Abstract
The architecture of nanoparticles of biological origin, generally also known as bionanoparticles, presents several features that are ideal for their use in developing diagnostics, therapeutics, and vaccines. In this regard, particles formed by viral proteins using recombinant DNA technology resemble authentic virus particles. However, they lack infectivity due to the absence of genetic components such as DNA or RNA. Hence, they are designated as virus-like particles (VLP). VLPs possess the following characteristics: (1) they can be generated by either a single or a few viral proteins; (2) their size, formed by viral proteins, is in the range of 20 to100 nm; (3) the number of protein molecules required for particle assembly is from hundreds to thousands, depending on the VLP; (4) the protein(s) responsible for their assembly are amenable for manipulation; and (5) multiple proteins/peptides can be incorporated into a VLP. The potential advantages of VLPs directed by retroviral proteins are discussed in this review.
My Personal Recollection of Hilary Koprowski
In thinking back on Dr. Hilary Koprowski, I am reminded of an individual with an infectious enthusiasm for science and for life. His joie de vivre, in my view, served him well to overcome the highs and lows of life in research. Dr. Koprowski is credited with a multitude of innovations, including a safer rabies virus vaccine, and he enjoyed a long and storied career. I witnessed his exuberant spirit and passion for science firsthand as I was fortunate to be associated with Dr. Koprowski for nearly two decades in the latter part of his career, at both The Wistar Institute and Thomas Jefferson University. He is an exalted mentor—a very special individual who inspired my scientific journey.
My formal association with Dr. Koprowski began in 1989 when I joined The Wistar Institute as a faculty member. My transition from a radiation biology/chemical carcinogenesis background to a molecular virology and cancer biology background took place under the guidance of Drs. Premkumar Reddy and Stuart Aaronson at NCI. This training, and a brief stint at the Centers for Disease Control and Prevention in Atlanta, paved the way for carrying out productive collaborative studies with Dr. Koprowski. He was a scientist with strong convictions. This was evident in his efforts towards searching for a potential etiologic agent in multiple sclerosis (MS). However, it should be noted that Dr. Koprowski also had the wisdom to move forward despite setbacks. Formidable obstacles never slowed Dr. Koprowski down in his quest for understanding the disease and exploring therapeutic options for MS. It was an invaluable lesson for young investigators.
After leaving Wistar, my collaboration with Dr. Koprowski continued at Thomas Jefferson University, as a part of The Institute of Biotechnology and Advanced Molecular Medicine, until 2008. Dr. Koprowski's interest in virology and cancer gave me plenty of opportunities to witness a master at work. In addition to his enthusiasm and commitment to science, he was always able to see the larger picture and connect dots in a way that no one else could.
To this day, I vividly remember an incident highlighting his commitment to the advancement of science. Dr. Koprowski was admitted to Bryn Mawr Hospital on the Main Line for a procedure and he was being fed intravenously. Despite his condition, I received a telephone call from him asking me about a deadline for an NIH grant proposal. Later that week, I went to his home in Wynnewood for a discussion related to our project objectives. My impression is that he was like a child, forever bubbly and inquisitive, never waning in his enthusiasm for research.
I am glad that the journal “Monoclonal Antibodies in Immunodiagnosis and Immunotherapy” is devoting a special issue to celebrate the achievements of Dr. Koprowski. Given his seminal contributions in the area of monoclonal antibodies, this edition is certainly a fitting tribute.
Lastly, thinking outside the box came naturally to Hilary Koprowski, particularly since he had such a vast range of knowledge. The theme of this article is one of his favorite topics, as he had devoted a considerable amount of time in generating and testing vaccines using diverse platforms, including viruses and plants. Dr. Koprowski's legacy will be kept alive through the continued work of the scientists who had the privilege of associating with him and the institutions where he worked.
