Abstract
On a clonal level, certain antibodies and T cells can interact with dissimilar antigens found in microbes and in host cells. More than 5% of over 800 monoclonal antibodies derived from multiple RNA and DNA viruses, as well as from a large number of T cell clones, engage in such interactions. Several of these cross-reactions, which we termed molecular mimicry, are against unique host proteins involved in autoimmune responses and diseases. Thus, molecular mimicry initiated as a host response to a virus or a microbial infection, but alternatively cross-reacting with an appropriate host-antigen, can be a mechanism for instigating an autoimmune disease. Molecular mimicry provides an explanation for the genetic observation that identical twins rarely manifest the same autoimmune disease and the documented epidemiologic evidence that microbial and/or viral infections often precede autoimmune disorders.
Prologue
In the early 1980s, one of my postdoctoral fellows, Robert Fujinami, was involved in generating monoclonal antibodies to the measles virus. The ability to generate such antibodies had recently been published by Kolmer and Milstein for which they later received the Nobel Prize.(1) It was common knowledge that, in addition to the Kolmer/Milstein team at Cambridge University, Harris at Sir William Dunn School of Pathology at the University of Oxford and Koprowski at The Wistar Institute were similarly engaged. Both were at the cusp of similar discoveries using myeloma cells and just steps behind Kolmer and Milstein. From Koprowski, I obtained his enormously competent technician, Hanna Lewicki, who first brought the hybridoma technique to Scripps and our San Diego area. Using monoclonal antibodies, Fujinami and I found that a monoclonal antibody against the phosphoprotein of measles virus cross-reacted with an intermediate filament protein of human cells, vimentin.(2) The molecular weight of the measles virus phosphoprotein is 70,000, whereas that of vimentin is 52,000. Cross-reactivity was documented by immunofluorescent staining of infected and uninfected cells and by immunoblotting. After we discussed our findings with Koprowski, he recommended that we publish them,(2) while adding his independent observations with Wroblewska and Frankel on the cross-reaction of a 146,000 molecular weight herpes simplex 1 protein with the 52,000 Kd vimentin. Importantly and of related interest, the monoclonal antibody against measles virus phosphoprotein did not react against the herpes simplex 1 virus protein and vice versa, indicating that these monoclonal antibodies recognized distinct antigenic determinants on the intermediate filament molecule. The implication of these experiments and of one conducted by Lane and Hoeffler(3) with SV40 was this: if an infectious agent induced a cross-reactive response with a host cell determinant involved in autoimmunity (e.g., the acetylcholine receptor and myasthenia gravis; thyroglobulin and autoimmune thyroid disease; insulin and diabetes; encephalitogenic protein(s) and multiple sclerosis), the outcome might be autoimmune disease (reviewed(4,5)).
We were well aware that, on the level of whole organisms, autoimmune responses or diseases associated with viral or other microbial infections could occur by mechanisms in addition to molecular mimicry. Examples are the action of viruses on polyreactive B cells expanding a pre-existing clone that reacts with self, the ability of viruses to release antigens not ordinarily recognized by the host immune system, viruses breaking immune tolerance, and altering cellular DNA sufficiently to allow formation of anti-DNA antibodies. Additionally, viruses can signal via Toll-like receptors to alter DNA. Nevertheless, my laboratory co-workers and I embarked on the journey outlined here to explore the feasibility of molecular mimicry as a cause of autoimmunity.
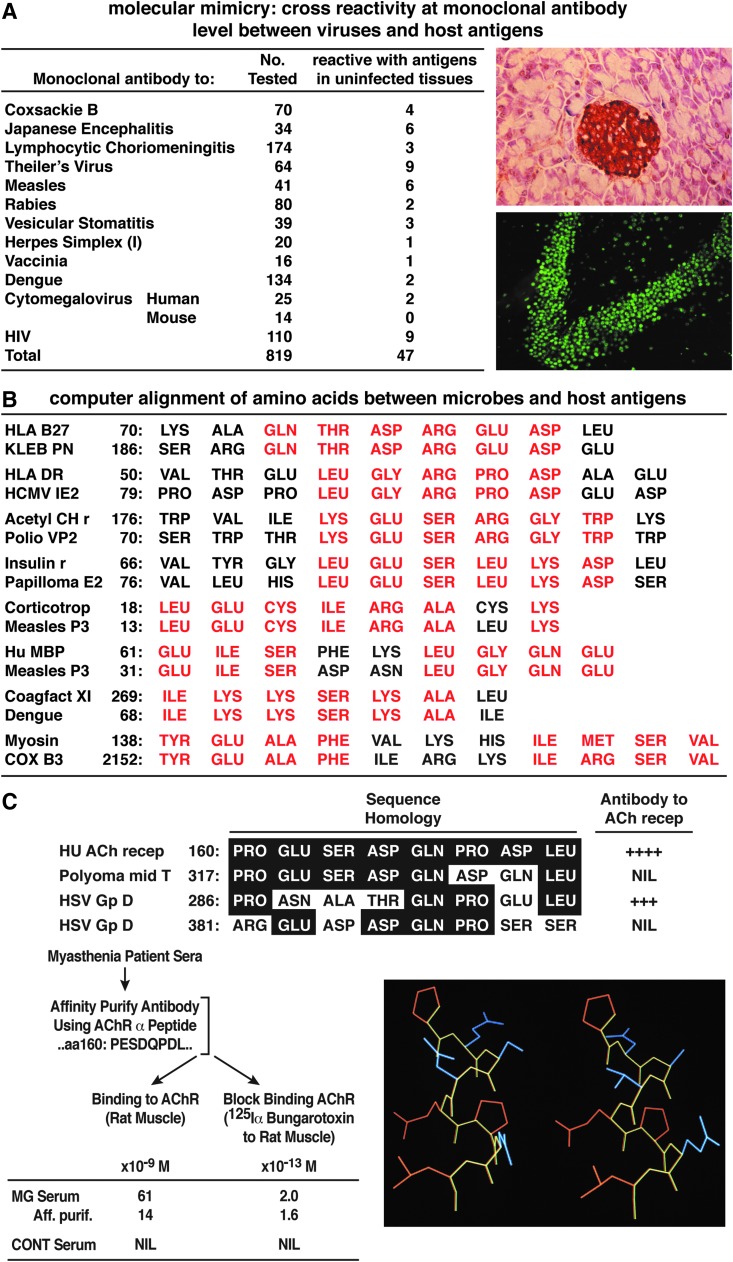
