Abstract
Objective: A meta-analysis of all published randomized controlled trials of the effectiveness of gentamicin/collagen sponges for preventing surgical site infections (SSIs).
Background: Despite routine use of systemic prophylactic antimicrobial agents, SSIs continue to be associated with substantial morbidity. Results conflict of studies of the efficacy of gentamicin/collagen sponges for preventing SSIs. However, many of these studies have assessed sponge use in only a single specific type of operation. The general effect of sponge use among different types of operations has not been previously assessed.
Methods: The PubMed and Cumulative Index to Nursing and Allied Health Literature (CINAHL) databases were searched for articles appearing from 1990 through January 2012 that were related to gentamicin/collagen sponge use and SSIs. Summary estimates were obtained through a random effects model. After reviewing 714 article abstracts and reviewing 22 articles in detail, we pooled the odds ratios (OR) for 13 independent study populations (cardiac, n=4; colorectal, n=4; pilonidal sinus, n=2; hernia, n=2; gastrointestinal, n=1) in which the association between prophylactic use of gentamicin/collagen sponges and SSIs was assessed.
Results: Pooling of the results of all studies included in the review in a random effects model showed a significant protective effect of prophylactic use of gentamicin/collagen sponges against SSI (pooled OR: 0.66; 95% confidence interval [CI]: 0.45, 0.97; n=13). However, when the data were stratified by type of operation, a significant protective effect was observed in cardiac procedures (pooled OR: 0.59; 95% CI: 0.37, 0.96; n=4) but not in colorectal procedures (pooled OR: 0.74; 95% CI: 0.29–1.92; n=4).
Conclusion: Use of gentamicin/collagen sponges was associated with a reduced risk of SSI following cardiac operations but not following colorectal procedures.
Surgical site infections (SSIs) are among the most common types of healthcare-associated infections [1]. Despite the routine use of systemic prophylactic antimicrobial agents, SSIs continue to be associated with substantial morbidity following various types of surgery. Alternative methods of antimicrobial prophylaxis have therefore been studied, including the application of antimicrobial agents locally within a surgical incision [2–4].
A number of studies have examined the efficacy of implanting gentamicin/collagen sponges in surgical incisions to reduce the risk of SSI [2,5–17]. Currently, this method of delivering antimicrobial agents is approved in 54 countries and these sponges have been used in more than two million patients; however, they are not approved for use in the United States [18]. Gentamicin/collagen sponges are composed of highly purified collagen, and deliver high local concentrations of gentamicin, but without its serum concentration reaching the level of toxicity. The collagen of these sponges is absorbed slowly by the body within 1–8 wks after their application, and does not require removal [19].
Results conflict of studies of the efficacy of gentamicin/collagen sponges for preventing SSIs. However, these studies often assess sponge use only in one specific type of operation. The general effect of using gentamicin/collagen sponges in different types of operations has not been assessed previously. We conducted a study to evaluate published randomized controlled trials of gentamicin/collagen sponges in reducing SSIs and to assess their overall effectiveness in this regard.
Materials and Methods
Search strategy
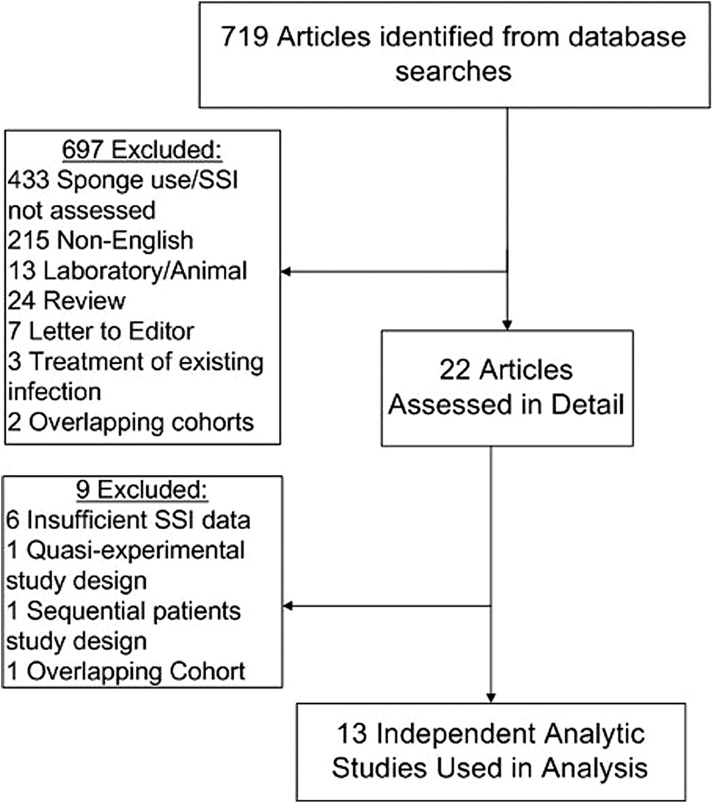
Our study included a meta-analysis conducted with the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [20]. A systematic search was completed of published studies in the PUBMED data bases, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and clinicaltrials.gov from January 1, 1990 through January 31, 2012, using the search terms: “Surgical wound infection,” “absorbable implants,” and “gentamicin-collagen” or “gentamicin” or “collagen.” Additionally, the reference lists of the articles retrieved in the search were reviewed to further identify studies that were not identified by the preliminary literature search (Fig. 1).
FIG. 1.
Selection of trials included in meta-analysis of published randomized controlled trials of the effectiveness of gentamicin/collagen sponges for preventing surgical site infections (SSI).
Inclusion and exclusion criteria
Studies were included if they were randomized controlled trials involving human subjects, published in English, and used gentamicin/collagen sponges in a prophylactic manner. Studies also had to include the data needed to calculate a relative risk (RR) or odds ratio (OR) and a 95% confidence interval (CI). Studies were excluded if gentamicin/collagen sponges were used to treat existing infections, if they involved animals, or if they were done in a laboratory. Review articles and articles written in a non-English language were also excluded. When more than one article reported data for the same study population, the articles were grouped to avoid including the same data more than once.