Alagarsamy Srinivasan
Background
Nanoparticles have recently emerged as a new platform and possess several advantageous features for their applications in diagnostics, therapeutics, and vaccine development.(1) Nanoparticles are defined as particles predominantly between 20 and 100 nm in diameter, and are broadly divided into two types based on their composition. The first group encompasses nanoparticles, which are made from biocompatible and biodegradable materials, such as solid lipids, or polymers, which can be natural (gelatin and albumin) or synthetic (polylactides and polyalkylcyanoacrylates). The second group comprises nanoparticles of biological origin. These particles are formed by specific proteins of cellular and viral origin. An example of cellular protein is human ferritin, an iron storage protein, which assembles into particles with a hollow shell structure in the range of ∼12 nm in diameter. The particles consist of 24 subunits of heavy and light chains. It has also been shown that 24 heavy chain subunits alone can assemble into particulate structures.(2) Ferritin-based nanoparticles have been used as antigen carriers for diagnostic applications.(3) Similarly, proteins of several viruses have been shown to assemble in a symmetric manner from hundreds of copies of a single or multiple proteins.(4–12) These structures are morphologically similar to authentic virus particles. Hence, these structures are known as virus-like particles (VLPs) and are in the range of ∼20 to 120 nm in diameter. An advantage of VLPs is that they are amenable to genetic and chemical modifications. This enables VLPs to be ideal carriers for antigens of interest.(12,13) Based on the size and number of copies of protein needed to assemble particles, nanoparticles formed by viral proteins can be designed to carry multiple antigenic epitopes for the detection of diverse viral agents in a single assay format. Therefore it is possible to use VLPs for the detection of single or multiple infectious agents in infected individuals, as a carrier of therapeutic proteins and peptides, and also as a scaffold for vaccines against bacterial/viral pathogens, as well as cancer.
Virus-like Particles Directed by Retroviruses
Retroviruses are enveloped RNA viruses that replicate by a virion-associated reverse transcriptase enzyme, which converts genomic RNA to DNA in infected cells.(14,15) The viral DNA then becomes a part of the host cell's genome upon integration, and directs the generation of RNA for viral protein synthesis and assembly. Mature retrovirus particles exhibit an ordered structure in comparison to immature virus particles. The retrovirus particle is comprised of Gag, Gag-Pro-Pol, and envelope proteins, and is covered by a lipid membrane derived from the host cell. The precursor proteins Gag and Gag-Pro-Pol, while uncleaved in the immature virus particles, are cleaved by viral protease during maturation.(16,17) The precursor Gag protein has been shown to carry out several functions, including budding, directing virion size, and incorporating other viral proteins and RNA. Characteristically, the size of virions of a diverse number of retroviruses is in the range of 80 to 100 nm in diameter, which is determined by a component of Gag protein known as the capsid.(18) Interestingly, Gag and Gag-Pro-Pol proteins are present inside the virus particle and the envelope protein is present on the surface of virus particles. While the targeting of the Gag protein to the membrane is mediated by the myristylation of Gag, the localization of the Env protein at the membrane is mediated by the membrane-spanning domain of Env. This property of Env proteins can be exploited for anchoring candidate vaccine proteins or diagnostic antigens on the surface of particles.
During infection by retroviruses in appropriate target cells, the release of empty, non-infectious virus particles lacking the RNA genome has also been noted, in addition to the release of bona fide infectious virus particles. These empty particles have been shown to contain the viral structural proteins in different combinations, including Gag-Pol-Env, Gag-Pol, Gag-Env, and only Gag.(14) This observation was the spark for several studies, which ultimately identified that the retroviral structural protein Gag alone has the ability to form VLPs.(10,19,20) These VLPs are distinguishable from infectious viruses by their lack of reverse transcriptase, protease, and integrase enzymes, as well as RNA and envelope proteins. As shown in Figure 1, VLPs are protein cages surrounded by a lipid membrane, morphologically similar to immature virus particles. The monomeric building block is the Gag protein. All retroviral Gag proteins contain matrix, capsid, and nucleocapsid domains.
FIG. 1.