In the same year as our work with measles virus and Koprowski's with herpes simplex virus,(2) Samuel Dales, took a sabbatical in my laboratory. There, he found that mice immunized with lysates from vaccinia virus-infected cells or with pure vaccinia virus generated hybridomas that made the anticipated monoclonal antibodies against several vaccinia proteins, but also produced antibodies against other proteins like intermediate filaments or vimentin.(6) Of particular interest was the fact that monoclonal antibodies to intermediate filaments cross-reacted with vaccinia virus hemagglutinin as determined by immunoblot, immunofluorescence, and electron microscopy. With these results on hand, our laboratory initiated studies with our in-house collection of monoclonal antibodies against viral proteins and worked collaboratively with The Wistar Institute laboratories of Koprowski and Notkins at the NIH.(7) Together, as shown in Figure 1A, we evaluated over 800 monoclonal antibodies to a wide spectrum of DNA and RNA viruses. Included were the herpes virus group, vaccinia virus, myxoviruses, paramyxoviruses, arenaviruses, flaviviruses, alphaviruses, rhabdoviruses, coronaviruses, and human retroviruses. Considering that five to six amino acids are needed to induce a monoclonal antibody response,(8) the probability that 20 amino acids will occur in six identical residues between two proteins is 206 or 1:128,000,000. Our calculation of the frequency of cross-reactivity for the 800+ antiviral monoclonals revealed that 5% of these 800+ monoclonal antibodies from 16 different viruses also reacted with host-cell antigenic determinants expressed on or in uninfected tissues.(7) Thus, for molecular mimicry to occur, the disparate proteins or peptides must be close enough in homology, between self and microbe, to share amino acid (aa) determinants or conformational shapes but distant enough to be recognized as foreign by the immune system (Fig. 1B). If the homology is identical, then immune tolerance will occur. Further, to elicit an autoimmune response or disease, the homology must be with a host self-determinant (epitope) having an important biologic activity. For example, an immune attack on the encephalitogenic peptide of myelin can injure oligodendrocytes leading to demyelination, or an attack against the acetylcholine receptor can incite myasthenia gravis (Fig. 1C).
FIG. 1.
(A) List of monoclonal antibodies from a variety of RNA and DNA viruses that were studied for cross-reactivity with host-antigens in/on uninfected tissues (see Shrinivasappa and colleagues(7) for details). At the extreme right are single immunofluorescent panels demonstrating the reaction of two of these viral monoclonal antibodies. The upper panel depicts a monoclonal antibody to herpes simplex virus that reacts with insulin in the islets of Langerhans. A monoclonal antibody to Coxsackie B provided a similar picture. At lower right is the portrait of a monoclonal antibody against Japanese encephalitis virus that reacts with host-antigen(s) in hippocampal neurons of the central nervous system. (Data originated in the Koprowski, Notkins, and Oldstone laboratories and continued in Oldstone's facility). (B) Partial list of the more interesting amino acid homologies between a microbial protein and host-antigen with implications for an immunopathologic disease. Our immunochemical study of such amino acid homologies(10–12) provided criteria for the success or failure of such homologies to generate cross-reactive immune responses and are in part illustrated in Figs. 1C and 2. (C) The immunochemistry needed to determine whether an amino acid is biologically meaningful. In this example, antibody to herpes simplex virus (HSV) GpD aa 286–293 generated high-affinity cross-reactive antibodies to the human acetylcholine receptor (HuAChR), but antibodies to polyoma middle T peptide aa 317–324 or HSV GpD aa 381–388 did not. Sera from patients with myasthenia gravis or affinity purified antibody to either HuAChR aa 160–167 or to HSV GpD aa 286–293 both bound at high affinity to AChR, indicating biologic activity (see Schwimmbeck and colleagues(45) for details). The aa stick structure of the HuAChR aa 160–167 is given at left and is compared to aa structure of HSV GpD aa 286–293 at right.