Data extraction
Titles and abstracts of articles identified by our searches were evaluated for relevance according to inclusion and exclusion criteria and full reviews were completed for all potentially pertinent articles. Two authors extracted the following data from each of the potentially relevant articles: author, year of publication, country in which the study was conducted, primary study outcome, how the study was defined (e.g., U.S. Centers for Disease Control and Prevention (CDC) criteria), duration of follow-up, and other methods of prophylaxis used in the study, if any. Data collected included the antimicrobial agents used in a study, timing of their administration, and duration of their administration. Additional data extracted included detailed information about the sponge(s) used in a study, including brand or type, number of sponges used, sponge dimensions, and quantity of gentamicin present in each sponge. The number of SSIs was recorded among study participants randomized to the intervention and those randomized to the control group of a study. The previously validated Jadad score was used to assess study quality, and we calculated the mean Jadad score for all studies [21]. Studies were categorized as being of high or low quality on the basis of their individual Jadad score in relation to the mean score. Studies with a Jadad score of 3 points or higher were considered as being of high quality whereas those with a score below 3 points were considered as being of low quality.
Two reviewers extracted the data independently for each article; any discrepancies were resolved by consensus. The initial literature search identified 719 articles. Twenty-two articles were potentially relevant and were reviewed in detail. Of these, 13 were included in the final analysis [5–17].
Statistical analysis
Using the extracted raw data, the natural logarithm of the OR and the variance of the OR were calculated. None of the included studies adjusted their analyses for potential confounders. Therefore, only raw unadjusted data were included in our analyses. Statistical analysis was completed with RevMan 5.1 software (The Nordic Cochrane Centre, Copenhagen Denmark) [22]. Both fixed-effects and random effects models were used to obtain pooled estimates of RR, but only random-effects models were reported because they are more conservative estimates. Pooled ORs were calculated for each study using a random-effects model (Mantel–Haenszel method). Stratified analyses were done on the basis of a priori categories. These categories included surgery type, sponge-only vs. sponge plus systemic prophylaxis, and study quality. Statistical heterogeneity was calculated with the Mantel–Haenszel Q statistic and the I2 index, and publication bias was assessed through visual inspection of a funnel plot.
Results
Studies meeting the inclusion criteria were those that assessed the efficacy of the gentamicin/collagen sponge in the following types of operations: Cardiac, colorectal (anal fistula or anorectal abcess), hernia (groin, abdominal), gastrointestinal (including bariatric), and dermatologic (pilonidal sinus). Among the 13 studies included in this analysis, four assessed colorectal operations, four assessed cardiac operations, two each assessed hernia and pilonidal sinus operations, and one assessed a gastrointestinal operation. Table 1 provides the details of these studies. Surgical site infections were defined according to the criteria of the CDC [23].
Table 1.
Studies Included in Meta-Analysis of Studies for Evaluating the Efficacy of Gentamicin/Collagen Sponges in Preventing Surgical Site Infection
| First author, Year (sample size) | Location | Type of surgery | Outcome assessment | Sponge type (number of sponges) | Sponge dimensions (amount of gentamicin) | Sponge Placement | Antibiotics given (intervention) | Antibiotics given (control) | When antibiotics given | Placebo? | Study Quality (Jadad Score – out of 5) | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Andersson 2010 (159) | Sweden | Pilonidal sinus excision | Assessed by outpatient nurse | Collatamp (1) | Not stated (not stated) | Cavity resulting from excision packed with sponge before wound closure | None | None | N/A | No | Low (2.5) | 0.92 (0.46–1.84) |
| Bennett-Guerrero, Aug 2010 (1,502) | United States | Cardiac (non-emergent CABG or valve repair or replacement surgery through full median sternotomy) | Assessed by surgeon and infection control staff/consultant | Not stated (2) | 10×10 cm (130 mg) | Between the sternal halves along the full length of the sternum immediately before closure | IV: β-lactam, vancomycin Other: mupirocin nasal swab | IV: β-lactam, vancomycin Other: mupirocin nasal swab | 60 min prior to incision | No | High (3) | 0.96 (0.67–1.38) |
