Combining lapatinib and trastuzumab with taxane chemotherapy may offer clinical benefit to patients with cancer. Dose-limiting toxicities, safety, and tolerability of this combination was assessed. Of the triplet combinations tested, the cohort receiving 750 mg/day dose of lapatinib had the lowest incidence of diarrhea; therefore, this dose should be used in further studies on the treatment of metastatic breast cancer.
Keywords: Breast cancer, HER2, Lapatinib, Paclitaxel, Trastuzumab
Abstract
Background.
Recent data support the hypothesis that combining lapatinib and trastuzumab with taxane chemotherapy may offer added clinical benefit to patients with human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer (MBC). This study examined the safety of the triplet combination in first-line HER2-positive MBC.
Patients and Methods.
Patients were enrolled into three sequential cohorts; the last two cohorts were added by protocol amendment following review of safety data from cohort 1. Patients in cohort 1 received lapatinib (1000 mg/day) plus paclitaxel (80 mg/m2 per week, 3 of every 4 weeks); cohort 2 received lapatinib (1000 mg/day) plus paclitaxel (70 mg/m2 per week, 3 of every 4 weeks); and cohort 3 received lapatinib (750 mg/day) plus paclitaxel (80 mg/m2 per week, 3 of every 4 weeks). All received standard trastuzumab dosing. The primary objective was assessment of dose-limiting toxicities, safety, and tolerability of this combination.
Results.
The most frequent adverse events (AEs) for all cohorts were diarrhea (89%), rash (79%), fatigue (73%), alopecia (63%), nausea (63%), and vomiting (40%). In cohorts 1 and 2, the incidence of grade 3 diarrhea was 62% and 50%, respectively; in cohort 3, the incidence was 25% (with prophylactic loperamide). Dehydration was the most frequent serious AE (10%). Across cohorts, overall response rate was 75%.
Conclusions.
The dose-limiting toxicity of paclitaxel, trastuzumab, and lapatinib in first-line HER2-positive MBC was diarrhea. Of the triplet combinations tested, the cohort receiving 750 mg/day dose of lapatinib had the lowest incidence of diarrhea; therefore, this dose should be used in further studies on the treatment of MBC.
Implications for Practice:
Dual targeting of the HER2 receptor using trastuzumab and lapatinib has been shown to be effective in HER2-positive metastatic breast cancer. In this study, we evaluated the safety of paclitaxel in combination with trastuzumab and lapatinib. The main side effect was diarrhea, which occurred in the majority of patients at the standard dosing of all three drugs. A pharmacokinetic interaction was found between paclitaxel and lapatinib, resulting in increased exposure of both drugs. We evaluated three dose levels of lapatinib and paclitaxel (all patients received standard trastuzumab dosing). A dose of lapatinib 750 mg/day had the lowest incidence of diarrhea in combination with paclitaxel 80 mg/m2 per week and trastuzumab 2 mg/kg per week. These doses should be used if the triplet is considered for further development in patients with HER2-positive metastatic breast cancer.
Introduction
The human epidermal growth factor receptor 2 (HER2) is overexpressed in approximately one third of breast cancers and is associated with a more malignant phenotype, resistance to chemotherapy, and poor prognosis [1–5]. Inhibition of HER2 signaling has been shown to be a clinically relevant goal, and several currently approved therapeutic strategies are available [6].
One strategy involves trastuzumab, a monoclonal antibody that targets the extracellular domain of HER2. A second strategy involves lapatinib, an orally active small molecule tyrosine kinase inhibitor with intracellular activity against HER2 and epidermal growth factor receptor. Although both trastuzumab [7] and lapatinib [8] have demonstrated clinical benefit as monotherapies, the efficacy of these agents in HER2-positive disease is greatly enhanced when combined with first-line taxane chemotherapy [9, 10].
