Abstract
The current availability of and access to biomarker testing for personalized cancer therapy is reviewed.

Bruce A. Chabner

Leif W. Ellisen

A. John Iafrate
The concept of “Personalized Medicine” has captured the imagination of the world medical community in general, and the community of oncologists in particular. The essential notion is that molecular tests, or biomarkers, of patient and/or tumor can match the patient to the most effective and least toxic treatment, and thereby improve outcomes, avoid useless toxicity, reduce costs of care, and place therapeutics on a more rational basis. Obviously, the care of patients in all fields, including oncology, has been “personalized” for decades, as doctors tailor treatment to many aspects of the individual patient's circumstance: social, financial, and medical factors all playing a role. However, the newest iteration of personalized medicine, and perhaps it is a misnomer, focuses on treatment tailored to the specific genetic causes underlying the disease.
Nowhere has the concept of therapy based on genetics caught tighter hold than in cancer research, where substantial advances in treatment have resulted from identifying specific somatic mutations in tumors and treating with drugs that target those mutations. This paradigm has been successful for treating unique molecular subsets of breast cancer (trastu-zumab) [1], chronic myeloid leukemia (imatinib and congeners) [2], gastrointestinal stromal tumors (imatinib) [3], melanoma (the BRAF inhibitor vemurafenib) (4), and non-small cell lung cancer (the EGFR and EML-4/ALK inhibitors) [5]. In the past decade, the number of successful targeted therapies, with attendant mutational biomarkers, has steadily increased. Six new cancer drugs, targeted for specific genetic mutations, were approved for marketing in just the past two years [6]. For each of these drugs, a specific biomarker test selects the drug for the patient [7]. A list of commonly used molecular tests and their approved indications is provided in Table 1.
Table 1.
Biomarkers for approved targeted therapies, 2013
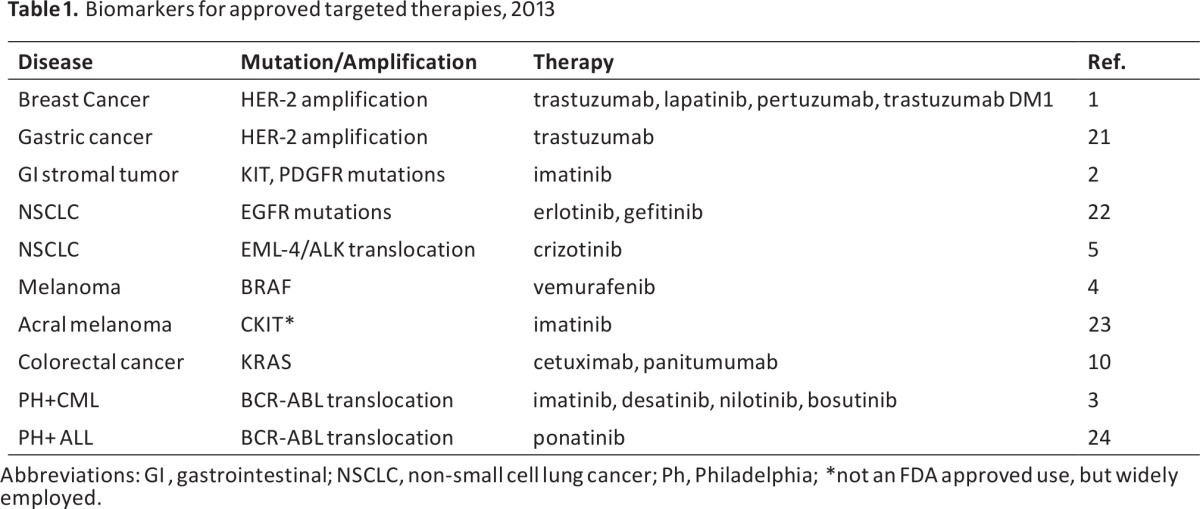
Abbreviations: Gl, gastrointestinal; NSCLC, non-small cell lung cancer; Ph, Philadelphia; *not an FD Aapproved use, but widely employed.