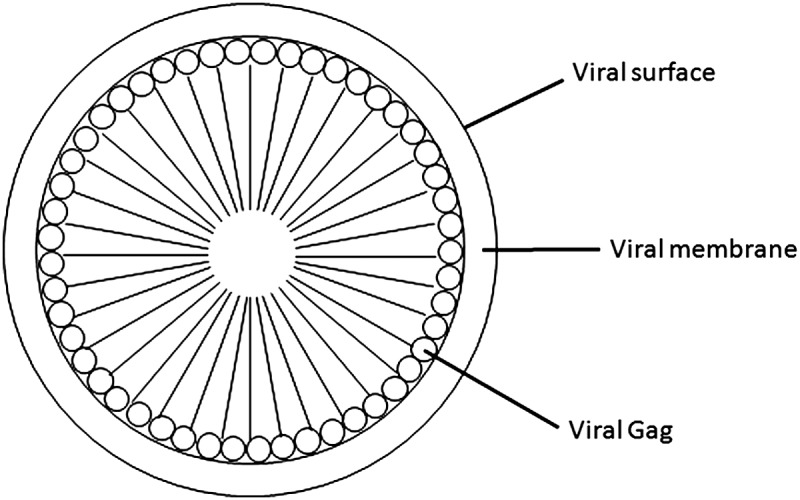
Schematic representation of retroviral VLP. The N-terminus of Gag is localized to the inner side of the cell membrane and the C-terminus is pointed inward.
Structural and functional information is available for several retroviral Gag proteins, including HIV-1. This has enabled investigators to carry out site-specific mutagenesis studies for identifying essential and nonessential regions of the Gag protein, in terms of viral assembly and incorporation of antigens of interest into VLPs.(18,21)
Modes of Incorporation of Protein/Peptide into VLP
VLPs directed solely by Gag thus represent a class of virus-based nanoparticles (VBNPs). These are also designated as nanobags, nanocontainers, or nanoboxes. Because of their unique features, non-replicating nanoparticles such as VLPs can be harnessed for the display of proteins/peptides on their surface and for carrying proteins internally. Hence, VLPs have the potential for both diagnostic and therapeutic utility. Retroviral VLPs can be produced from diverse host systems such as yeast, insect, and mammalian cells, through the process of budding.(7,11,22) Thus, the VLP system can be considered as a eukaryotic display system.(11) VLPs provide multiple surfaces for the presentation of antigenic, immunogenic, and functional proteins/peptides, such as the exterior surface of the VLP, the interior surface of the VLP, and the chimeric form of an assembly protein. These three locations can be exploited for carrying proteins/peptides without disrupting the VLP assembly.
Exterior surface of VLP
It has been demonstrated that retrovirus particles can incorporate envelope glycoproteins encoded by both homologous and heterologous viruses, in addition to membrane proteins of cellular origin (Fig. 2). The exterior surface can be used for displaying a candidate protein/peptide and requires a membrane-anchoring domain. This feature is useful for presenting target vaccines and diagnostic proteins. The number of protein molecules that can be incorporated onto the VLP surface is less than the number of protein molecules needed for the assembly of a VLP. Co-expression of the protein with the structural protein Gag is required to incorporate the protein on the exterior surface.
FIG. 2.
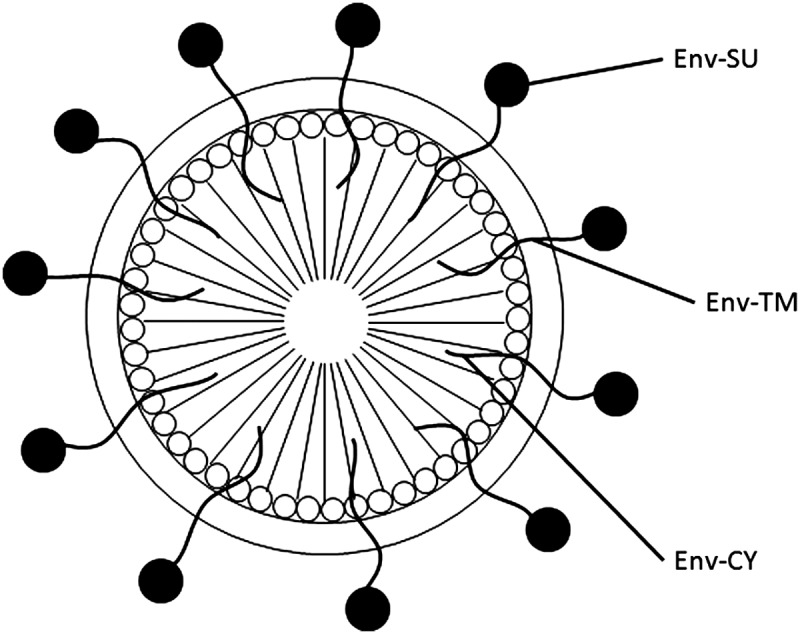
Display of viral envelope proteins on the surface of retroviral VLP. Env-SU, envelope surface domain present outside of the virion; Env-TM, membrane-spanning domain of the envelope protein; Env-CY, cytoplasmic tail of the envelope protein.