T Cell Clones and Molecular Mimicry
In addition to molecular mimics performed by antibodies, a variety of T cell clones sensitized to myelin basic protein (MBP), proteolipid of myelin, and glutamic decarboxylase have also been reported to cross-react with proteins or peptides from selected viruses.(9) The constraints on peptide selection for generating cross-reactive antibodies or cross-reactive cytotoxic T cells have been analyzed elsewhere(8–12) and are illustrated in part in Figure 2. The crystallographic structural requirements for binding of an immunodominant MBP peptide to MHC DR2 isotypes for recognition by human T cell clones have been solved.(13,14) Further, the permissivity of the T cell receptor to a wide variety of peptides(15–17) indicates that the issue was not the probability of multiple recognitions but how the host limited such cross-reactivity. The technological explosion in molecular biology allowed the banking of multiple sequence homologies that, by computer search and alignment, suggested the possible cross-reaction of viruses with host sequences and thus foreshadowed the potential for autoimmune disease. These extensive data allowed one to experimentally verify or dismiss molecular mimicry as a cause of autoimmunity (Figs. 1 and 2). A partial list of such possibilities appears in our earlier publication(18) and in Figure 1B. For example, sufficient amino acid sequences shared between specific coagulation proteins and dengue virus might play a role in dengue hemorrhagic shock syndrome; those shared between measles virus and MBP as well as corticotropin could participate in measles virus-induced demyelination and immunosuppression. Further, sequences in common between the campylobacteria jejuni and myelin gangliosides could take part in Guillain-Barré syndrome. Herpes simplex virus and corneal antigens may be associated with herpes stromal keratitis, and insulin-dependent diabetes with antigens from Coxsackie B, rotaviruses, hepatitis C virus, rhinoviruses, and others that cross-react with islet antigen glutamate decarboxylase 65, proinsulin carboxypeptidase H, and so on.(19–28)
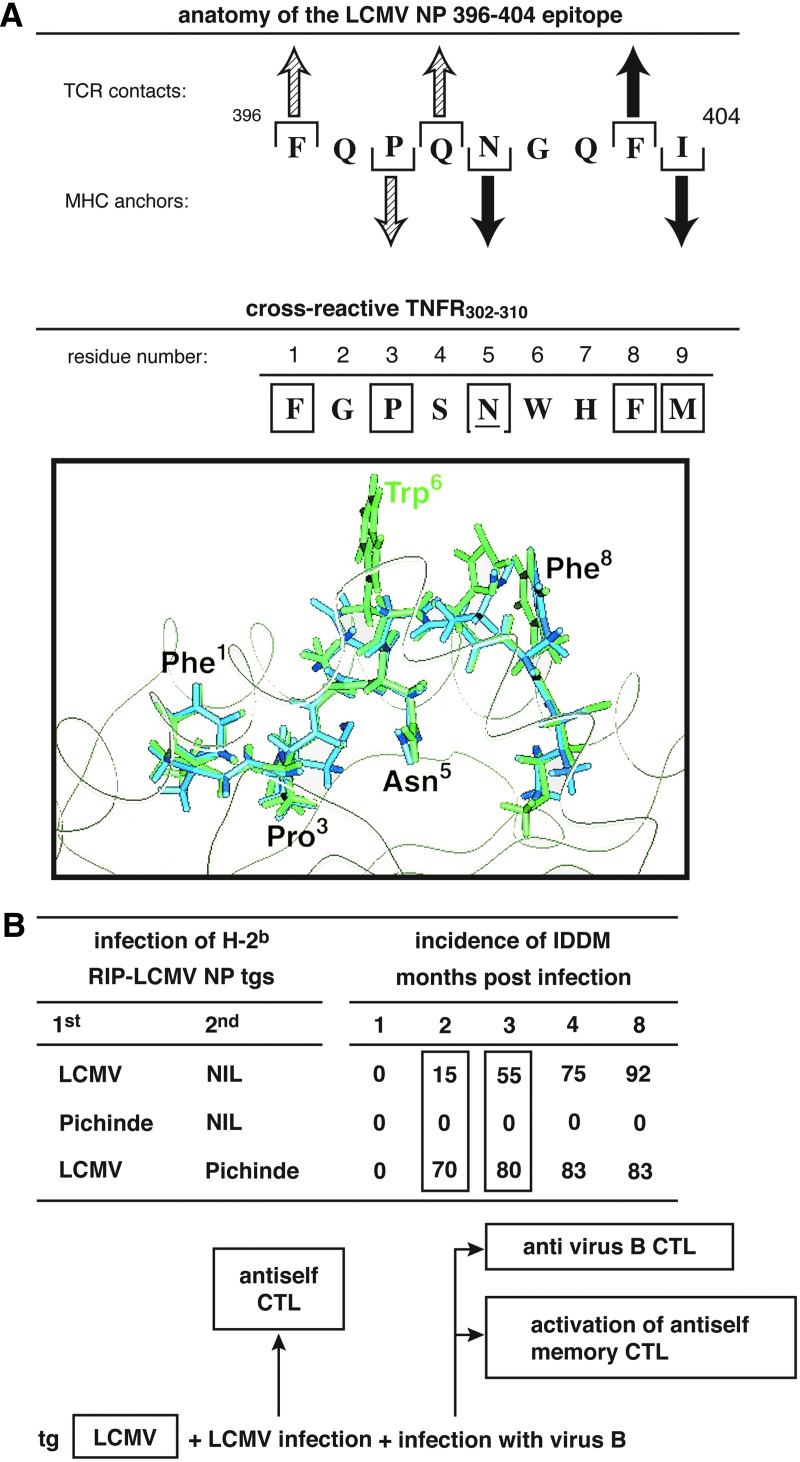
FIG. 2.