Biomarkers used to select patients for new treatments have guided patient selection in many successful drug trials in recent years and have led to a sea change in the process for drug approval. Crizotinib was approved three years after entering the clinic, based on one phase I trial and a single confirmatory phase II study in EML-4/ALK translocated NSCLC[8].
Not only has the use of biomarkers accelerated the pace of cancer drug development; the discovery of unique genomic subsets of common tumors has changed our basic concept of cancer. No longer are histological categories of major tumors sufficient to define treatment. Lung, breast, and colon cancer, as well as melanoma, are now recognized as collections of molecular subsets of cancer, with each subset having its own natural history and responsiveness to treatment [9]. In non-small cell lung cancer (NSCLC), EGFR mutation, EML-4/ALK translocation, ROS1 kinase translocation, RET mutation, and potentially a number of other categories of disease define therapeutically relevant subsets of disease [10].
The implementation of personalized cancer therapy rests on the availability of genomic tumor testing, both for rapid drug development and for clinical practice. At present, probably no more than 50% of cancer patients could meaningfully profit from genetic profiling of their tumors for routine clinical management. That calculation would include all metastatic melanoma (BRAF), breast and gastric cancer (HER2), and NSCLCs (EGFR, ALK). A case could be made as well for profiling all colorectal cancer patients for KRAS and BRAF mutations, which influence prognosis and response to EGFR monoclonal antibodies [11]. These same biomarkers are of potential use in other epithelial cancers, in which subsets of patients express the mutations; thus BRAFV600E mutations are of interest in colon, lung, salivary gland, and thyroid tumors. Given the continuously increasing number of new agents under development and the expanding range of targeted genetic changes, the population of patients who would profit from tumor profiling can be anticipated to increase, particularly if one takes into account the need to select patients for clinical trials based on test results. The trend is unmistakable. Oncology research, and the practice of medical oncology in the coming decade will increasingly depend on access to molecular assays.
What is the current availability of testing tumor genetics in the United States? Some hospitals are addressing this need by establishing their own genotyping facilities. The Cancer Center at Massachusetts General Hospital (MGH) set up the first such service in 2008, the Translational Research Laboratory (TRL), and focused its effects on screening for 20 genes and 160 specific mutations and translocations prominent in melanoma, lung, breast, and colorectal cancer. The assays it currently offers are primarily PCR-based tests for mutations or FISH-based assays for translocations and amplifications. With the development of rapid DNA sequencing, the approach will change to deep sequencing of 1000 cancer-related genes. The service at MGH is CLIA approved. Results are recorded in the patient charts.
Reimbursement for home-grown assays is variable. MGH bills insurance companies for its services in cases for which the result will determine eligibility for an approved therapy. The hospital receives variable reimbursement, depending on the insurer and the clinical setting, and these funds have defrayed the cost of running the service. Recent changes in Medicare coding for genetic tests have created uncertainty about future reimbursements. It is clear that most insurers, including Medicare, will only pay for assays that are tied to therapeutic decisions involving approved agents. The actual costs of performing multiplex assays for the actionable cancer mutations will likely lie in the range of $2,000-$10,000 for each patient, but will depend on the scope of the analysis and the technology involved. With the rapid evolution of sequencing technology, costs will likely decrease with time. Nonetheless, the existing MGH facility and others like it, such as the facilities at Dana Farber and Vanderbilt, have proven increasingly useful for routine care, as more and more targeted drugs have been approved for routine clinical practice.
In addition to the benefits afforded for clinical practice, the TRL facility supports clinical research and has developed a number of novel assays, some of which have been adopted for commercialization, including those for EGFR mutations and EML-4/ALK translocations. For MGH patients and researchers, the TRL provides the expertise for developing new genotyping assays that serve as selective biomarkers for experimental drug trials and for unapproved indications of approved drugs. Thus, the TRL has significantly enhanced our ability to attract early phase trials for targeted therapies. Promising trials are now in progress targeting tumors with ROS1 translocations, and breast cancers and other tumors with phosphoinositide-3 kinase pathway mutation, deletion or amplifications.