For example, the combination of either trastuzumab or lapatinib with paclitaxel is well-tolerated and offers significant clinical benefits to patients with HER2-positive metastatic breast cancer (MBC) [9, 11–14]. The combination of paclitaxel (175 mg/m2 every 3 weeks × 6 cycles or 80 mg/m2 every week) with trastuzumab as first-line therapy in HER2-overexpressing MBC was shown to be superior to paclitaxel alone [9, 11]. Similarly, a phase III, first-line study (EGF30001) in women with HER2-negative and HER2-uncharacterized MBC showed that the addition of lapatinib to paclitaxel (175 mg/m2 every 3 weeks) improved time to progression, overall response rate (ORR), and clinical benefit rate in a preplanned subgroup of HER2-positive patients [12]. Recent work by Jagiello-Gruszfeld et al. [13] produced an encouraging ORR for first-line paclitaxel plus lapatinib (51% and 77% as assessed by the independent review committee and investigator, respectively). In addition, a phase III randomized, double-blind study comparing lapatinib plus paclitaxel versus paclitaxel alone showed that lapatinib plus paclitaxel improved median survival by 7.3 months and significantly reduced the risk of disease progression by 48% over paclitaxel alone [14].
Combination treatment with trastuzumab and lapatinib has also yielded promising results. These two agents have the advantage of targeting different domains of HER2 signaling and of having partially nonoverlapping mechanisms of action [15–18]. Results from a phase III study (EGF104900) showed that the combination of lapatinib plus trastuzumab significantly improved progression-free survival (PFS) and clinical benefit rate over lapatinib alone and tended to offer a survival benefit [19].
Taken together, these data offer an appealing hypothesis that the combination of anti-HER2 therapies and taxanes may improve clinical benefit in patients with HER2-positive MBC. We report results of an open-label phase I study conducted to assess the safety and tolerability of the triplet combination of lapatinib, trastuzumab, and paclitaxel as a first-line treatment for HER2-positive MBC.
Patients and Methods
Study Population
Patients were women aged ≥18 years with histologically confirmed invasive stage IV breast cancer. All had tumors overexpressing HER2 (defined as 3+ by immunohistochemistry [IHC] or HER-2/neu gene amplification by fluorescence in situ hybridization [FISH] or 0 to 2+ by IHC and HER-2/neu gene amplification by FISH). All women had a lesion measurable by Response Evaluation Criteria in Solid Tumors (RECIST) or assessable disease. Adequate organ function, a left ventricular ejection fraction (LVEF) within institutional normal range, and Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 were also required for enrollment. Previous adjuvant treatment with trastuzumab was permitted if 12 months had elapsed since its discontinuation; previous neoadjuvant/adjuvant treatment with taxanes was permitted if progression had occurred 12 or more months after its completion.
Patients with prior treatment for metastatic disease, a history of central nervous system metastases, uncontrolled or symptomatic angina, arrhythmias, congestive heart failure, or persistent peripheral neuropathy ≥ grade 2 or with certain gastrointestinal or hepatobiliary diseases were excluded.
Study Design
EGF104383 was initially designed as a randomized, double-blind, placebo-controlled phase III study comparing the efficacy and tolerability of paclitaxel plus trastuzumab plus lapatinib with paclitaxel plus trastuzumab plus placebo in women with HER2-amplified MBC. Prior to the start of the planned randomized phase of this trial, an open-label safety study was conducted. The results of this safety study are presented here. The phase III randomized phase of this study did not occur because of the high rates of grade 3 diarrhea at standard doses of lapatinib (1,000 mg) in this triple combination and the subsequent time taken to recruit patients into the three cohorts.
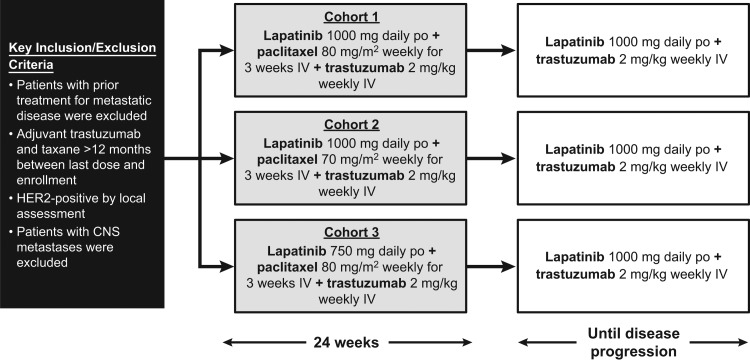
The study design is presented in Figure 1. Patients were initially enrolled in the open-label safety cohort between December 2005 and October 2006 and were treated with a combination of paclitaxel (80 mg/m2 intravenously weekly for 3 weeks of a 4-week cycle), trastuzumab (2 mg/kg weekly intravenously, including a loading dose of 4 mg/kg), and oral lapatinib (1,000 mg daily). Paclitaxel was administered for a minimum of six cycles and could be continued at investigator discretion or discontinued if the patient progressed, refused treatment, or experienced an unacceptable toxicity. If unacceptable toxicity related to paclitaxel was experienced or at least six cycles of paclitaxel were received, paclitaxel could be halted and lapatinib and trastuzumab continued until disease progression. If either lapatinib or trastuzumab was discontinued, all treatments were halted.