| Bennett-Guerrero, Sept 2010 (602) | United States | Colorectal (1 of 13 colorectal operations; not otherwise specified) | Assessed by infection control staff/consultant | Not stated (2) | 5×20 cm (130 mg) | Inserted anteriorly to the fascia, along full length of incision, immediately before closure | IV: β-lactam, ciprofloxacin, clindamycin, metronidazole Oral: neomycin or erythromycin | IV: β-lactam, ciprofloxacin, clindamycin, metronidazole Oral: neomycin or erythromycin | 60 min prior to incision | No | High (3) | 1.63 (1.12–2.36) |
| Eklund, 2005 (542) | Finland | Cardiac (elective CABG ) ) |
Assessed by surgeon and infection control staff/consultant | Gentacoll (1) | 10×10 cm (130 mg) | Underneath sternum before wound closure | IV: β-lactam, vancomycin | IV: β-lactam, vancomycin | Not specified | No | High (3) | 0.67 (0.30–1.47) |
| Friberg, 2005 (1,905) | Sweden | Cardiac (any cardiac operation though median sternotomy, including operations on the ascending aorta) | Assessed by surgeon and infection control staff/consultant | Collatamp (2) | 10×10× 0.05 cm (130 mg) | Between sternal halves immediately before closure | IV: β-lactam, clindamycin Oral: chlorhexidine mouthwash at one center | IV: β-lactam, clindamycin Oral: chlorhexidine mouthwash at one center | Immediately before, 1–2 times during, and up to 48 h after surgery | No | High (4.5) | 0.45 (0.31–0.66) |
| Haase, 2005 (80) | Germany | Colorectal (loop-ileostomy closure) | Assessed by surgeon and researcher | Sulmycin (1) | Not stated (not stated) | Placed subcutaneously | IV: β-lactam, metronidazole | IV: β-lactam, metronidazole | After anesthesia induction | Yes | High (5) | 1.00 (0.23–4.31) |
| Miller, 1995 (104) | Austria | Gastrointestinal (vertical banded gastroplasty) | Did not specify who made the assessment | Septocoll (not stated) | 5×8 cm (58.3 mg) | Placed in subcutaneous layer | None | IV: β-lactam | In operating room | No | Low (2) | 1.00 (0.06–16.43) |
| Musella, 2001 (577) | Italy | Hernia (groin hernia repair) | Assessed by surgeon and blinded family physician | Collatamp (1) | 10×10×0.05 cm (130 mg) | Placed in front of prosthetic mesh and covered by the sutured aponeurosis of the external oblique muscle | IV: β-lactam, cephalosporins | IV: β -lactam, cephalosporins | Two times per day, at 1 h before and 12 h after surgery | No | Low (2) | 0.16 (0.02–1.33) |
| Nowacki, 2004 (218) | Poland | Colorectal (rectal cancer excision) | Did not specify who made the assessment | Garamycin (1) | Not stated (not stated) | Placed into presacral area, always below peritoneal reflection. If anterior resection, sponge wrapped around anastomosis | IV: β-lactam, metronidazole | IV: β-lactam, metronidazole | Not specified | No | Low (2.5) | 0.61 (0.21–1.75) |
| Praveen, 2009 (202) | Malaysia | Hernia (inguinal hernia repair) | Did not specify who made the assessment | Not stated (not stated) | Not stated (160 mg) | Not stated | None | IV: gentamicin | During anesthesia induction | No | Low (2) | 1.18 (0.41–3.39) |
| Rutten, 1997 (221) | The Netherlands | Colorectal (elective colorectal surgery: colonic resection, reversal of Hartmann pouch, abdomino-perineal resection, subtotal colectomy, low anterior resection) | Did not specify who made the assessment | Varied¥ (1) | Not stated (not stated | Placed directly upon the closed fascia directly adjacent to the surgical incision | IV: β-lactam, metronidazole | IV: β-lactam, gentamicin and metronidazole | Twice per day within 24 h of anesthesia induction | No | Low (2.5) | 0.26 (0.10–0.68) |
| Schimmer, 2012 (220) | Germany | Cardiac (operations performed on the heart and thoracic aorta via median sternotomy) | Assessed by researcher | Not stated (1) | 20×5×0.05 cm (2 mg) | Implanted retrosternally | IV: β-lactam | IV: β-lactam | Twice per day starting 30 min before incision for up to 48 h post-operation | Yes | High (5) | 0.37 (0.17–0.82) |
| Yetim, 2010 (80) | Turkey | Pilonidal sinus excision | Did not specify who made the assessment | Gentacoll (1) | 5×5×0.5 cm (50 mg) | Placed on sacral fascia | None | Oral quinolone and ornidazole combination | Once a day for 7 d postoperatively | No | Low (2) | 0.21 (0.04–1.06) |
CABG=coronary artery bypass graft; OR=odds ratio.
 Coronary artery bypass graft
Coronary artery bypass graft
¥ Sulmycin, Cronocol, Duracoll, Garacol, Garacoll, Garamacina, Garamycin, Gentecol/Gentacoline, Gentacoll, Gentalyn, Gentimplant, Verotin
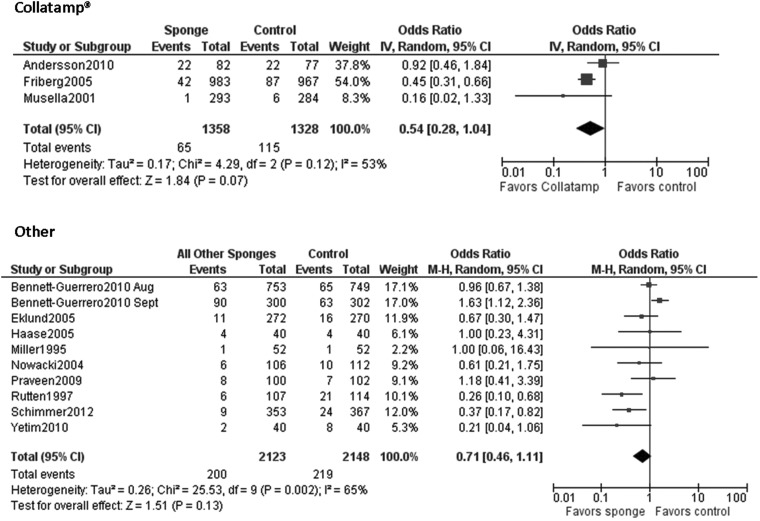
All but two of the studies were performed outside of the United States, and the same group of authors published both of the studies conducted in the United States [6,7]. The size and type of sponge used for the interventions varied across studies, and 14 different brands of sponges were included; one study utilized 12 of the 14 sponge brands [15]. Three studies used only Collatamp sponges (EUSA Pharma, Oxford, UK), and other studies used a variety of different sponges [3,9,12]. A pooled assessment of Collatamp sponges was conducted because three studies utilized this brand of sponge. However, similar analyses could not be completed for other brands. Only two studies used a placebo sponge [10,16].