Following the lead of MGH, many of the nation's comprehensive cancer centers, including Vanderbilt, Dana Farber, MD Anderson, Memorial Sloan Kettering, the Huntsman Cancer Center, and Ohio State have established in-house TRL-like facilities. A recent New York Times article details the proliferation of hospital-based genotyping centers throughout Manhattan [12]. For many cancer centers, these facilities provide assays for testing tumors from NSCLC, melanoma, and other patients. These centers have a variable and selective repertoire of genomic tests primarily aimed at supporting experimental studies. In conversations with other cancer center investigators, it is clear that most of these facilities are focused on research projects. Few centers currently have in place the capacity for routine genotyping of patients' tumors for all approved indications, and to our knowledge, none provide this service to outside investigators or clinicians on a regional basis. With the development of rapid and inexpensive technology for selective gene sequencing and even whole exome sequencing, it is likely that the major research centers will expand tumor genetic testing to include a higher percent-age of their patients in the next few years.
Given the growing importance of tumor profiling for routine practice and for experimental trials, how widely available is this technology? In the United States, there are currently serious gaps in our ability to deliver routine personalized treatment for all cancer patients. Companion assays for FDA-approved drugs are now available through commercial sources such as Bioference Labs, Foundation Medicine, Abbott Molecular Diagnostics, Roche Molecular Diagnostics, and other providers, and, depending on the specific indication, the costs may or may not be reimbursed by various insurers or by Medicare. For uninsured or underinsured patients, the commercial testing option is expensive, or not available. If a researcher wishes to test for the presence of a mutation that would place the patient on a clinical trial, the costs of the test are likely not to be covered by insurance.
For approved drugs, access to testing is incomplete. Pfzer oncology director Mace Rothenberg estimates that only 60% of U.S. patients with ALK-translocated NSCLC are currently receiving crizotinib during the course of their illness. The reasons for this deficiency are unknown, but Rothenberg speculates that several factors are believed to play a role, including: (1) the expense and time required to purchase the test from a commercial source, (2) the cost of the assay, which may or may not be reimbursed by insurance, (3) the need to provide an adequate tissue sample for testing, and (4) the lack of physician awareness of superiority of crizotinib treatment as compared to chemotherapy. The results of a randomized trial demonstrating that superiority will soon be published [13]. Aside from this anecdote about crizotinib and ALK mutation, at this time we do not have accurate information on the percentage of lung cancer patients (smoker or nonsmoker) in the U.S. who are screened for EGFR mutation, or the percentage of melanoma patients who are screened for BRAF mutation. Our suspicion is that chemotherapy is tried first in many advanced NSCLC patients, despite data from phase III randomized trials demonstrating the superiority of EGFR inhibitors for patients with EGFR-mutant tumors [14]. Similarly, interferon or IL-2 is likely tried first in many melanoma patients for the reasons enumerated above. Studies are needed to confirm these suspicions.
Beyond the availability of tests for approved indications, the majority of cancer patients who are cared for in community practices (not at a major cancer center) will likely have very limited access to genomic tests that can direct them to clinical trials of promising new agents when conventional treatment fails.
Unlike the U.S., other countries have taken the lead in making genetic profiling of cancer available on a national basis. Queen's University in Northern Ireland has received a £32 million grant from foundations and from the United Kingdom Research Partnership Investment Fund to build a Centre for Experimental Medicine, which, among its other facilities, will provide country-wide routine genotyping for patient care, and for clinical trials research [15]. Genotyping will be pad for by the country's health care service. A similar tumor genotyp-ing service is planned for other parts of the United Kingdom [16]. A nationwide network of regional cancer genotyping services is under development in France [17] and in Germany [18]. In France, regional centers are providing genotyping for 28 actionable mutations and amplifications. No such national system, either for routine practice or for research purposes, is yet envisaged by the National Cancer Institute, Medicare, insurers, or hospitals in the United States.