Figure 1.
Study design. Trastuzumab 4 mg/kg loading dose.
Abbreviations: CNS, central nervous system; HER2, human epidermal growth factor receptor 2; IV, intravenous; po, by mouth.
After reports of gastrointestinal adverse events (AEs; seven patients experienced grades 3 and 4 diarrhea), the protocol was amended to close enrollment into cohort 1 and initiate enrollment into cohort 2. A second cohort was added to explore a lower dose of paclitaxel (70 mg/m2) plus the same doses of trastuzumab and lapatinib. If a patient tolerated the lower dose for two cycles, at the discretion of the investigator, the paclitaxel dose could be increased to 80 mg/m2, which the patient continued to receive in subsequent cycles. Following a review of the cohort 2 safety data, enrollment in a third cohort investigating a lower dose of lapatinib (750 mg/day) plus paclitaxel (80 mg/m2) plus trastuzumab was initiated. If the first two cycles were well-tolerated, lapatinib could be increased to 1,000 mg/day for the subsequent cycles, at the discretion of the investigator. The protocol amendment required all patients in all three cohorts to receive prophylactic loperamide for diarrhea management.
This study was conducted in accordance with good clinical practice, all applicable regulatory requirements, and the guiding principles of the Declaration of Helsinki. An institutional review board of each participating center approved the study protocol, and each patient or her guardian provided informed consent.
Study Endpoints and Assessments
The primary objective of this open-label safety study was assessment of dose-limiting toxicities, safety, and tolerability of lapatinib when administered with paclitaxel and trastuzumab. To that end, an emphasis was placed on descriptive comparisons of the safety cohorts. Primary safety endpoints for the open-label phase included extent of exposure, AEs, deaths, and characteristics of diarrhea, as well as vital signs, hematology, clinical chemistry, and toxicity laboratory data. AEs were graded according to National Cancer Institute Common Toxicity Criteria v3.0 (NCI CTC).
Echocardiograms or multiple-gated acquisition scans were performed every 8 weeks. Cardiac dysfunction was defined as any grade 3 deterioration in LVEF function or a ≥20% relative decrease in LVEF from baseline that is below the lower limit of normal. Patients with an NCI CTC AE grade 3 or 4 left ventricular systolic dysfunction were withdrawn from lapatinib and trastuzumab.
Blood samples for plasma lapatinib concentrations were collected from a small subset of patients in each cohort (cohort 1, n = 3; cohort 2, n = 4; cohort 3, n = 6) on day 1 of cycles 1 and 2 at predose, immediately prior to termination of paclitaxel infusion (to determine the paclitaxel concentration) and 4 hours postlapatinib. Efficacy data were summarized for each cohort and in total across cohorts; however, no formal hypothesis testing was performed. The primary efficacy endpoint was ORR, which was defined as the percentage of patients achieving either a complete (CR) or partial (PR) response per RECIST 1.0 during the study. Secondary efficacy endpoints were clinical benefit, PFS, time to response, and duration of response. Clinical benefit was defined as the percentage of patients achieving CR, PR, or stable disease (SD) for ≥24 weeks. PFS was defined as the time from first dose to the earliest disease progression or death due to any cause. Time to response was defined as the time from first dose until CR or PR. Duration of response was defined as the time from first evidence of PR or CR until the first sign of disease progression or death. ORR, clinical benefit, PFS, and disease progression were based on investigator assessments per RECIST 1.0.
Statistical Methods
The efficacy, safety, and tolerability analyses were based on the open-label population (all patients who took study medication). Continuous variables were summarized with the statistics mean, median, standard deviation, and minimum and maximum; categorical variables were summarized with frequency counts and percentages. All confidence intervals (CIs) were two-sided and used 95% confidence levels. Any analysis requiring significance testing used a two-sided test at the 0.05 significance level, unless otherwise specified. Patients with unknown or missing responses were treated as nonresponders and were included in the denominator when calculating the percentage.