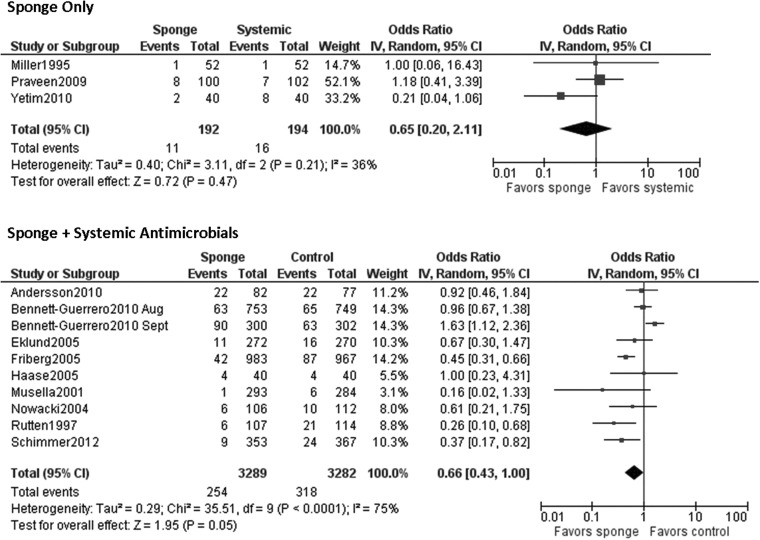
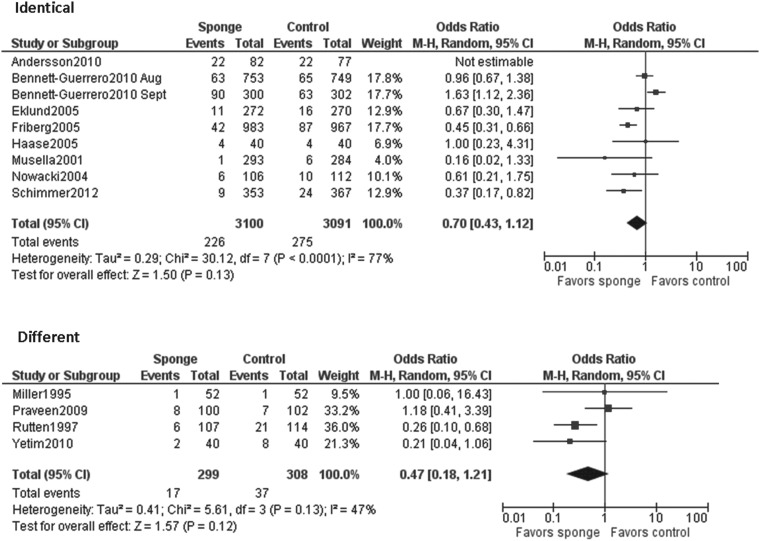
Identical systemic prophylactic antimicrobial regimens were given to both the intervention and control groups in eight studies [6–10,12,13,16]. One study used different antimicrobial regimens for the intervention and control groups [15]. In the four remaining studies, in which only the gentamicin/collagen sponge was used, was compared with a control group that was given systemic antimicrobial prophylaxis [11,14,17]. Although different combinations of antimicrobial agents were administered in the studies included in our analysis, 11 of 12 studies provided β-lactam antimicrobials (Table 1).
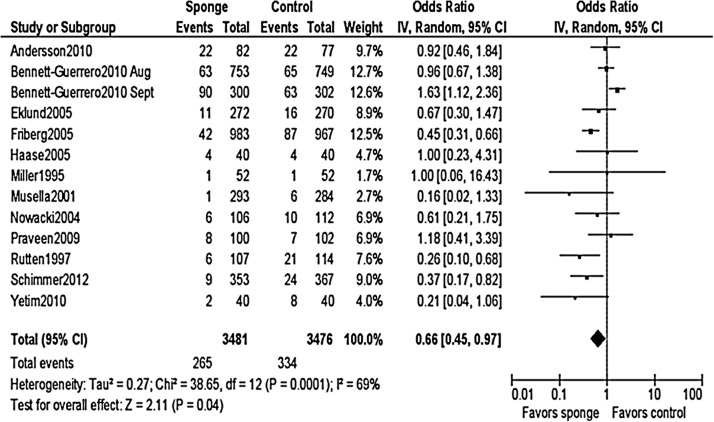
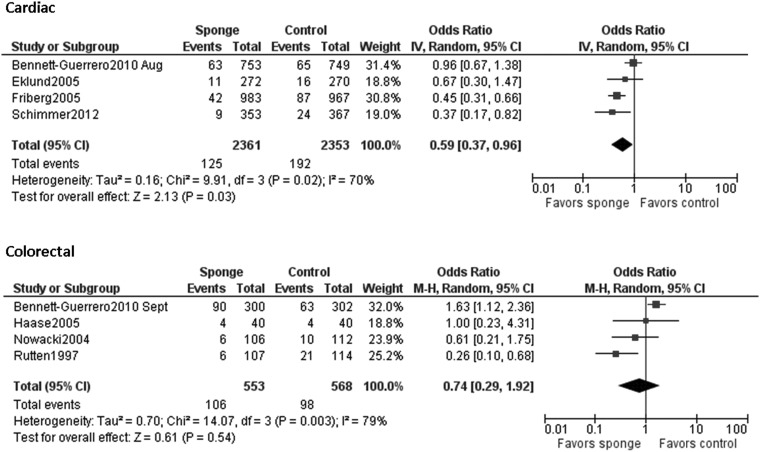
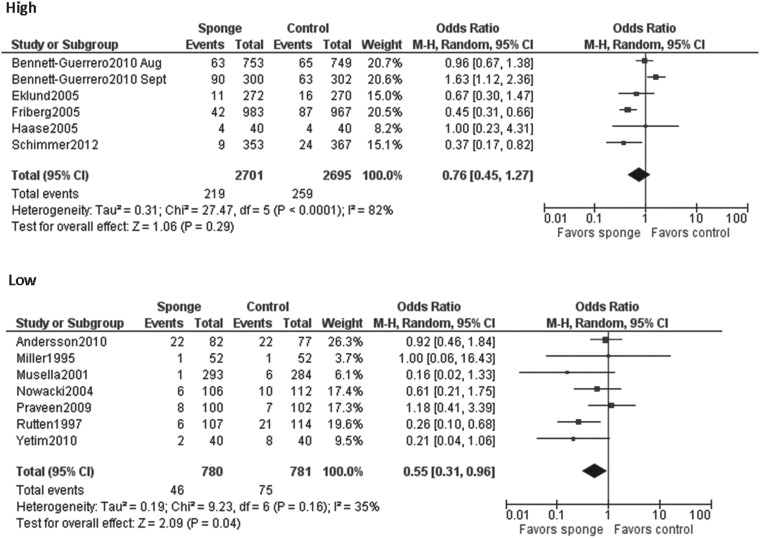
When all studies were pooled in a random-effects model, a significant protective effect against SSI was observed with the prophylactic use of a gentamicin/collagen sponge (pooled OR: 0.66; 95% CI: 0.45–0.97; n=13) (Fig. 2). Stratification of the data for this effect according to sponge type, sponge-only vs. sponge plus systemic prophylaxis, and identical vs. different antimicrobial regimens in the intervention and control groups did not change the results. However, when the data were stratified by type of operation, a significant protective effect was observed in cardiac procedures (pooled OR: 0.59; 95% CI: 0.37–0.96; n=4) but not in colorectal procedures (pooled OR: 0.74; 95% CI: 0.0.29–1.92; n=4) (Table 2). Moreover, a statistically significant protective effect was found when data from low-quality studies were pooled, whereas a non-significant protective effect was found when data from high-quality studies were pooled. Forest plots for the stratified data are included in the Appendix.