Interior surface of VLP
An ideal approach is to use a chimeric form of the assembly protein (Fig. 3). This is by far the most efficient, as the number of antigen molecules in each VLP will be the same as that of the viral structural proteins. It is well known that a high number of structural protein molecules (over 2500) are required for the VLP assembly. Therefore, this assembly mode allows a large number of proteins/peptides of interest to be incorporated into the VLP.
FIG. 3.
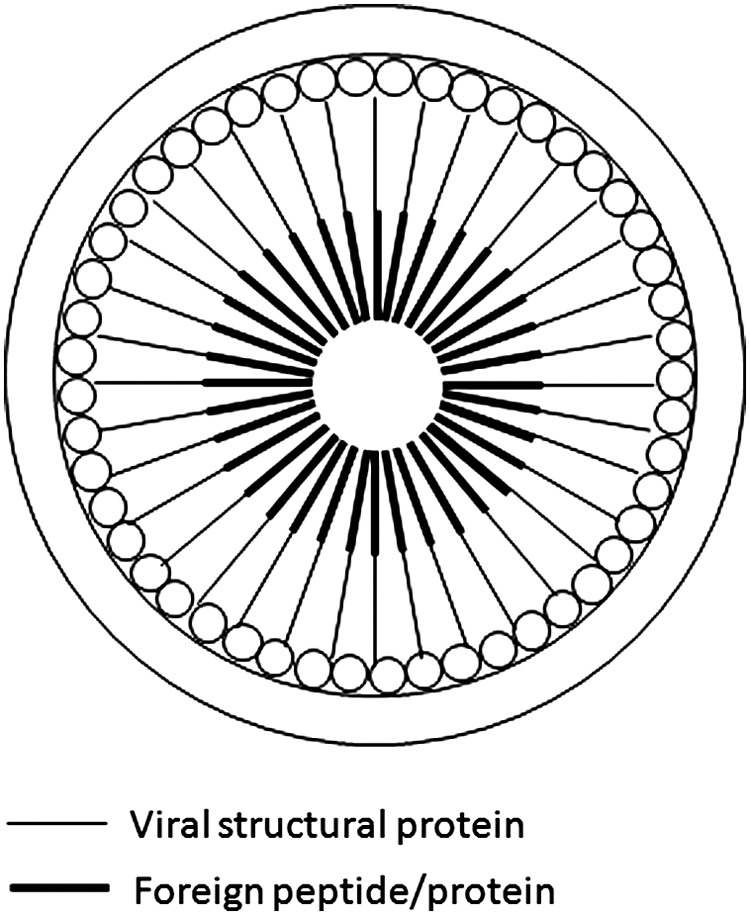
Intravirion display of antigen/peptide, utilizing a chimeric Gag protein approach. Chimeric Gag containing a single antigen/peptide is fused to the C-terminus.
Alternatively, a chimeric protein approach can be used to incorporate proteins in the interior cavity of VLPs. Specifically a chimeric protein can be generated in which the antigen of interest is fused to a domain derived from a cellular protein that interacts with the structural protein Gag. The interaction will result in its incorporation into the particles. The number of protein molecules incorporated into VLPs by this method may or may not be similar to the number of viral structural proteins.