Results
Study Population and Disposition
As of March 2010 (the data cutoff date for this analysis), 63 patients were enrolled in one of three cohorts, the first patient was enrolled in December 2005, and the last patient was enrolled in April 2009. Cohort 1 received lapatinib (1,000 mg daily) plus paclitaxel (80 mg/m2 weekly for 3 weeks in a 4-week cycle). Following a review of the safety data from cohort 1, the protocol was amended to add an additional two cohorts. Cohort 2 received oral lapatinib (1,000 mg daily) plus paclitaxel (70 mg/m2 weekly for 3 weeks in a 4-week cycle), and cohort 3 received lapatinib (750 mg daily) plus paclitaxel (80 mg/m2 weekly for 3 weeks in a 4-week cycle). All cohorts received trastuzumab (2 mg/kg weekly plus a loading dose of 4 mg/kg) concurrently with lapatinib and paclitaxel. After the protocol was amended, all patients also received prophylactic loperamide. No patients withdrew before the start of treatment, resulting in a safety population of 63 patients. Patients were enrolled sequentially into the cohorts; therefore, the members of cohort 3 spent the shortest time on treatment before the clinical cutoff date.
Baseline demographics and prior therapies are presented in Table 1. At the data cutoff date (March 2010), the open-label safety enrollment was complete and preliminary data were available for all patients. A total of 44 patients were still enrolled in the trial; 9 patients had withdrawn; 10 patients had died; and the last patient enrolled had completed 11 months of the study and was still undergoing treatment.
Table 1.
Baseline demographics and prior treatments
aTwo patients in each cohort.
Treatment Summary
Lapatinib was permanently discontinued by 52 (83%) patients (27 patients in cohort 1, 11 patients in cohort 2, and 14 patients in cohort 3). Of those discontinuations, 21 (33%) were due to disease progression and 15 (24%) due to AEs. Five patients decided to withdraw from the study (n = 3, 5%) or from treatment (n = 2, 3%), and the investigator decided that six patients should withdraw (10%). Other reasons for stopping lapatinib permanently were protocol violations (n = 1, 2%), noncompliance (n = 1, 2%), or prolonged interruption of study drug (n = 1, 2%).
Safety
Overall, the most frequently reported AEs by all patients were diarrhea (89%), rash (79%), fatigue (73%), alopecia (63%), nausea (63%), vomiting (40%), constipation (29%), myalgia (29%), cough (29%), and insomnia (22%) (Table 2). Most AEs reported during the study were grade 1 or 2.
Table 2.
Summary of frequently reported AEs
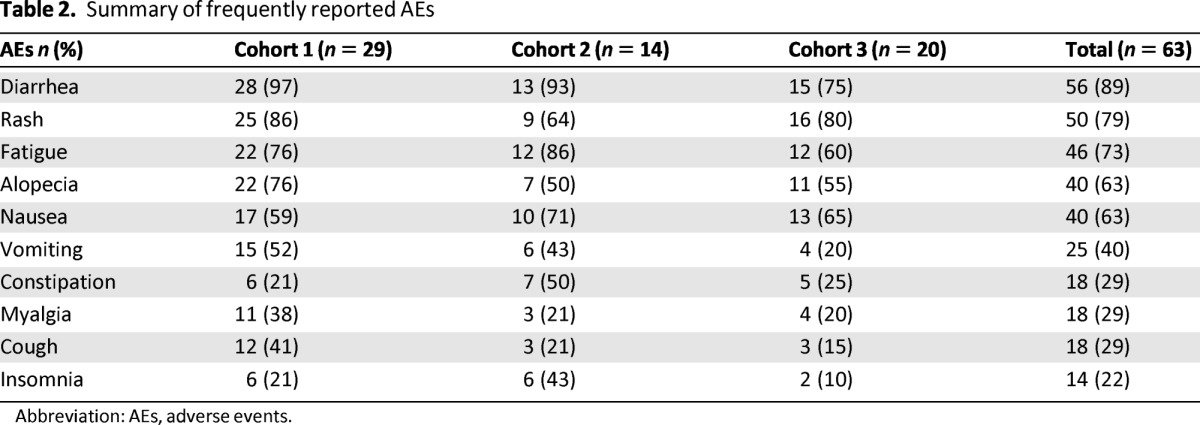
Abbreviation: AEs, adverse events.
