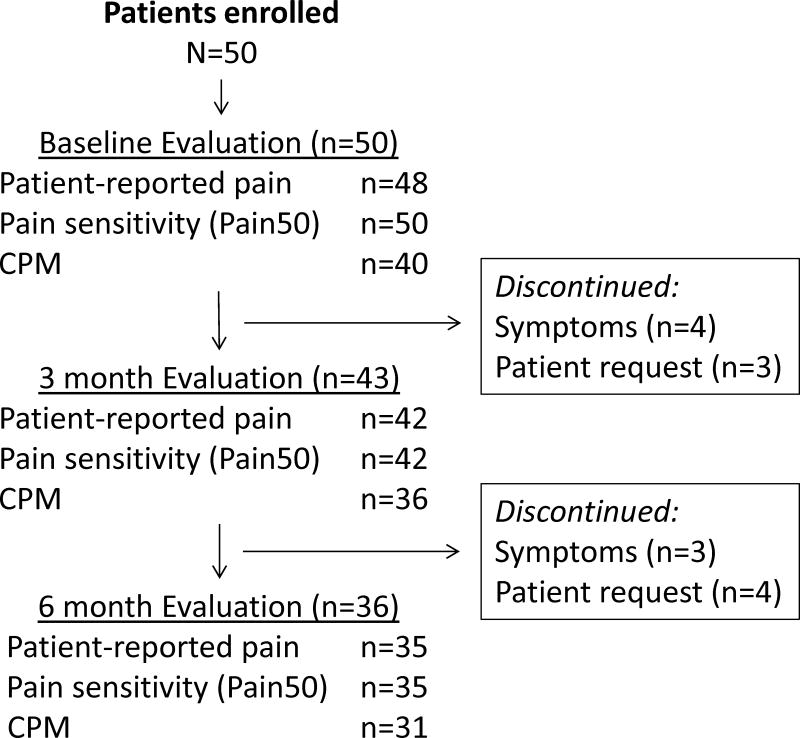
A total of 50 postmenopausal patients enrolled and underwent baseline evaluation (Figure 1). Baseline characteristics are listed in Tables 1 and 2. Thirty percent of patients had received prior tamoxifen, and 46% had been treated with chemotherapy. Eighty percent of patients received anastrozole. Baseline patient-reported pain was 1.7 (standard deviation (SD) 1.3) on a 0–10 scale.
Figure 1.
Patient flow diagram.
Table 1.
Baseline patient characteristics.
| Characteristic | Total cohort (n=50) |
|---|---|
|
| |
| Median age, years (range) | 60 (38–77) |
|
| |
| Race | |
| - White | 47 (94%) |
| - Black | 2 (4%) |
| - Other | 1 (2%) |
|
| |
| Mean weight, kg (SD) | 80.7 (17.8) |
|
| |
| Mean body mass index (SD) | 30.0 (6.5) |
|
| |
| Aromatase inhibitor | |
| - Anastrozole | 40 (80%) |
| - Exemestane | 1 (2%) |
| - Letrozole | 9 (18%) |
|
| |
| Prior chemotherapy | 23 (46%) |
|
| |
| Prior taxane | 20/23 (87%) |
|
| |
| Time since chemotherapy completion, year (range) | 0.3 (0.1–3.6) |
|
| |
| Prior tamoxifen | 14 (30%) |
|
| |
| Mean baseline quantitative sensory testing measures (SD) | |
| Pain50, kg/cm2 | 4.3 (1.5) |
| Baseline Conditioned Pain Modulation | 7.9 (14.7) |
|
| |
| Mean baseline serum estradiol concentration, pg/ml (SD) | 6.0 (7.6) |
SD: standard deviation.
Table 2.