FIG. 2.
Pooled results of meta-analysis of randomized controlled trials of effectiveness of gentamicin/collagen sponges for preventing surgical site infections.
Table 2.
Variables and Results with Stratification of Data in Meta-Analysis of Studies of Efficacy of Gentamicin/Collagen Sponges in Preventing Surgical Site Infection
| Number of studies | I2 | OR (95% CI) | |
|---|---|---|---|
| Type of surgery | |||
| Cardiac | 4 | 70% | 0.59 (0.37–0.96) |
| Colorectal | 4 | 79% | 0.74 (0.29–1.92) |
| Sponge type | |||
| Collatamp | 3 | 53% | 0.54 (0.28–1.04) |
| Other | 10 | 65% | 0.71 (0.46–1.11) |
| Intervention group | |||
| Sponge only | 3 | 36% | 0.65 (0.20–2.11) |
| Sponge+systemic antimicrobial agents | 10 | 75% | 0.66 (0.43–1.00) |
| Systemic antimicrobial regimen (intervention vs. control) | |||
| Identical | 8 | 77% | 0.70 (0.43–1.12) |
| Different | 4 | 47% | 0.47 (0.18–1.21) |
| Study quality | |||
| High | 6 | 82% | 0.76 (0.45–1.27) |
| Low | 7 | 35% | 0.55 (0.31–0.96) |
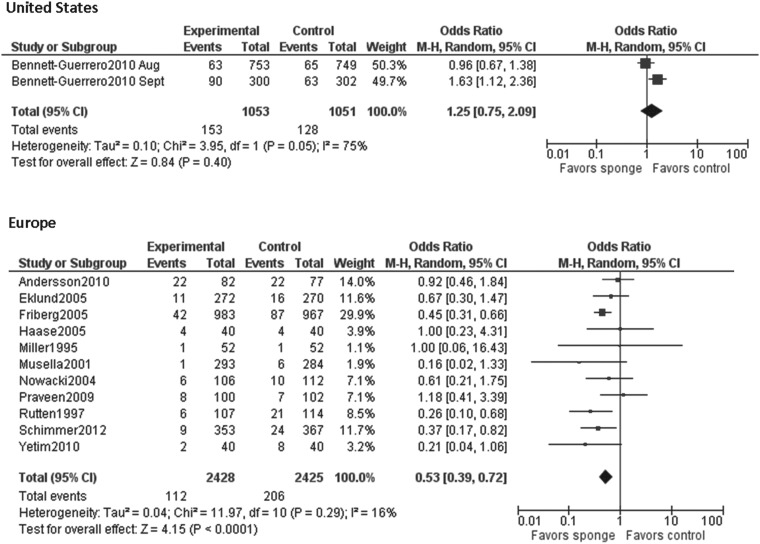
| Region | |||
| United States | 2 | 75% | 1.25 (0.75–2.09) |
| Europe | 11 | 16% | 0.53 (0.39–0.72) |
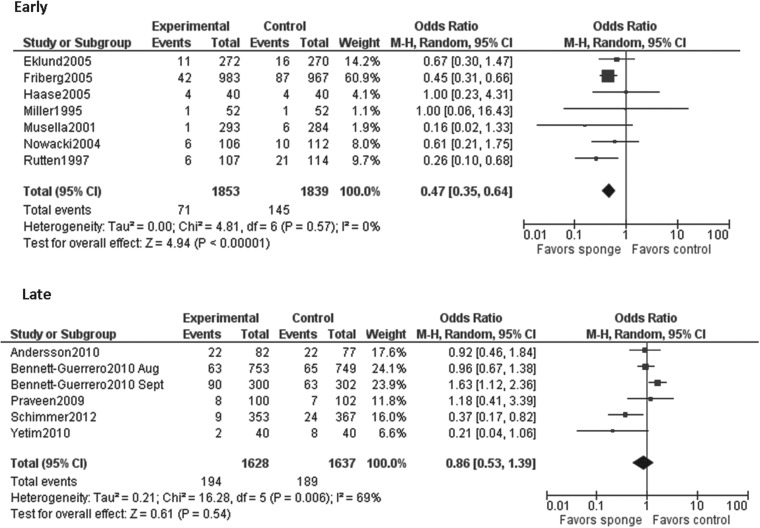
| Time period | |||
| Early (1995–2005) | 7 | 0% | 0.47 (0.35–0.64) |
| Late (2009–2012) | 6 | 69% | 0.86 (0.53–1.39) |
CI=confidence interval; I2=heterogeneity statistic (percent variability from heterogeneity among studies); OR=odds ratio.