Display of several rules for mimicry. (A) Molecular mimicry at the CD8 T cell level between an immunodominant LCMV NP peptide epitope aa 394–404 and tumor necrosis factor (TNF) receptor (r) aa 302–310. CD8 T cells recognize linear peptides bound within the MHC groove. However, there are restrictions. Specific aa must form an anchor for MHC while other aa(s) are required to interact with the T cell receptor (see Lewicki and colleagues(46) for details on mapping H-2Db-restricted LCMV NP immunodominant epitope aa 396–404). Structures of both peptides appear below. (B) Demonstration of how experimental molecular mimicry can enhance an autoimmune disease, in this instance, insulin-dependent type 1 diabetes. In this model a transgenic (tg) mouse is constructed in which the LCMV NP is expressed in beta cells of the pancreatic islets of Langerhans by using the rat insulin promoter. Although the challenge of such tg mice with LCMV leads to type 1 diabetes in over 90% of acutely infected animals, infection with Pichinde virus (another member of the arenaviridae family, like LCMV), fails to cause diabetes. However, type 1 diabetes is significantly accelerated following an initial LCMV challenge to such tg mice given Pichinde virus. The cause is cross-reactivity on the CD8 T cell epitope level between Pichinde and LCMV; this process expands the number of aggressive LCMV CD8 T cells that cause the diabetes. Pichinde virus alone generates lower affinity and fewer CD8 T cells and cannot, by itself, cause the diabetes. A major consequence of this observation is that the viral infection (in this case Pichinde) does not cause diabetes itself but can be involved (and isolated) as the potentiator of the disease long after the initiating virus that caused the disease cleared and is no longer isolatable from the host. That is a two-hit phenomenon in which the infectious agent causing the diabetes would most likely be missed, having already been cleared from the host.(4)
Our laboratory then tested the molecular mimicry hypothesis in several experimental models in vivo,(4,5,8,29–34) two of which are illustrated and discussed here. First, we knew that T cell immune responses to MBP led to the autoimmune disease allergic encephalomyelitis (EAE) in several animal species. That discovery came after Fujinami and I, over two decades ago, designed experiments providing the initial evidence that molecular mimicry could cause an autoimmune disease in vivo.(8) Our computer search revealed that MBP of rabbits aa 69 Thr-Gly-Ser-Pro-Gln was identical to the hepatitis B virus polymerase aa 592–597. When we inoculated New Zealand rabbits with 10 aa virus peptide 589-Ile-Gly-Cys-Tyr-Gly-Ser-Leu-Pro-Gln-Glu, the rabbits generated specific T and B lymphocyte responses against the shared 6 aa acid sequence of MBP; importantly, this response was accompanied by histopathologic evidence of EAE. Over 10 other non-identical sequences, despite their origin from hepatitis B virus, failed to elicit EAE when inoculated into multiple rabbits. Although this was the first experimental evidence for autoimmune disease caused by molecular mimicry in living animals, it was an artificial system in that the virus used was not a normal pathogen of rabbits. However, the observation clearly indicated that our hypothesis of molecular mimicry was worth pursuing.