Baseline patient-reported symptoms by treatment-discontinuation status.
| Characteristic | Total (n=50) | Discontinued AI by 6 months due to MSK symptoms (n=7) | All Others (n=43) | p-value* | |||
|---|---|---|---|---|---|---|---|
| Mean | St Dev | Mean | St Dev | Mean | St Dev | ||
| Pain diary (0–10) | 1.7 | 1.3 | 1.9 | 1.2 | 1.6 | 1.3 | 0.52 |
| Depression (CESD) | 7.6 | 6.9 | 14.0 | 9.5 | 6.6 | 5.9 | 0.033 |
| Sleep (MOS-Sleep, SPDX2) | 31.2 | 18.8 | 47.1 | 17.9 | 28.6 | 17.8 | 0.013 |
| Fatigue (MFI) | |||||||
| General fatigue | 11.5 | 4.5 | 16.9 | 3.6 | 10.6 | 4.1 | 0.003 |
| Physical fatigue | 10.4 | 4.3 | 14.9 | 2.7 | 9.7 | 4.1 | 0.004 |
| Reduced activity | 9.3 | 4.4 | 12.9 | 3.9 | 8.7 | 4.3 | 0.021 |
| Reduced motivation | 7.6 | 4.0 | 11.2 | 2.3 | 7.0 | 4.0 | 0.006 |
| Mental fatigue | 8.3 | 4.3 | 9.0 | 3.7 | 8.1 | 4.4 | 0.49 |
| Cognitive function (MASQ) | |||||||
| Language | 14.0 | 3.4 | 15.0 | 4.9 | 14.4 | 3.8 | 0.86 |
| Visual-perception ability | 10.7 | 3.1 | 10.3 | 2.0 | 10.8 | 3.2 | 0.97 |
| Verbal memory | 16.3 | 3.6 | 17.3 | 1.6 | 16.1 | 3.8 | 0.42 |
| Visual-spatial memory | 14.0 | 3.3 | 14.7 | 2.1 | 13.9 | 3.4 | 0.31 |
| Attention/concentration | 15.5 | 3.4 | 16.8 | 2.9 | 15.4 | 3.5 | 0.31 |
At baseline, there was no statistically significant association between patient-reported pain and depression. The average pain reported by the 5 patients with CESD scores within the possibly or probably depressed range (CESD ≥ 16) was 2.5 (SD 1.9), compared to an average of 1.6 (SD 1.3) for those with CESD scores less than 16 (p=0.19). Baseline patient-reported pain was also associated with both poor sleep quality (r=0.56, p<0.0001) and general fatigue (r=0.49, p=0.0006), but not with poor cognitive function (r=0.14, p=0.41).
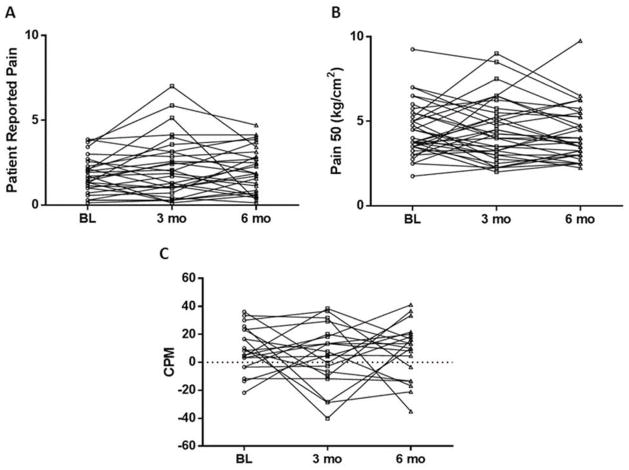
There was considerable inter-patient variability in measures of pain sensitivity prior to AI initiation (Figure 2). Mean baseline Pain50 was 4.3 kg/cm2 (SD 1.5). During assessment for CPM, mean scores for the test stimulus increased from 44.2 (SD 20.2) to 50.7 (SD 20.6) with the conditioning stimulus, which equates to a mean baseline CPM magnitude of 7.9 (SD 14.7). Of the 40 patients who underwent CPM assessment at baseline, 26 (65%) had impaired (>0) levels. The mean baseline CPM for those who received (n=19) and did not receive (n=21) chemotherapy was 14.6 and 2.0, respectively (p=0.006; Figure 3). No differences were noted according to prior chemotherapy for baseline Pain50 (4.4 vs 4.2, p=NS) or patient reported pain (1.8 vs 1.6, p=NS).