The only statewide effort in the U.S. to provide genotyping was undertaken in Texas in 2009 [19], as part of the state's $3 billion, 10-year commitment to support cancer research. Clinical Trials Network of Texas (CTNeT), a statewide consortium of 22 regional cancer centers and practices, was established, together with a sophisticated genomics center at Baylor University. The center intended to genotype up to 2,500 tumor specimens for multiple mutations, amplifications, and translocations, and planned to bank tumor samples for use in laboratory research and for identifying patients for trials. Its first clinical trials were set to begin this year. However, due to the complex political and financial hurdles of pulling these academic centers and practices together under one umbrella, that attempt failed, to the great disappointment of many of its participants. Because of serious administrative mishaps, the state abruptly terminated CTNeT in January of 2013 and suspended its investment in clinical trials of “personalized medicine” [20].
Genomics-based medicine remains the most rational and promising new thrust of cancer therapeutics, and is largely the product of U.S. technology and medical science. The U.S. public invested in the research that discovered these treatments, and the critical trials were largely conducted in U.S. academic centers. Genomic-based therapy offers the prospect of improving the quality of life and survival for thousands of cancer patients, for many of whom there is no effective alternative therapy. In effect, it simplifies the current complexity of cancer treatment Thus it seems illogical that, in the United States, and within the world's most expensive health care system, we cannot guarantee access to genotyping for all patients with lung cancer, colorectal cancer, melanoma, and other cancers. If a patient happens to go to the right cancer center, or finds the right clinical trial, he or she may have the tumor genotyped. However, if the patient is uninsured, or underinsured, or resides in an underserved part of the country, the patient will not have access to this service. The enthusiasm about “personalized medicine” in the United States seems likely to be an unfulfilled promise for these many cancer patients.
If the tests in Table 1, and others surely to come, were routinely available to all patients, many patients would be spared inappropriate and highly toxic treatment, outcomes of care would improve, money would be saved, more patients would accrue to trials, trials would be completed, drugs would be approved more rapidly, and progress in cancer research would accelerate. At this point, the evidence for the financial benefit of personalized therapeutics remains conjectural, but its value seems intuitive, given the higher response rates, longer time to progression, convenience, and lesser toxicity of oral targeted therapies, as compared to infusional chemotherapy. We would be willing to wager that genomic testing does pay dividends for approved treatments and for allocating patients to rational trials. And, for what better purpose have we established a national network of comprehensive cancer centers than to do such testing on a regional basis? It should be part of their mission.
Footnotes
Editor's Note: Please see the accompanying article on pages 644–645 of this issue.
Disclosures
Bruce A. Chabner: Sanofi, Epizyme, PharmaMar, GlaxoSmithKline, Pharmacyclics, Ariad, and Pfizer (Data Monitoring Committee) (C/A); Eli Lilly (H); Gilead, Epizyme, Celgene, Exelixis (Ol). A. John lafrate: Pfizer, Bioreference Labs, Chugai (C/A); Snapshot (IP); Archer Dx (OI)
The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (Ol) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Reference
- 1.Slamon DF, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER 2 for metastatic breast cancer that overexpresses HER2. N Eng J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 2.Demetri GD, Margaretvon Mehren, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 347:472–280. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 3.Druker BJ. Translation of the Philadelphia chromosome into therapy for CML Blood. 2008;112:4808–4817. doi: 10.1182/blood-2008-07-077958. [DOI] [PubMed] [Google Scholar]
- 4.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809–19. doi: 10.1056/NEJMoa1002011. New drugs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwak AL, Bang Y-J, Camidge R, et al. N Eng J Med. 2010;363:1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. http://www.fda.gov/drugs/lnformationon-drugs/Approved.