A foreign protein or peptide can also be incorporated into the VLPs using oligomerization. Components of the viral protein Gag, such as the capsid, are known to oligomerize. By exploiting this feature, a chimeric protein containing oligomerization sequences can be incorporated into VLPs. Since both the chimeric and native capsid proteins combine to form VLPs, the number of chimeric protein molecules that can be incorporated may vary.
Lastly, antigenic proteins/peptides can be incorporated into VLPs through nonstructural viral proteins.(23–26) VLPs formed by Gag protein of lentiviruses provide an additional avenue to incorporate an antigenic protein/peptide of interest. In the case of HIV-1, three nonstructural viral proteins are specifically incorporated into virus particles, which are known as passengers of the virus particles. These include Vpr-Vif-Nef and Vpr-Vpx-Vif-Nef for HIV-1 and HIV-2/SIV, respectively.(14,15) The extent of incorporation of the nonstructural proteins has been shown to be variable. Based on this, it is possible that passenger proteins can also be used as carriers of antigenic proteins/peptides. Further, the ability to incorporate multiple passenger proteins enables the VLP system to detect multiple pathogens.
Applications of Nanoparticles
Of the nanoparticles directed by cellular and viral proteins, particles formed by the structural protein Gag have attracted considerable attention. The underlying reason is that each particle is assembled with ∼2500 copies of the Gag protein. Hence, a chimeric protein approach utilizing Gag can enable the incorporation of an equal number of foreign antigens of interest. In addition, the particles possess flexibility such that vaccine antigens or therapeutic proteins can be inserted in multiple locations of Gag, without disrupting particle formation. These applications of nanoparticles are elaborated further:
1. Nanoparticles for the delivery of therapeutic proteins/peptides. An important feature of VLPs is that proteins/peptides, such as a therapeutic protein or viral antigen, are covered with both a protective protein shell and a lipid membrane. Therefore the proteins/peptides are not susceptible to degradation by proteases. Further, it is also possible to modify VLPs to carry therapeutic proteins/peptides(11,27) and transduce them to appropriate cells through targeted delivery.
2. Nanoparticles for the development of vaccines. Structural and biochemical analyses of virus particles revealed the presence of virally encoded glycoproteins on their surface. During normal infection of a host by a virus, the host immune system initially encounters surface viral proteins. The generation of an immune response against the viral glycoproteins has been shown to inhibit virus replication, which has paved the way for the development of successful live attenuated, inactivated, and subunit viral vaccines against several pathogenic agents. Using a VLP platform, the display of a surface protein can generally be achieved by using a viral glycoprotein and/or through manipulation of a viral glycoprotein. The VLP platform also enables a larger number of surface antigen molecules or peptides to be presented due to spacing and orientation, than the number of protein molecules present on the surface of an authentic virus particle. The unique features of the VLP system, making it well suited for the display of proteins, are as follows: (1) The viral structural proteins follow a defined pathway to assemble into VLPs. This ensures that there is uniformity in the number of molecules used for assembly and the shape of particles. This is clearly an advantage over particles that can be generated with synthetic materials; (2) The VLP system has sufficient flexibility to incorporate glycoproteins of heterologous origin, similar to homologous viral glycoproteins.
3. Advantages of VLP surface display versus phage display. In phage display, a heterologous peptide or protein is displayed on the surface of the phage through fusion with a coat protein. This system allows displaying a peptide or a protein of reasonable size on the surface. However, the bacterial host system, which is used for the generation of recombinant phages, lacks the post-translational modifications needed for the structure and function of viral and eukaryotic proteins. This is important for the proteins involved in diagnostics, therapeutics, and vaccines. This requirement points towards a need for an alternative display system based on eukaryotes. Hence, the retroviral VLP system can serve as a platform for displaying eukaryotic envelope proteins.
4. VLP: an avenue to enrich proteins for diagnostic purposes and candidate vaccines. When viral or other marker antigens are expressed in a prokaryotic system, the proteins are largely present in the inclusion bodies. This poses a problem for purification of the antigen for diagnostic and therapeutic purposes. Proteins expressed as chimeric structural proteins, therefore, provide an avenue for purification. The VLPs are released into the medium of cells that express Gag or modified Gag protein (yeast, insect, or mammalian cells) and are inexpensive to purify. Thus, VLPs provide a simplified means of purifying proteins of interest.