Figure 2. Effect of estrogen depletion on quantitative sensory testing measures.
Change in (A) patient-reported pain, (B) pressure pain threshold (Pain50) and (B) conditioned pain modulation (CPM) with aromatase inhibitor therapy. Individual patients are represented by circles at baseline (BL), squares at 3 months (mo), and triangles at 6 months. Points above the horizontal dotted line in C reflect impaired CPM.
Figure 3. Mean conditioned pain modulation (CPM) for those who were or were not treated with chemotherapy (chemo).
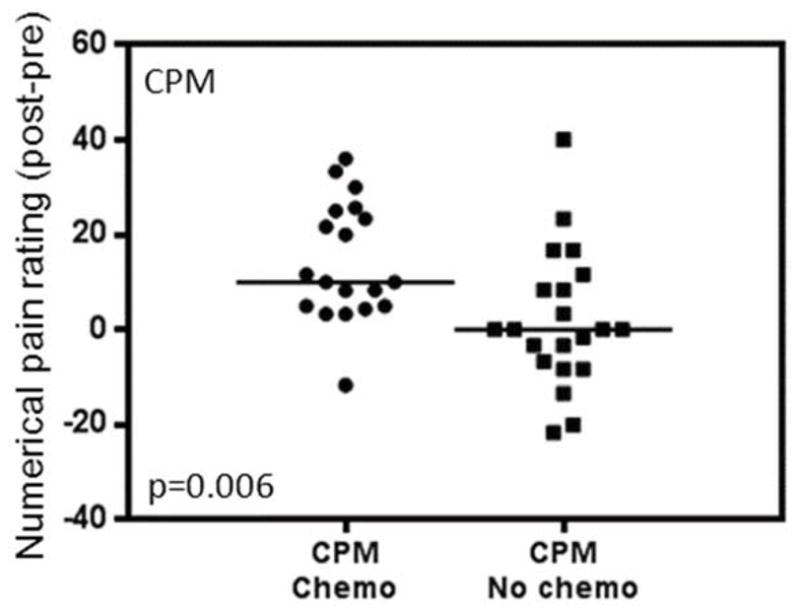
Circles represent individual patients who received chemotherapy, squares represent individual patients who did not receive chemotherapy, and horizontal lines represent mean values.
No associations were detected with either prior tamoxifen or BMI and baseline patient-reported pain, Pain50, or CPM. Baseline general fatigue was associated with baseline CPM (r=0.33, p=0.04) but not with baseline Pain50 (r=−0.06, p=NS). No associations were identified between baseline depression, sleep quality, or cognitive function and either baseline Pain50 or CPM.
Change in pain sensitivity with AI-induced estrogen deprivation
Average baseline serum estradiol concentration was 6.0 pg/ml (SD 7.6; Supplemental Figure 3). After 3 months of AI therapy, 38 of 43 (88%) assessed patients had undetectable serum estradiol concentrations (<0.625 pg/ml). The estradiol concentrations of the other 5 patients at the 3 month time point ranged from 0.67 to 3.38 pg/ml, which were all within the lower portion of the postmenopausal range (<10 pg/ml).
No change with AI therapy in mean patient-reported pain, Pain50, or CPM was identified (Figure 2, Table 3). There were no associations detected between change in mean patient-reported pain, Pain50, or CPM and either prior chemotherapy or prior tamoxifen. In addition, no associations were detected between change in patient-reported pain and change in either Pain50 or CPM between baseline and 3 months (data not shown).
Table 3.