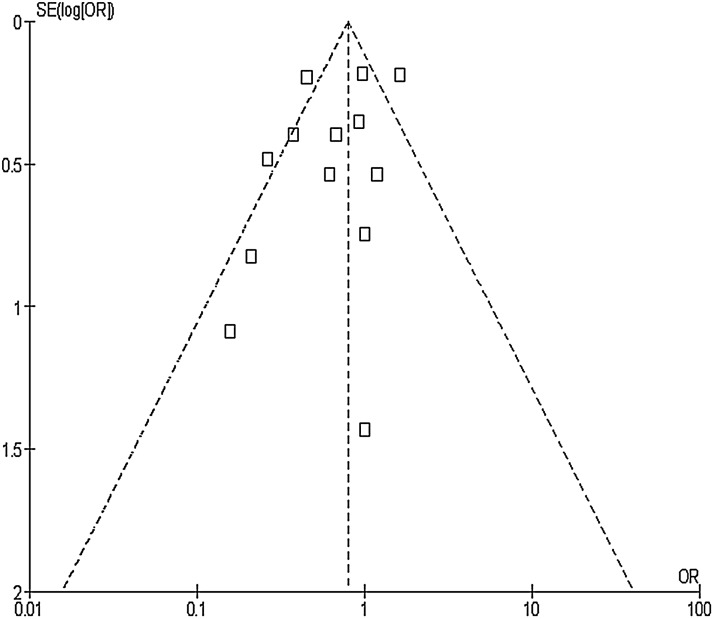
When the results of all studies were included in the analysis, the I2 index demonstrated a moderate amount of heterogeneity (p<0.001; I2=69%). To discover the source of this heterogeneity, data were stratified by region (United States and Europe) and by publication date. Studies published between 1995 and 2005 were considered “early” and studies published between 2009 and 2012 were considered “late.” No studies were published between 2005 and 2009. The results of the early studies were homogenous (p=0.57, I2=0%), whereas the results of the late studies were moderately heterogeneous (p=0.006, I2=69%). When stratified by region, results of the studies done in the United States were highly heterogeneous (p=0.05, I2=75%) and those of the European studies were fairly homogeneous (p=0.29, I2=16%). Visual inspection of the funnel plot did not identify apparent publication bias (Fig. 3).
FIG. 3.
Funnel plot of standard error in the lograrithm of the odds ratio (OR) vs. OR for assessing publication bias in meta-analysis of randomized controlled trials of effectiveness of gentamicin/collagen sponges for preventing surgical site infections.
Discussion
Although multiple studies have assessed the association between gentamicin/collagen sponge use and SSIs, results of these studies have varied widely and have often been inconsistent. Our overall results show that gentamicin/collagen sponges may reduce the frequency of SSIs after cardiac but not colorectal surgical procedures. Stratification of these data by sponge type and sponge-only vs. sponge-plus-systemic prophylaxis did not change the results.
To date, published meta-analyses have not assessed the association of gentamicin/collagen sponges and SSIs across different types of surgical operations. de Bruin et al. conducted a systematic review of published studies assessing gentamicin/collagen sponges and SSIs in various types of gastrointestinal operations [2]. They reviewed nine individual studies of medium-to-high risk operations and divided the procedures into the three categories of rectal operations, abdominal operations, and pilonidal sinus excision. Only one study in this review failed to find a significant difference in the rates of SSI in patients who were treated with sponges in conjunction with systemic antimicrobial agents and those in a control group of patients who received systemic antimicrobial agents alone. The authors who reported this latter study noted that the control group in the study received a collagen placebo sponge that may have promoted wound healing and therefore obscured the effect of the gentamicin-containing sponges. Moreover, the rate of SSI (10%) in the control group in this study was lower than that generally reported in the literature for patients undergoing loop-ileostomy closure (20%–40%)[2, 24–27]. de Bruin et al. concluded that prophylactic use of gentamicin/collagen sponges can reduce rates of SSI after high-risk gastrointestinal operations and can also improve wound healing after pilonidal sinus excision [2].
The overall pooled results of our meta-analysis were moderately heterogeneous. Our stratified analysis of subsets chosen a priori found that study location (United States vs. Europe) may have been the source of the heterogeneity. A single group of researchers, Bennett-Guererro et al., conducted the two studies done in the United States. These studies were weighted heavily in the overall analysis because their sample sizes were large. Importantly, one of these two studies was the only study to find a significant risk of SSI among the patients treated with gentamicin/collagen sponges [6,7].
The studies conducted in the United States may have differed from the European studies in several ways, including study design (e.g., quality control measures, duration of follow-up, number of hospitals included), the way in which sponges were prepared before they were implanted, the organisms causing SSIs (e.g., gram-positive organisms vs. gram-negative organisms or level of gentamicin resistance), in the study patient populations (e.g., race, ethnic groups, high risk groups of patients [e.g., obese patients or diabetic patients]), and in their baseline rates of SSIs [28,29]. For example, a recent study found that soaking gentamicin/collagen sponges in saline before they were implanted decreased the amount of gentamicin in the sponge and may therefore reduce the antimicrobial effect of the sponge [30]. Additionally, the studies done in the United States, which were published in 2010, followed the guidelines of the Surgical Care Improvement Project (SCIP) and were therefore more likely to have used appropriate antibiotics at the appropriate times than were the earlier European studies [31]. Thus, the additional benefit of local prophylaxis may not be as great when systemic prophylaxis is used appropriately.