- 7.Rudon J, Saura C, Dienstmann R, et al. Molecular prescreening to select patient population in early clinical trials. Nat. Rev Clinical Oncol. 2012 doi: 10.1038/nrclinonc.2012.48. [DOI] [PubMed] [Google Scholar]
- 8.Ou S-H, Bartlett CH, Mino-Kenudson M, et al. Crizotinib for the treatment of ALK-rearranged non-small cell lung cancer: A success story to usher in the second decade of molecular targeted therapy in oncology. The Oncologist. 2012;17:1351–1375. doi: 10.1634/theoncologist.2012-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arcila M, Lau C, Nafa K, et al. Detection of KRAS and BRAF mutations in colorectal carcinoma roles for high-sensitivity locked nucleic acid-PCR sequencing and broad-spectrum mass spectrom-etry genotyping. J Mol Diagn. 2011;13:64–73. doi: 10.1016/j.jmoldx.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergethon K, Shaw AT, Ou S-H I, et al. ROS1 Rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;35:6345. doi: 10.1200/JCO.2011.35.6345. Epub 2012 Jan 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pentheroudakis G, Kotoula V, De Roock W, et al. Biomarkers of benefit from cetuximab-based therapy in metastatic colorectal cancer; interaction of EGFR ligand expression with RAS/RAF, PI3KCA genotypes. BMC Cancer. 2013 Feb 2;13:49. doi: 10.1186/1471-2407-13-49. doi: 10.1186/1471–2407-13–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartocollis, Anemona. Cancer centers racing to map patients' genes. New York Times. [Accessed June 3, 2013]. Published April 21, 2013. Available at http://www.nytimes.com/2013/04/22/health/patients-genes-seen-as-future-of-cancer-care.html?pagewanted=all&_r=0.
- 13.Shaw A, Kim D-W, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-rearranged lung cancer. N Engl J Med doi. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 14.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;24:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 15.The Northern Ireland Molecular Pathology Laboratory. [Accessed June 3,2013]. Available at https://vimeo.com/55830092.
- 16.Hollmer, Mark. U.K.'s NHS debuts multi-gene sequencing cancer Dx. [Accessed June 3,2013]. http://www.fiercemedi-caldevices.com/story/uk-nhs-debuts-multi-gene-sequencing-cancer-dx. Published March 26, 2013.
- 17.Butler, Declan. France puts €260 million into research infrastructure. Nature. [Accessed June 3, 2013]. Published online 8 March 2011. Available at: http://www.nature.com/news/2011/110308/full/news.2011.145.html.
- 18.German Cancer Research Center. Joining forces against cancer - German consortium for translational cancer research gets started. [Accessed June 3,2013]. Published April 15, 2011. Available at: http://www.dkfz.de/en/presse/pressemitteilungen/2011/dkfz-pm-11–24-German-Consortium-for-Trans-lational-Cancer-Research-Gets-Started.php.
- 19.Cancer Prevention and Research Institute of Texas. [Accessed June 3,2013]. Available at: http://www.cprit.state.tx.us.
- 20.Copelin, Laylan. CPRIT fallout: Cancer network ceases operations. [Accessed June 3, 2013]. Published January 29, 2013. Available at: http://www.statesman.com/news/business/cprit-fallout-clinical-trials-net-work-suspends-ope/nT9SH/
- 21.Bang Y-J, Van Cutsem E, Feyereislova, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-esopha-geal junction cancer (ToGA): a phase 3, open-label randomized controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 22.Lynch TJ, Bell DW, Sondella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 21.Jiang X, Zhou J, Yuen NK, et al. lmatinib target-ing of KIT-mutant oncoprotein in melanoma. Clin Cancer Res. 2008;14:7726–32. doi: 10.1158/1078-0432.CCR-08-1144. [DOI] [PubMed] [Google Scholar]
- 22.Cortes JE, Kantarjian H, Shah NP, et al. Pona-tinib in refractory Philadelphia chromosome-positive leukemias. N Engl J. Med. 2012;367:2075–2088. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]