Patient-reported pain and quantitative sensory testing measures at all three timepoints.
| Measure | All Patients | P value | Stopped early | Continued AI | P value |
|---|---|---|---|---|---|
|
| |||||
| Patient-reported pain | |||||
| - Baseline | 1.7 (1.3) | 0.75 | 1.9 (1.2) | 1.6 (1.3) | 0.52 |
| - 3 months | 2.1 (1.8) | 4.2 (1.3) | 1.9 (1.8) | 0.021 | |
| - 6 months | 1.7 (1.4) | n/a | 1.7 (1.4) | ||
|
| |||||
| Pain50, kg/cm2 | |||||
| - Baseline | 4.3 (1.5) | 0.41 | 3.5 (1.3) | 4.4 (1.6) | 0.24 |
| - 3 months | 4.2 (1.8) | 2.3 (0.6) | 4.4 (1.8) | 0.01 | |
| - 6 months | 4.2 (1.6) | n/a | 4.2 (1.6) | ||
|
| |||||
| Conditioned pain modulation | |||||
| - Baseline | 7.9 (14.7) | 0.66 | 14.2 (8.0) | 7.2 (15.2) | 0.25 |
| - 3 months | 6.3 (18.4) | 15.0 (20.3) | 5.1 (18.2) | 0.52 | |
| - 6 months | 10.4 (18.9) | n/a | 10.4 (18.9) | ||
Values given are mean values with standard deviations listed in parentheses.
Association between pain sensitivity and discontinuation of AI therapy because of musculoskeletal symptoms
Seven patients (14%) discontinued therapy by 6 months because of musculoskeletal symptoms. No association was detected between baseline Pain50 or CPM and discontinuation of therapy because of musculoskeletal toxicity (data not shown). Patients who discontinued AI therapy by 6 months had an average Pain50 of 2.25 (SD 0.61) at the three month time point, whereas those who continued treatment had an average Pain50 of 4.40 (SD 1.77) at 3 months (p=0.01) (Table 3, Supplemental Figure 4). No association was identified between average CPM at 3 months and treatment discontinuation by 6 months.
Association between patient-reported symptoms and discontinuation of AI therapy because of musculoskeletal symptoms
Mean patient-reported pain in this patient cohort was 1.7 (SD 1.3) at baseline, 2.1 (SD 1.8) at 3 months, and 1.7 (SD 1.4) at 6 months. Patients who discontinued AI therapy by 6 months reported an average pain of 4.17 (SD 1.31) at the three month time point, whereas those who continued treatment reported an average pain of 1.85 (SD 1.76) at 3 months (p=0.021). No association was detected between baseline patient-reported pain and discontinuation of therapy because of musculoskeletal toxicity.
Patients were also assessed for non-pain symptoms present prior to initiation of AI therapy using validated questionnaires (Table 2). Patients who discontinued AI therapy by 6 months were more likely to report higher baseline levels of depression (CESD 14.0 (SD 9.5) vs 6.6 (SD 5.9), p=0.033), poor sleep quality (SPDX2 47.1 (SD 17.9) vs 28.6 (SD 17.8), p=0.013), and fatigue (MFI general fatigue 16.9 (SD 3.6) vs 10.6 (SD 4.1), p=0.003) compared to those who continued treatment beyond 6 months. No associations were noted between baseline cognitive function and treatment discontinuation.
Discussion
In this prospective study utilizing QST, we were unable to confirm our hypothesis that pre-existing high pain sensitivity or impaired descending pain inhibitory pathways predispose women to AIMSS. In addition, we failed to confirm our second hypothesis that inter-patient differences in estrogen deprivation leads to an increase in pain sensitivity or impairment in CPM in AI-treated postmenopausal breast cancer survivors. Therefore, it is unlikely that estrogen deprivation within the CNS leading to a generalized increase in evoked pain sensitivity is contributing substantially to the increase in musculoskeletal pain, which is experienced by about half of AI-treated women.