In our stratified analysis, we found that the low-quality studies and the studies that were performed in earlier years were more likely to find a significantly protective effect of gentamicin/collagen sponges than were the higher quality studies performed in later years. Approximately 57% of the low-quality studies in our review were classified as early studies. This could have resulted from the nature of scientific research, in which smaller, earlier studies see a significant effect, with less of an effect seen in higher quality studies performed at later times. Additionally, the greater benefit of gentamicin/collagen sponges seen in the low-quality and earlier studies than in the higher quality later studies could have come from improvements in systemic antibiotic prophylaxis over time.
Our meta-analysis has several limitations. First, meta-analyses are only as valid as the studies that contribute to the pooled OR in these analyses. To decrease the effect of study quality, we rated studies according to quality and compared the risk estimates for the high- and low-quality studies in our analysis. Second, the control groups among the studies investigating the use of gentamicin/collagen sponges were heterogeneous. For example, some control groups received placebo sponges, most of the control groups received the same systemic antimicrobials as the intervention groups, and one control group received neither placebo sponges nor systemic antimicrobial therapy. In addition, patients in some intervention groups received only sponges and the control groups received only prophylactic systemic antimicrobials. Moreover, the specific systemic antimicrobial agents used prophylactically varied substantially among the studies in our analysis. β-Lactam agents were given most commonly, but these agents were often given in combination with another class of antimicrobial agent. Thus, it was difficult to make comparisons across studies.
Our finding that gentamicin/collagen sponges may protect against SSI after cardiac operations is especially important because SSIs after these procedures are associated with poor clinical and economic outcomes and adversely affect the outcome economics of health-care facilities [32–34]. Recently, the Centers for Medicare and Medicaid ruled that extra cost related to treating mediastinitis would no longer be reimbursed [35]. Therefore, if interventions such as the use of gentamicin/collagen sponges are effective, they may reduce the incidence of SSIs and could be cost-effective from the hospital perspective.
In conclusion, the results of studies assessing the efficacy of gentamicin/collagen sponges for preventing SSI have conflicted with one another. Our meta-analysis found that these sponges may have a protective effect against SSIs after cardiac procedures. Before a recommendation can be made about whether gentamicin/collagen sponges should be used to prevent SSIs, additional high-quality studies must be performed. These additional high-quality studies may identify subpopulations of patients who may benefit from the prophylactic use of gentamicin/collagen sponges.
Appendix
APPENDIX FIG. 1.
Individual pooled estimates for surgery type.
APPENDIX FIG. 2.
Individual pooled estimates for sponge type.
APPENDIX FIG. 3.
Individual pooled estimates for intervention group.
APPENDIX FIG 4.
Individual pooled estimates for antimicrobial regimen in intervention vs. control groups.
APPENDIX FIG. 5.
Individual pooled estimates for study quality.
APPENDIX FIG. 6.
Individual pooled estimates for region.
APPENDIX FIG. 7.
Individual pooled estimates for study period.
Acknowledgments
This was an unfunded study. MLS was supported by the University of Iowa CTSA NIH/NCRR- 3KL2 RR024980-04S1. The authors thank the Iowa City VA Health Care System for its support.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Hidron AI, Edwards JR, Patel J, et al. , National Healthcare Safety Network Team, Participating National Healthcare Safety Network Facilities. NHSN annual update: Antimicrobial-resistant pathogens associated with healthcare-associated infections: Annual summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2006–2007. Infect Control Hosp Epidemiol 2008;29:996–1011 [DOI] [PubMed] [Google Scholar]
- 2.de Bruin AF, Gosselink MP, van der Harst E, et al. Local application of gentamicin collagen implants in the prophylaxis of surgical site infections following gastrointestinal surgery: A review of clinical experience. Tech Coloproctol 2010;14:301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McHugh SM, Collins CJ, Corrigan MA, et al. The role of topical antibiotics used as prophylaxis in surgical site infection prevention. J Antimicrob Chemother 2011;66:693–701 [DOI] [PubMed] [Google Scholar]
- 4.O'Neill KR, Smith JG, Abtahi AM, et al. Reduced surgical site infections in patients undergoing posterior spinal stabilization of traumatic injuries using vancomycin powder. Spine J 2011;11:641–646 [DOI] [PubMed] [Google Scholar]
- 5.Andersson RE, Lukas G, Skullman S, et al. Local administration of antibiotics by gentamicin-collagen sponge does not improve wound healing or reduce recurrence rate after pilonidal excision with primary suture: A prospective randomized controlled trial. World J Surg 2010;34:3042–3048 [DOI] [PubMed] [Google Scholar]
- 6.Bennett-Guerrero E, Ferguson TB, Jr, Lin M, et al. Effect of an implantable gentamicin-collagen sponge on sternal wound infections following cardiac surgery: A randomized trial. JAMA 2010;304:755–762 [DOI] [PubMed] [Google Scholar]
- 7.Bennett-Guerrero E, Pappas TN, Koltun WA, et al. Gentamicin-collagen sponge for infection prophylaxis in colorectal surgery. N Engl J Med 2010;363:1038–1049 [DOI] [PubMed] [Google Scholar]