Molecular Mimicry and Autoimmune Disease
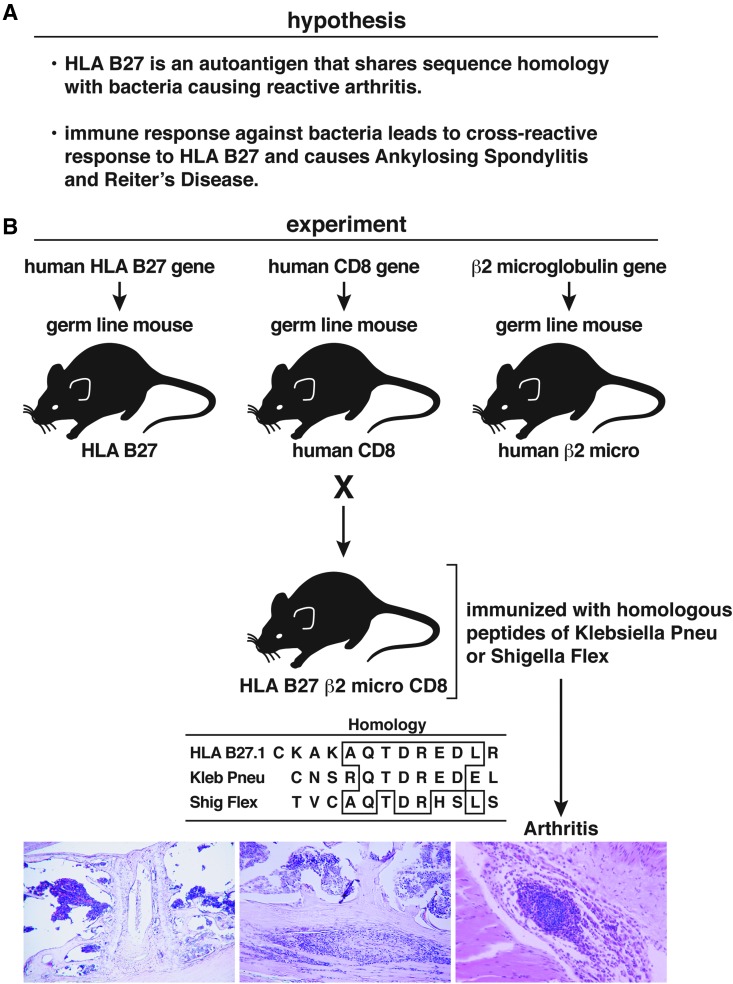
We next explored the association between HLA-B27 and the arthritic disease Reiter's syndrome, as well as ankylosing spondylitis (AS) and several selected bacterial infections (Fig. 3). The results provided a firm foundation for the hypothesis that molecular mimicry is a potential cause of autoimmune disease. Compelling epidemiologic studies of several bacterial infections in humans expressing HLA-B27 have yielded statistically significant connections between Reiter's syndrome and the development of AS. Moreover, Reiter's and AS are associated with the HLA-B27 haplotype and with infections due to Shigella flexneri, Yersinia enterocolitica, and Klebsiella pneumonia.(29,30,35–38) Over 90% of individuals who develop AS have the HLA-B27 haplotype, with HLA-B27 present in only approximately 10% of the normal population. Genetic studies indicate that monozygotic twins show a discordance for AS,(39) indicating a compelling role for environmental factor(s). With these observations on hand, my postdoctoral fellow Peter Schwimmbeck and I profiled by computer HLA-B27 hypervariable regions and bacteria associated with Reiter's syndrome.(4,5,29,30) We found the homologies shown in Figure 3B. In addition to the homology of six consecutive aa between HLA-B27.1 residues 72–77 and K. pneumoniae nitrogenase residues 188–193, these shared sequences are hydrophilic, suggesting locations of molecules exposed to the cell surface. Immunochemical analysis(29) indicated that 18 of 24 (75%) serum samples from patients with Reiter's syndrome and 7 of 24 samples (29%) from individuals with AS contained antibodies that bound to a synthesized peptide sequence aa 69–84 of the hypervariable region of HLA-B27.1. In contrast, only 1 of 22 sera (<5%) from individuals bearing HLA-B27 but free from Reiter's syndrome or AS bound to the bacterial residue aa 188–193 (p<0.01). Sera from three HLA-B27nil individuals with Reiter's syndrome did not have antibodies to the HLA-B27 peptide. Further, >40% of HLA-B27+ individuals with either Reiter's syndrome or AS had antibodies to K. pneumoniae nitrogenase aa 184–193 (p<0.01), whereas none of the arthritis-free, normal HLA-B27 subjects did. These results strongly suggested that the autoimmune response to HLA-B27 elicited by a bacterium via molecular mimicry is associated with and might be a causal pathogenic mechanism for a subset of individuals developing Reiter's syndrome and/or AS.
FIG. 3.