A smaller than expected number of patients in our study discontinued treatment by 6 months because of new or worsened musculoskeletal symptoms. Baseline pain, pain sensitivity, and conditioned pain modulation were not predictive of early treatment discontinuation. As expected, average patient-reported pain was higher at the three month time point in those patients who subsequently discontinued AI therapy by 6 months because of musculoskeletal symptoms. Interestingly, average pain sensitivity was significantly lower at the three month time point in those patients who discontinued AI therapy by 6 months. Although the sample size is limited, these findings suggest that in some patients, there may be an association between increased sensitivity to pain and intolerance of AI therapy.
Prior chemotherapy has been identified as a risk factor for development of AI-associated musculoskeletal symptoms in multiple clinical trials.3, 19, 32 In this study we found an association between prior treatment with chemotherapy and impairment of the descending inhibitory pain system at baseline, prior to AI therapy. This finding suggests that chemotherapy-induced nerve damage might “prime” an individual for the subsequent development of pain in part by leading to impaired CPM. The recent studies showing that beneficial response to duloxetine in diabetic neuropathic pain can be predicted by less efficient baseline CPM, as well as the evidence that duloxetine is effective in treating chemotherapy-induced pain, offer additional support for this hypothesis.34, 40 In addition, we previously demonstrated that duloxetine has activity in treatment of AI-associated musculoskeletal pain.20
Furthermore, prior research has demonstrated that some women who are unable to tolerate one AI medication are able to tolerate a second AI medication. Since the degree of estrogen level suppression is similar for all AI medications, these data suggest that estrogen deprivation alone is not the cause of AI-associated arthralgias.1, 19 Therefore, it remains unclear why some women experience AI-associated musculoskeletal pain and others do not.
Our results also have important implications for pain research more generally. Previously reported studies in premenopausal women with chronic pain have suggested a link between low estrogen periods during the menstrual cycle and higher reported pain.18, 23 In contrast, we identified no change in pain sensitivity, conditioned pain modulation, or patient-reported pain with the profound one logarithm reduction in estradiol associated with aromatase inhibition in this cohort of postmenopausal women. Although there was considerable intra-patient variability in QST measures in patients over time, the mean values during estrogen deprivation did not differ from the pre-treatment values. The lower baseline serum estrogen concentrations in postmenopausal women in this study, coupled with the immediate effect of AIs on estrogen, are in contrast to the slow and intermittent variations that occur during the menstrual cycle. Thus, our study is a different test of whether estrogen levels directly affect pain processing than the designs used in these previous studies.
In addition to evaluating the effect of pain sensitivity measures on treatment discontinuation, we also investigated associations between persistence with therapy and baseline patient-reported symptoms that were already present at the time of AI initiation. We failed to identify an association between pre-existing pain and treatment discontinuation due to pain, which is similar to previously reported findings in a different trial.19 However, in this patient cohort we identified statistically significant associations between baseline depression, poor sleep quality, and fatigue and early discontinuation of AI therapy because of musculoskeletal symptoms. This finding that increased global symptom burden, rather than just pain-related symptoms, may impact persistence with adjuvant endocrine therapy suggests that management of the symptom cluster rather than focusing specifically on analgesia may be more effective.
One limitation of our study is relatively small sample size, which could limit our power to detect differences in measures of pain sensitivity in AI-treated women. However, despite the small sample size we had 80% power to detect relatively small changes in pain sensitivity, and were unable to detect changes of that magnitude. In addition, given the stability of Pain50 and CPM values between the baseline and 3 month time points (Figure 2), in conjunction with the biochemically verified suppression of circulating estradiol, it is therefore unlikely that a clinically significant change in pain sensitivity parameters would be identified in a larger patient cohort.
The unexpectedly low incidence of development of moderate or severe pain in our study population (17% at 3 months) could also limit our power to detect associations between pain sensitivity measures and patient-reported symptoms. Finally, the findings could be biased toward the null if patients with missing data either because of early treatment discontinuation due to symptoms or because of patient request to discontinue participation in the overall study or to undergo CPM assessment at the 3 month time point had substantial decreases in their pain sensitivity.