Conclusions
Advances in recombinant DNA technology have revolutionized the field of biology and our understanding of human diseases in addition to therapeutic options to treat them. Designer vaccines against infectious agents and protein/peptide-based therapeutics are some of the success stories of recombinant protein technologies. VLPs, by their structural similarities to native virus particles, represent an excellent platform that can be exploited for developing vaccines and therapeutics. Specifically, VLPs formed by retroviral Gag proteins are most attractive as they can be modified to accommodate proteins containing ∼500 amino acids or more. This feature is advantageous as nanoparticles can be generated containing multiple antigens or a combination of vaccines with a molecular adjuvant. Thus, VLPs, perfected by nature, are ideal structural scaffolds for multiple applications.
Author Disclosure Statement
The authors have no financial interests to disclose.
DoD Disclaimer
The views expressed in this manuscript are those of the authors and do not reflect the official policy of the Department of the Army, Department of Defense or the U.S. Government.
References
- 1.Peek LJ, Middaugh CR, and Berkland C: Nanotechnology in vaccine delivery. Adv Drug Del Rev 2008;60(8):915–928 Epub 2008/03/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaaskelainen A, Soukka T, Lamminmaki U, Korpimaki T, and Virta M: Development of a denaturation/renaturation-based production process for ferritin nanoparticles. Biotechnol Bioeng 2009;102(4):1012–1024 Epub 2008/10/30. [DOI] [PubMed] [Google Scholar]
- 3.Svenson S, and Prud'homme RK: Multifunctional Nanoparticles for Drug Delivery Applications: Imaging, Targeting, and Delivery; Springer, New York, 2012 [Google Scholar]
- 4.Kozlovska TM, Cielens I, Vasiljeva I, Strelnikova A, Kazaks A, Dislers A, et al. : RNA phage Q beta coat protein as a carrier for foreign epitopes. Intervirology 1996;39(1–2):9–15 Epub 1996/01/01. [DOI] [PubMed] [Google Scholar]
- 5.Borisova G, Borschukova Wanst O, Mezule G, Skrastina D, Petrovskis I, Dislers A, et al. : Spatial structure and insertion capacity of immunodominant region of hepatitis B core antigen. Intervirology 1996;39(1–2):16–22 Epub 1996/01/01. [DOI] [PubMed] [Google Scholar]
- 6.Bellier B, and Klatzmann D: Virus-like particle-based vaccines against hepatitis C virus infection. Expert Rev Vacc 2013;12(2):143–154 Epub 2013/02/19. [DOI] [PubMed] [Google Scholar]
- 7.Zeltins A: Construction and characterization of virus-like particles: a review. Mol Biotechnol 2013;53(1):92–107 Epub 2012/09/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q, and Lai H: Plant-derived virus-like particles as vaccines. Hum Vacc Immunother 2013;9(1):26–49 Epub 2012/09/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pushko P, Pumpens P, and Grens E: Development of virus-like particle technology from small highly symmetric to large complex virus-like particle structures. Intervirology 2013;56(3):141–165 Epub 2013/04/19. [DOI] [PubMed] [Google Scholar]
- 10.Deml L, Speth C, Dierich MP, Wolf H, and Wagner R: Recombinant HIV-1 Pr55gag virus-like particles: potent stimulators of innate and acquired immune responses. Mol Immunol 2005;42(2):259–277 Epub 2004/10/19. [DOI] [PubMed] [Google Scholar]
- 11.Schott JW, Galla M, Godinho T, Baum C, and Schambach A: Viral and non-viral approaches for transient delivery of mRNA and proteins. Curr Gene Ther 2011;11(5):382–398 Epub 2011/07/27. [DOI] [PubMed] [Google Scholar]
- 12.Dalba C, Bellier B, Kasahara N, and Klatzmann D: Replication-competent vectors and empty virus-like particles: new retroviral vector designs for cancer gene therapy or vaccines. Mol Ther 2007;15(3):457–466 Epub 2007/01/25. [DOI] [PubMed] [Google Scholar]