- 8.Eklund AM, Valtonen M, Werkkala KA. Prophylaxis of sternal wound infections with gentamicin-collagen implant: Randomized controlled study in cardiac surgery. J Hosp Infect 2005;59:108–112 [DOI] [PubMed] [Google Scholar]
- 9.Friberg O, Svedjeholm R, Soderquist B, et al. Local gentamicin reduces sternal wound infections after cardiac surgery: A randomized controlled trial. Ann Thorac Surg 2005;79:153–161; discussion: 161–162 [DOI] [PubMed] [Google Scholar]
- 10.Haase O, Raue W, Bohm B, et al. Subcutaneous gentamycin implant to reduce wound infections after loop-ileostomy closure: A randomized, double-blind, placebo-controlled trial. Dis Colon Rectum 2005;48:2025–2031 [DOI] [PubMed] [Google Scholar]
- 11.Miller K, Lang B, Hell E. Local vs systemic antibiotics to decrease wound complications following vertical banded gastroplasty: Results of a prospective randomized trial. Obes Surg 1995;5:293–297 [DOI] [PubMed] [Google Scholar]
- 12.Musella M, Guido A, Musella S. Collagen tampons as aminoglycoside carriers to reduce postoperative infection rate in prosthetic repair of groin hernias. Eur J Surg 2001;167:130–132 [DOI] [PubMed] [Google Scholar]
- 13.Nowacki MP, Rutkowski A, Oledzki J, et al. Prospective, randomized trial examining the role of gentamicin-containing collagen sponge in the reduction of postoperative morbidity in rectal cancer patients: Early results and surprising outcome at 3-year follow-up. Int J Colorectal Dis 2005;20:114–120 [DOI] [PubMed] [Google Scholar]
- 14.Praveen S, Rohaizak M. Local antibiotics are equivalent to intravenous antibiotics in the prevention of superficial wound infection in inguinal hernioplasty. Asian J Surg 2009;32:59–63 [DOI] [PubMed] [Google Scholar]
- 15.Rutten HJ, Nijhuis PH. Prevention of wound infection in elective colorectal surgery by local application of a gentamicin-containing collagen sponge. Eur J Surg Suppl 1997;578:31–35 [PubMed] [Google Scholar]
- 16.Schimmer C, Ozkur M, Sinha B, et al. Gentamicin-collagen sponge reduces sternal wound complications after heart surgery: A controlled, prospectively randomized, double-blind study. J Thorac Cardiovasc Surg 2012;143:194–200 [DOI] [PubMed] [Google Scholar]
- 17.Yetim I, Ozkan OV, Dervisoglu A, et al. Effect of gentamicin-absorbed collagen in wound healing in pilonidal sinus surgery: A prospective randomized study. J Int Med Res 2010;38:1029–1033 [DOI] [PubMed] [Google Scholar]
- 18.Medical Devices Database [database online]. Silver Spring, MD: U.S. Food and Drug Administration; Available at: http://www.fda.gov/medicaldevices/deviceregulationandguidance/databases/default.htm Accessed May24, 2012 [Google Scholar]
- 19.Buimer MG, Ankersmit MF, Wobbes T, et al. Surgical treatment of hidradenitis suppurativa with gentamicin sulfate: A prospective randomized study. Dermatol Surg 2008;34:224–227 [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. , The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin Trials 1996;17:1–12 [DOI] [PubMed] [Google Scholar]
- 22.Review Manager (RevMan)[Computer Program]. Version 5.1. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2011 [Google Scholar]
- 23.Mangram AJ, Horan TC, Pearson ML, et al. Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 1999;27:97–132 [PubMed] [Google Scholar]
- 24.Ersoy OF, Karaca S, Kayaoglu HA, et al. Comparison of different surgical options in the treatment of pilonidal disease: Retrospective analysis of 175 patients. Kaohsiung J Med Sci 2007;23:67–70 [DOI] [PubMed] [Google Scholar]
- 25.Kirkil C, Boyuk A, Bulbuller N, et al. The effects of drainage on the rates of early wound complications and recurrences after limberg flap reconstruction in patients with pilonidal disease. Tech Coloproctol 2011;15:425–429 [DOI] [PubMed] [Google Scholar]
- 26.Sondenaa K, Nesvik I, Andersen E, et al. Recurrent pilonidal sinus after excision with closed or open treatment: Final result of a randomised trial. Eur J Surg 1996;162:237–240 [PubMed] [Google Scholar]
- 27.Spivak H, Brooks VL, Nussbaum M, et al. Treatment of chronic pilonidal disease. Dis Colon Rectum 1996;39:1136–1139 [DOI] [PubMed] [Google Scholar]
- 28.Corn T. Treating sternal wound infections after cardiac surgery with an implantable gentamicin-collagen sponge. JAMA 2010;304:2123. [DOI] [PubMed] [Google Scholar]
- 29.de Bruin AF, Gosselink MP, Rutten HJ. Gentamicin sponge for infection prophylaxis in colorectal surgery. N Engl J Med 2010;363:2566. [DOI] [PubMed] [Google Scholar]
- 30.Lovering AM, Sunderland J. Impact of soaking gentamicin-containing collagen implants on potential antimicrobial efficacy. Int J Surg 2012;10Suppl 1:52–54 [DOI] [PubMed] [Google Scholar]
- 31.Bratzler DW, Hunt DR. The surgical infection prevention and surgical care improvement projects: National initiatives to improve outcomes for patients having surgery. Clin Infect Dis 2006;43:322–330 [DOI] [PubMed] [Google Scholar]
- 32.Demmy TL, Park SB, Liebler GA, et al. Recent experience with major sternal wound complications. Ann Thorac Surg 1990;49:458–462 [DOI] [PubMed] [Google Scholar]
- 33.Fry DE. The economic costs of surgical site infection. Surg Infect (Larchmt) 2002;3Suppl 1:S37–43 [DOI] [PubMed] [Google Scholar]
- 34.Tang GH, Maganti M, Weisel RD, et al. Prevention and management of deep sternal wound infection. Semin Thorac Cardiovasc Surg 2004;16:62–69 [DOI] [PubMed] [Google Scholar]
- 35.Centers for Medicare and Medicaid Services. CMS improves patient safely for Medicare and Medicaid by addressing never events. Available at: http://www.cms.hhs.gov/apps/media/press/factsheet.asp?Counter=3224&intNumPerPage=10&checkDate=&checkKey=&srchType=1&numDays=3500&srchOpt=0&srchData=&keywordType=All&chkNewsType=6&intPage=&showAll=&pYear=&year=&desc=false&cboOrder=date Accessed May9, 2012