Another challenge is the lack of an established definition of AIMSS in the literature. This is in part due to the difficulty accounting for variability in patient-reported pain symptoms with AI therapy (e.g., arthralgia, myalgia, tendonitis), the impact of concomitant over-the-counter and prescription medications, and the need to parse out change in pain in patients who may have pre-existing pain from prior surgery or chemotherapy or from co-morbidities common in this postmenopausal population, especially osteoarthritis. We therefore chose to use treatment discontinuation due to musculoskeletal symptoms as a surrogate endpoint for AIMSS, as we have done in previous publications.19, 22
In summary, estrogen deprivation with AI therapy did not impact experimental pain sensitivity or descending pain modulation. Additional studies are needed to better understand how effects of prior chemotherapy might contribute to the pain and intolerance to therapy that a substantial proportion of breast cancer survivors, including those treated with adjuvant AI therapy, develop. These studies might then lead to better interventions designed to increased persistence with these life-saving medications. In this regard, we recently initiated a large placebo-controlled trial of duloxetine for patients with AIMSS, which should shed further light on this frequent and problematic toxicity.
Supplementary Material
Footnotes
Disclosures
Research funding: NLH is a Damon Runyon-Lilly Clinical Investigator supported (in part) by the Damon Runyon Cancer Research Foundation (grant number CI-53-10). This work was also supported in part by the Michigan Institute for Clinical and Health Research (NLH) and the Fashion Footwear Association of New York/QVC Presents Shoes on Sale (DFH). This publication was made possible by Grant Number UL1RR024986 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
Potential conflicts of interest: NLH receives research funding from AstraZeneca, Lilly Pharmaceuticals, and sanofi aventis. DFH received research funding from AstraZeneca, Novartis, Pfizer, and Veridex LLC. DJC has received consulting income and grant support from Pfizer, Forest, Wyeth, Cerephex and Nuvo, and consulting income from Lilly, UCB, Cerephex, Tonix, and Purdue Labs. SEH has received grant support from Cerephex, Merck and Forest Laboratories. No other authors reported conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Briot K, Tubiana-Hulin M, Bastit L, Kloos I, Roux C. Effect of a switch of aromatase inhibitors on musculoskeletal symptoms in postmenopausal women with hormone-receptor-positive breast cancer: the ATOLL (articular tolerance of letrozole) study. Breast Cancer Res Treat. 2010;120:127–134. doi: 10.1007/s10549-009-0692-7. [DOI] [PubMed] [Google Scholar]
- 2.Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J, Mamounas EP, Rowden D, Solky AJ, Sowers MR, Stearns V, Winer EP, Somerfield MR, Griggs JJ. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, Sierra A, Hershman DL. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25:3877–3883. doi: 10.1200/JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]
- 4.Dixon JM, Renshaw L, Young O, Murray J, Macaskill EJ, McHugh M, Folkerd E, Cameron DA, A’Hern RP, Dowsett M. Letrozole suppresses plasma estradiol and estrone sulphate more completely than anastrozole in postmenopausal women with breast cancer. J Clin Oncol. 2008;26:1671–1676. doi: 10.1200/JCO.2007.13.9279. [DOI] [PubMed] [Google Scholar]
- 5.Dowsett M, Cuzick J, Ingle J, Coates A, Forbes J, Bliss J, Buyse M, Baum M, Buzdar A, Colleoni M, Coombes C, Snowdon C, Gnant M, Jakesz R, Kaufmann M, Boccardo F, Godwin J, Davies C, Peto R. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol. 2010;28:509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 6.Felson DT, Cummings SR. Aromatase inhibitors and the syndrome of arthralgias with estrogen deprivation. Arthritis Rheum. 2005;52:2594–2598. doi: 10.1002/art.21364. [DOI] [PubMed] [Google Scholar]