- 13.Lai D, Singh SP, Cartas M, Murali R, Kalyanaraman VS, and Srinivasan A: Extent of incorporation of HIV-1 Vpr into the virus particles is flexible and can be modulated by expression level in cells. FEBS Lett 2000;469(2–3):191–195 Epub 2000/03/14. [DOI] [PubMed] [Google Scholar]
- 14.Goff SP: Retroviridae: the retroviruses and their replication. In: Fields' Virology, 4th ed, Knipe DM. (ed). Philadelphia, Lippincott Williams and Wilkins, 2001. pp. 1871–1940 [Google Scholar]
- 15.Freed EO, and Martin MA: HIVs and their replication. In: Fields' Virology, 4th ed, Knipe DM, (ed). Philadelphia, Lippincott Williams and Wilkins, 2001. pp. 1971–2041 [Google Scholar]
- 16.Bell NM, and Lever AM: HIV Gag polyprotein: processing and early viral particle assembly. Trends Microbiol 2013;21(3):136–144 Epub 2012/12/26. [DOI] [PubMed] [Google Scholar]
- 17.Lee SK, Potempa M, and Swanstrom R: The choreography of HIV-1 proteolytic processing and virion assembly. J Biol Chem 2012;287(49):40867–40874 Epub 2012/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ako-Adjei D, Johnson MC, and Vogt VM: The retroviral capsid domain dictates virion size, morphology, and coassembly of gag into virus-like particles. J Virol 2005;79(21):13463–13472 Epub 2005/10/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gheysen D, Jacobs E, de Foresta F, Thiriart C, Francotte M, Thines D, et al. : Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell 1989;59(1):103–112 Epub 1989/10/06. [DOI] [PubMed] [Google Scholar]
- 20.Karacostas V, Nagashima K, Gonda MA, and Moss B: Human immunodeficiency virus-like particles produced by a vaccinia virus expression vector. Proc Natl Acad Sci USA 1989;86(22):8964–8967 Epub 1989/11/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Accola MA, Strack B, and Gottlinger HG: Efficient particle production by minimal Gag constructs which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain. J Virol 2000;74(12):5395–5402 Epub 2000/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tobin GJ, Li GH, Williamson JC, Nagashima K, and Gonda MA: Synthesis and assembly of chimeric human immunodeficiency virus gag pseudovirions. Intervirology 1996;39(1–2):40–48 Epub 1996/01/01. [DOI] [PubMed] [Google Scholar]
- 23.Pandey RC, Datta D, Mukerjee R, Srinivasan A, Mahalingam S, and Sawaya BE: HIV-1 Vpr: a closer look at the multifunctional protein from the structural perspective. Curr HIV Res 2009;7(2):114–128 Epub 2009/03/12. [DOI] [PubMed] [Google Scholar]
- 24.Majumder B, Venkatachari NJ, Srinivasan A, and Ayyavoo V: HIV-1 mediated immune pathogenesis: spotlight on the role of viral protein R (Vpr). Curr HIV Res 2009;7(2):169–177 Epub 2009/03/12. [DOI] [PubMed] [Google Scholar]
- 25.Serio D, Rizvi TA, Cartas M, Kalyanaraman VS, Weber IT, Koprowski H, et al. : Development of a novel anti-HIV-1 agent from within: effect of chimeric Vpr-containing protease cleavage site residues on virus replication. Proc Natl Acad Sci USA 1997;94(7):3346–3351 Epub 1997/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cartas M, Singh SP, Serio D, Rizvi TA, Kalyanaraman VS, Goldsmith CS, et al. : Intravirion display of a peptide corresponding to the dimer structure of protease attenuates HIV-1 replication. DNA Cell Biol 2001;20(12):797–805 Epub 2002/03/07. [DOI] [PubMed] [Google Scholar]
- 27.Liu F, Wu X, Li L, Liu Z, and Wang Z: Use of baculovirus expression system for generation of virus-like particles: successes and challenges. Prot Express Purific 2013;90(2):104–116 Epub 2013/06/08. [DOI] [PMC free article] [PubMed] [Google Scholar]