(A) Presentation of the hypothesis that molecular mimicry could cause an immune-mediated autoimmune disease based on cross-reactive aa between HLA-B27 and certain bacteria based on epidemiologic and clinical observations. (B) Experimental approach to test the hypothesis, offering proof of concept for the hypothesis.(5)
To prove this association, we created an animal model of human Reiter's and AS (Fig. 3B). First, using transgenic technology, we humanized mice to express human HLA.(40) To obtain HLA-B27-restricted T cell function, it was necessary to create a triple-transgenic mouse that expressed not only human HLA-B27 but also human beta 2 microglobulin and human CD8.(41) Next, we inoculated peptide sequences that contained the homologous residues of K. pneumoniae or S. flexneri (Fig. 3B),(5) which corresponded to the hypervariable region of HLA-B27 into transgenic mice that expressed human HLA-B27.(41) K. pneumoniae was selected on the basis of our prior immunochemical analysis(29,30); S. flexneri has a strong correlation with Reiter's syndrome and AS. In the 1940s, Finland reported an epidemic of S. flexneri infections involving about 150,000 humans, 344 of whom developed Reiter's syndrome. Of those with Reiter's syndrome, 82 individuals went on to develop AS. In our transgenic model, injections with either K. pneumoniae or S. flexneri led to inflammatory responses in which 60% of such mice had T cells, macrophages and polymorphonuclear leukocytes localized primarily in joints and vertebral columns (Fig. 3B); however, none of the inoculated non-transgenic mice were so affected.(5) Inoculation of the S. flexneri and K. pneumoniae peptides into non-HLA-B27 transgenic mice failed to elicit joint or vertebral inflammation or antibodies to HLA-B27.
Independently, Hammer and colleagues(42) described a transgenic rat model that expressed HLA-B27. Their transgenic HLA-B27 rats spontaneously developed an AS-like disease when housed in a normal vivarium. However, the AS-like disease did not occur when the HLA-B27 transgenic rats were housed in a germ-free vivarium.(43) When “germ-free rats” were colonized with bacteria, they developed arthritis.(44) Further, antibiotics given to HLA-B27 rats housed in a normal vivarium blunted the development and appearance of the AS-like arthritis.
Conclusion
In summary, proving cause and effect for human disease is difficult. Nevertheless, an accumulation over the last three decades of clear associations between sequence homology and T and B cell cross-reactivity between selected host antigens identified as essential for the development of autoimmune diseases and microbial agents strongly supports a causative role for molecular mimicry in that process. A current survey of PubMed reveals such associations for a plethora of diseases that afflict the central nervous system, endocrine, and gastric organs as well as eyes, heart, and other organs. Molecular mimicry is but one mechanism for the development of autoimmune disorders occurring in association with infectious agents. Further, the concept of and lessons learned by exploring the parameters of molecular mimicry provide a blueprint for framing questions and initiating relevant approaches. Uncovering the infectious agent as well as recognizing the host “self” determinants and the pathogenic mechanism(s) involved offer hints for the future design of strategies to treat and prevent autoimmune disorders. The current availability of amino acid sequence information, bioinformatics, and high efficiency computers accelerates that process. Increasing knowledge of the units and principles of the immune system, initiation of immune responses, and immunologic recognition, combined with increasingly sophisticated experimental animal models, enables us and our successors to solve the puzzles of etiology and to unravel the complexities of autoimmune diseases.
Acknowledgments
This is publication no. 26035 from the Department of Immunology and Microbial Science, The Scripps Research Institute (La Jolla, CA). This work was supported by NIH grant AI009484 and past NIH grants AI07007 and AG04342.
Author Disclosure Statement
The author has no financial interests to disclose.
References
- 1.Kolmer G: The Nobel Lectures in Immunology. The Nobel Prize for Physiology or Medicine, 1984. Derivation and diversification of monoclonal antibodies. Scand J Immunol 1993;37:117–129 [DOI] [PubMed] [Google Scholar]
- 2.Fujinami RS, Oldstone MBA, Wroblewska Z, Frankel ME, and Koprowski H: Molecular mimicry in virus infection: cross-reaction of measles phosphoprotein or of herpes simplex virus protein with human intermediate filaments. Proc Natl Acad Sci USA 1983;80:2346–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane DP, and Hoeffler WK: SV40 large T shares an antigenic determinant with a cellular protein of molecular weight 68,000. Nature 1980;288:167–170 [DOI] [PubMed] [Google Scholar]