- 7.Foerster BR, Petrou M, Harris RE, Barker PB, Hoeffner EG, Clauw DJ, Sundgren PC. Cerebral blood flow alterations in pain-processing regions of patients with fibromyalgia using perfusion MR imaging. AJNR Am J Neuroradiol. 2011;32:1873–1878. doi: 10.3174/ajnr.A2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geisler J, Haynes B, Anker G, Dowsett M, Lonning PE. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol. 2002;20:751–757. doi: 10.1200/JCO.2002.20.3.751. [DOI] [PubMed] [Google Scholar]
- 9.Geisser ME, Glass JM, Rajcevska LD, Clauw DJ, Williams DA, Kileny PR, Gracely RH. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain. 2008;9:417–422. doi: 10.1016/j.jpain.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Geisser ME, Gracely RH, Giesecke T, Petzke FW, Williams DA, Clauw DJ. The association between experimental and clinical pain measures among persons with fibromyalgia and chronic fatigue syndrome. European journal of pain (London, England) 2007;11:202–207. doi: 10.1016/j.ejpain.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Giesecke J, Reed BD, Haefner HK, Giesecke T, Clauw DJ, Gracely RH. Quantitative sensory testing in vulvodynia patients and increased peripheral pressure pain sensitivity. Obstet Gynecol. 2004;104:126–133. doi: 10.1097/01.AOG.0000129238.49397.4e. [DOI] [PubMed] [Google Scholar]
- 12.Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, Clauw DJ. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheum. 2004;50:613–623. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- 13.Giesecke T, Gracely RH, Williams DA, Geisser ME, Petzke FW, Clauw DJ. The relationship between depression, clinical pain, and experimental pain in a chronic pain cohort. Arthritis Rheum. 2005;52:1577–1584. doi: 10.1002/art.21008. [DOI] [PubMed] [Google Scholar]
- 14.Giesecke T, Williams DA, Harris RE, Cupps TR, Tian X, Tian TX, Gracely RH, Clauw DJ. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis Rheum. 2003;48:2916–2922. doi: 10.1002/art.11272. [DOI] [PubMed] [Google Scholar]
- 15.Gracely RH, Lota L, Walter DJ, Dubner R. A multiple random staircase method of psychophysical pain assessment. Pain. 1988;32:55–63. doi: 10.1016/0304-3959(88)90023-1. [DOI] [PubMed] [Google Scholar]
- 16.Hassett AL, Epel E, Clauw DJ, Harris RE, Harte SE, Kairys A, Buyske S, Williams DA. Pain is associated with short leukocyte telomere length in women with fibromyalgia. J Pain. 2012;13:959–969. doi: 10.1016/j.jpain.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Hays RD, Stewart AL. Sleep measures. In: Stewart AL, Ware JE, editors. Measuring functioning and well-being: The Medical Outcomes Study approach. Duke University Press; Durham, N.C: 1992. pp. 235–259. [Google Scholar]
- 18.Hellstrom B, Anderberg UM. Pain perception across the menstrual cycle phases in women with chronic pain. Perceptual and motor skills. 2003;96:201–211. doi: 10.2466/pms.2003.96.1.201. [DOI] [PubMed] [Google Scholar]
- 19.Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, Hayden J, Tarpinian K, Yakim E, Flockhart DA, Stearns V, Hayes DF, Storniolo AM. Predictors of aromatase inhibitor discontinuation due to treatment-emergent symptoms in early-stage breast cancer. J Clin Oncol. 2012;30:936–942. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry NL, Banerjee M, Wicha M, Van Poznak C, Smerage JB, Schott AF, Griggs JJ, Hayes DF. Pilot study of duloxetine for treatment of aromatase inhibitor-associated musculoskeletal symptoms. Cancer. 2011;117:5469–5475. doi: 10.1002/cncr.26230. [DOI] [PubMed] [Google Scholar]
- 21.Henry NL, Giles JT, Stearns V. Aromatase inhibitor-associated musculoskeletal symptoms: etiology and strategies for management. Oncology (Williston Park) 2008;22:1401–1408. [PubMed] [Google Scholar]
- 22.Henry NL, Skaar TC, Dantzer J, Li L, Kidwell K, Gersch C, Nguyen AT, Rae JM, Desta Z, Oesterreich S, Philips S, Carpenter JS, Storniolo AM, Stearns V, Hayes DF, Flockhart DA. Genetic associations with toxicity-related discontinuation of aromatase inhibitor therapy for breast cancer. Breast Cancer Res Treat. 2013;138:807–816. doi: 10.1007/s10549-013-2504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LeResche L, Mancl L, Sherman JJ, Gandara B, Dworkin SF. Changes in temporomandibular pain and other symptoms across the menstrual cycle. Pain. 2003;106:253–261. doi: 10.1016/j.pain.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 24.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Napadow V, Lee J, Kim J, Cina S, Maeda Y, Barbieri R, Harris RE, Kettner N, Park K. Brain correlates of phasic autonomic response to acupuncture stimulation: An event-related fMRI study. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22091. epub April 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain. 2003;105:403–413. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 27.Petzke F, Harris RE, Williams DA, Clauw DJ, Gracely RH. Differences in unpleasantness induced by experimental pressure pain between patients with fibromyalgia and healthy controls. European journal of pain (London, England) 2005;9:325–335. doi: 10.1016/j.ejpain.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Phillips K, Clauw DJ. Central pain mechanisms in the rheumatic diseases: future directions. Arthritis Rheum. 2013;65:291–302. doi: 10.1002/art.37739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. J Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 30.Santen RJ, Demers L, Ohorodnik S, Settlage J, Langecker P, Blanchett D, Goss PE, Wang S. Superiority of gas chromatography/tandem mass spectrometry assay (GC/MS/MS) for estradiol for monitoring of aromatase inhibitor therapy. Steroids. 2007;72:666–671. doi: 10.1016/j.steroids.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Seidenberg M, Haltiner A, Taylor MA, Hermann BB, Wyler A. Development and validation of a Multiple Ability Self-Report Questionnaire. J Clin Exp Neuropsychol. 1994;16:93–104. doi: 10.1080/01688639408402620. [DOI] [PubMed] [Google Scholar]
- 32.Sestak I, Cuzick J, Sapunar F, Eastell R, Forbes JF, Bianco AR, Buzdar AU. Risk factors for joint symptoms in patients enrolled in the ATAC trial: a retrospective, exploratory analysis. Lancet Oncol. 2008;9:866–872. doi: 10.1016/S1470-2045(08)70182-7. [DOI] [PubMed] [Google Scholar]
- 33.Smets EM, Garssen B, Bonke B, De Haes JC. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39:315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 34.Smith EM, Pang H, Cirrincione C, Fleishman S, Paskett ED, Ahles T, Bressler LR, Fadul CE, Knox C, Le-Lindqwister N, Gilman PB, Shapiro CL. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. Jama. 2013;309:1359–1367. doi: 10.1001/jama.2013.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith YR, Stohler CS, Nichols TE, Bueller JA, Koeppe RA, Zubieta JK. Pronociceptive and antinociceptive effects of estradiol through endogenous opioid neurotransmission in women. J Neurosci. 2006;26:5777–5785. doi: 10.1523/JNEUROSCI.5223-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spritzer KL, Hays RD. A Manual for Use and Scoring, Version 1.0. Los Angeles, CA: 2003. MOS Sleep Scale. [Google Scholar]
- 37.Tracey I. Can neuroimaging studies identify pain endophenotypes in humans? Nat Rev Neurol. 2011;7:173–181. doi: 10.1038/nrneurol.2011.4. [DOI] [PubMed] [Google Scholar]
- 38.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152:S2–15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yarnitsky D, Crispel Y, Eisenberg E, Granovsky Y, Ben-Nun A, Sprecher E, Best LA, Granot M. Prediction of chronic post-operative pain: pre-operative DNIC testing identifies patients at risk. Pain. 2008;138:22–28. doi: 10.1016/j.pain.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 40.Yarnitsky D, Granot M, Nahman-Averbuch H, Khamaisi M, Granovsky Y. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain. 2012;153:1193–1198. doi: 10.1016/j.pain.2012.02.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.