- 4.Oldstone MBA: Molecular mimicry, microbial infection, and autoimmune disease: evolution of the concept. Curr Topics Microbiol Immunol 2005;296:1–17, 65–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oldstone MBA: Molecular mimicry and immune-mediated diseases. FASEB J 1998;12:1255–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dales S, Fujinami RS, and Oldstone MBA: Infection with vaccinia favors the selection of hybridomas synthesizing auto-antibodies against intermediate filaments, among them one cross-reacting with the virus hemagglutinin. J Immunol 1983;131:1546–1553 [PubMed] [Google Scholar]
- 7.Shrinivasappa J, Saegusa J, Prabhakar B, Gentry M, Buchmeier M, Wiktor T, Koprowski H, Oldstone M, and Notkins A: Molecular mimicry: frequency of reactivity of monoclonal antiviral antibodies with normal tissues. J Virol 1986;57:397–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujinami RS, and Oldstone MBA: Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science 1985;230:1043–1045 [DOI] [PubMed] [Google Scholar]
- 9.Wucherpfennig KW, and Strominger JL: Molecular mimicry in T-cell mediated autoimmunity: viral peptides activate human T-cell clones specific for myelin basic protein. Cell 1995;80:695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hudrisier D, Riond J, Burlet-Schiltz O, von Herrath MB, Lewicki H, Monsarrat B, Oldstone MBA, and Gairin JE: Structural and functional identification of major histocompatibility complex class-I-restricted self-peptides as naturally occurring molecular mimics of viral antigens. J Biol Chem 2001;276:19396–19403 [DOI] [PubMed] [Google Scholar]
- 11.Dyrberg T, and Oldstone MBA: Peptides as probes to study molecular mimicry and virus-induced autoimmunity. Curr Topics Microbiol Immunol 1986;130:25–37 [DOI] [PubMed] [Google Scholar]
- 12.Dyrberg T, Petersen JS, and Oldstone MBA: Immunological cross-reactivity between mimicking epitopes on a virus protein and a human autoantigen depends on a single amino acid residue. Clin Immunol Immunopathol 1990;54:290–297 [DOI] [PubMed] [Google Scholar]
- 13.Wucherpfennig KW, Sette A, Southwood S, Oseroff C, Matsui M, Strominger JL, and Hafler DA: Structural requirements for binding of an immunodominant myelin basic protein peptide to DR2 isotypes and for its recognition by human T cell clones. J Exp Med 1994;179:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith KJ, Pyrdol J, Gauthier L, Wiley DC, and Wucherpfennig KW: Crystal structure of HLA-DR2 (DRA*0101, DRB1*1501) complexed with a peptide from human myelin basic protein. J Exp Med 1998;188:1511–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mason D: A very high level of crossreactivity is an essential feature of the T-cell receptor. Immunol Today 1998;19:395–404 [DOI] [PubMed] [Google Scholar]
- 16.Yin Y, and Mariuzza RA: The multiple mechanisms of T cell receptor cross-reactivity. Immunity 2009;31:849–851 [DOI] [PubMed] [Google Scholar]
- 17.Macdonald WA, Chen Z, Gras S, Archbold JK, Tynan FE, Clements CS, Bharadwaj M, Kjer-Nielsen L, Saunders PM, Wilce MCJ, Crawford F, Stadinsky B, Jackson D, Brooks AG, Purcell AW, Kappler JW, Burrows SR, Rossjohn J, and McCluskey J: T cell allorecognition via molecular mimicry. Immunity 2009;31:897–908 [DOI] [PubMed] [Google Scholar]
- 18.Oldstone MBA: Molecular mimicry and autoimmune disease. Cell 1987;50:819–820 [DOI] [PubMed] [Google Scholar]
- 19.Hafer-Macko C, Hsieh ST, Li CY, Ho TW, Sheikh K, Cornblath DR, McKhann GM, Asbury AK, and Griffin JW: Acute motor axonal neuropathy: an antibody-mediated attack on axolemma. Ann Neurol 1996;40:635–644 [DOI] [PubMed] [Google Scholar]
- 20.van der Meche FG, and van Doorn PA: Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy: immune mechanisms and update on current therapies. Ann Neurol 1995;37:S14–S31 [DOI] [PubMed] [Google Scholar]
- 21.Yuki N, Tagawa Y, and Handa S: Autoantibodies to peripheral nerve glycosphingolipids SPG, SLPG, and SGPG in Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy. J Neuroimmunol 1996;70:1–6 [DOI] [PubMed] [Google Scholar]
- 22.Zhao Z-S, Granucci F, Yeh L, Schaffer P, and Cantor H: Molecular mimicry by herpes simplex virus type 1: autoimmune disease after viral infection. Science 1997;79:1344–1347 [DOI] [PubMed] [Google Scholar]
- 23.Atkinson MA, Bowman MA, Campbell L, Darrow BL, Kaufman DL, and MacLaren NK: Cellular immunity to a determinant common to glutamate decarboxylase and coxsackie virus in insulin-dependent diabetes. J Clin Invest 1994;94:2125–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacLaren NK, and Alkinson MA: Insulin-dependent diabetes mellitus: the hypothesis of molecular mimicry between islet cell antigens and microorganisms. Mol Med Today 1997;3:76–83 [DOI] [PubMed] [Google Scholar]
- 25.Karges WJ, Ilonen J, Robinson BH, and Dosch HM: Self and non-self antigen in diabetic autoimmunity: molecules and mechanisms. Mol Aspects Med 1995;16:79–213 [DOI] [PubMed] [Google Scholar]
- 26.Solimena S, and DeCamilli P: Coxsackieviruses and diabetes. Nat Med 1995;I:25–26 [DOI] [PubMed] [Google Scholar]
- 27.Rudy G, Stone N, Harrison LC, Colman PG, McNair P, Brusic V, French MB, Honeyman MC, Tait B, and Lew AM: Similar peptides from two beta cell autoantigens, proinsulin and glutamic acid decarboxylase, stimulate T cells of individuals at risk for insulin-dependent diabetes. Mol Med 1995;1:625–633 [PMC free article] [PubMed] [Google Scholar]
- 28.Honeyman MC, Stone NL, and Harrison LC: T-cell epitopes in type 1 diabetes autoantigen tyrosine phosphatase IA-2: potential for mimicry with rotavirus and other environmental agents. Mol Med 1998;4:231–239 [PMC free article] [PubMed] [Google Scholar]
- 29.Schwimmbeck PL, Yu TDY, and Oldstone MBA: Autoantibodies to HLA B27 in the sera of HLA B27 patients with ankylosing spondylitis and Reiter's syndrome: molecular mimicry with Klebsiella pneumoniae as potential mechanism of autoimmune disease. J Exp Med 1987;166:173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwimmbeck P, and Oldstone MBA: Klebsiella pneumoniae and HLA B27 associated diseases of Reiter's syndrome and ankylosing spondylitis. Curr Topics Microbiol Immunol 1989;145:45–67 [DOI] [PubMed] [Google Scholar]
- 31.von Herrath MG, Evans CF, Horwitz MS, and Oldstone MBA: Using transgenic mouse models to dissect the pathogenesis of virus-induced autoimmune disorders of the islets of Langerhans and the central nervous system. Immunol Rev 1996;152:111–143 [DOI] [PubMed] [Google Scholar]
- 32.Oldstone MBA, Nerenberg M, Southern P, Price J, and Lewicki H: Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell 1991;65:319–331 [DOI] [PubMed] [Google Scholar]
- 33.von Herrath M, Dockter J, and Oldstone MBA: How virus induces a rapid or slow onset insulin-dependent diabetes mellitus in a transgenic model. Immunity 1994;1:231–242 [DOI] [PubMed] [Google Scholar]
- 34.Evans CF, Horwitz MS, Hobbs MV, and Oldstone MBA: Viral infection of transgenic mice expressing a viral protein in oligodendrocytes leads to chronic central nervous system autoimmune disease. J Exp Med 1996;184:2371–2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brewerton D, Caffrey M, Hart F, James D, Nichols A, and Sturrock R: Ankylosing spondylitis and HLA B27. Lancet 1973;i:904–907 [DOI] [PubMed] [Google Scholar]
- 36.Gilliland B, and Mannik M:Ankylosing spondylitis and Reiter's syndromePrinciples of Internal Medicine, 10th Harrison TR (Ed.) McGraw Hill; New York: 19861986–1988. [Google Scholar]
- 37.Gilliland B, and Mannik M:Rheumatoid arthritisPrinciples of Internal Medicine, 10th Harrison TR (Ed.) McGraw Hill; New York: 19861977–1986. [Google Scholar]
- 38.Khare SD, Luthra HS, and David CS: Role of HLA-B27 in spondyloarthropathies. Curr Topics Microbiol Immunol 1996;206:85–97 [DOI] [PubMed] [Google Scholar]
- 39.Eastmond CJ, and Woodrow JC: Discordance for ankylosing spondylitis in monozygotic twins. Ann Rheum Dis 1977;36:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.LaFace DM, Vestberg M, Yang Y, Srivastava R, DiSanto J, Flomenberg N, Brown S, Sherman LA, and Peterson PA: Human CD8 transgene regulation of HLA recognition by murine T cells. J Exp Med 1995;182:1315–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tishon A, LaFace DM, Lewicki H, van Binnendijk RS, Osterhaus A, and Oldstone MBA: Transgenic mice expressing human HLA and CD8 molecules generate HLA-restricted measles virus cytotoxic T lymphocytes of the same specificity as humans with natural measles virus infection. Virology 2000;275:286–293 [DOI] [PubMed] [Google Scholar]
- 42.Hammer RE, Maika SD, Richardson JA, Tang J-P, and Taurog JD: Spontaneous inflammatory disease in transgenic mice expressing HLA-B27 and human β2m: an animal model of HLA-B27-associated human disorders. Cell 1990;63:1099–1112 [DOI] [PubMed] [Google Scholar]
- 43.Rath HC, Herfarth HH, Ikeda JS, Grenther WB, Hamm TE, Jr, Balish E, Taurog JD, Hammer RE, Wilson KH, and Sartor RB: Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest 1996;98:945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taurog JD, Richardson JA, Croft JT, Simmons WA, Zhou M, Fernandez-Sueiro JL, Balish E, and Hammer RE: The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic mice. J Exp Med 1994;180:2359–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schwimmbeck PL, Dyrberg T, Drachman DB, and Oldstone MBA: Molecular mimicry and myasthenia gravis. An autoantigenic site of the acetylcholine receptor alpha-subunit that has biologic activity and reacts immunochemically with herpes simplex virus. J Clin Invest 1989;84:1174–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewicki H, Tishon A, Borrow P, Evans CF, Gairin JE, Hahn KM, Jewell DA, Wilson IA, and Oldstone MBA: CTL escape viral variants. I. Generation and molecular characterization. Virology 1995;210:29–40 [DOI] [PubMed] [Google Scholar]