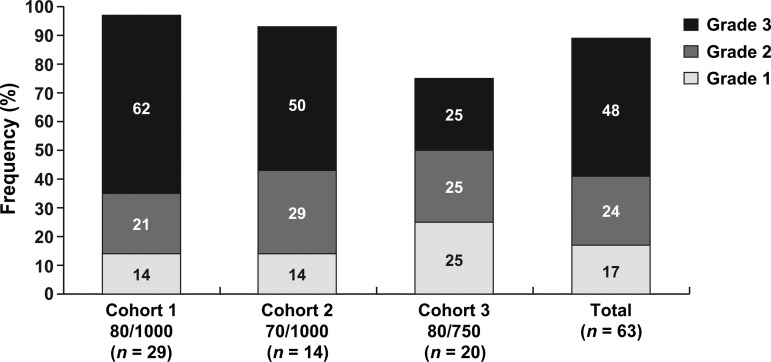
Diarrhea was reported by 97% of patients in cohort 1, 93% in cohort 2, and 75% in cohort 3 (Fig. 2). Thirty patients (48%) had grade 3 diarrhea, but no grade 4 diarrhea was reported. Two patients in cohort 1 reported diarrhea as a serious AE (SAE), and two patients withdrew from the study treatment because of diarrhea (one patient from cohort 1 and one patient from cohort 2). The incidence of grade 3 diarrhea was considerably lower in cohort 3 (25%) than in cohort 1 (62%) and cohort 2 (50%), and no patients from this cohort withdrew from study treatment.
Figure 2.
Incidence of diarrhea by maximum severity grade. Percentages within diarrhea grades are based on those patients who experience diarrhea.
Eight patients reported hepatobiliary AEs (cohort 1, six patients; cohort 3, two patients). All events were grade 1 to 2 in severity except one grade 3 event in cohort 1; none led to a withdrawal from lapatinib.
Twelve patients (19%) had decreases in LVEF reported as an AE. Of the 12 patients, eight were in cohort 1 (three withdrew from treatment), three were in cohort 2 (one withdrew from treatment), and one was in cohort 3. All events were grade 1 to 2 in severity except one grade 3 event in cohort 1.
SAEs were experienced by 37% of patients (Table 3). Dehydration was the most frequently reported SAE (10%); of the six patients with dehydration, five were in cohort 1. Other common SAEs included decreased LVEF (5%), diarrhea (3%), hypokalemia (3%), nausea (3%), pleural effusion (3%), and vomiting (3%). There were no fatal SAEs.
Table 3.
Most common SAEs
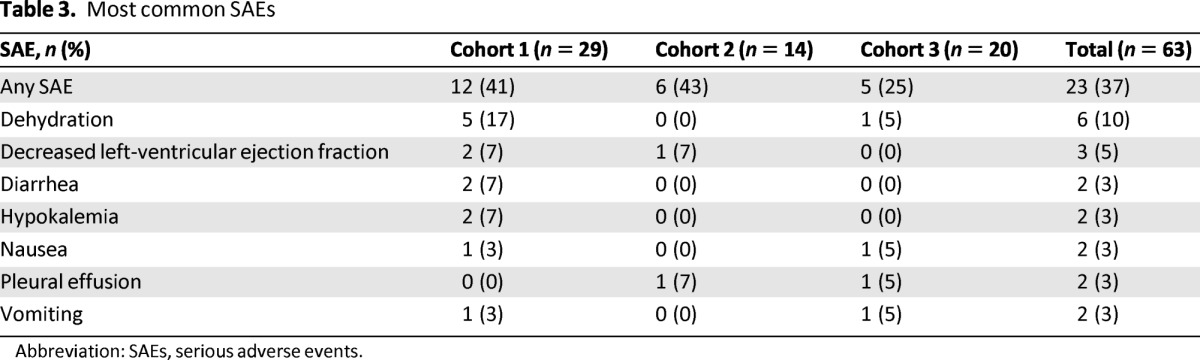
Abbreviation: SAEs, serious adverse events.
Pharmacokinetics
Plasma lapatinib concentrations were measured in three to six patients from each cohort. Maximum concentrations ranged from 543 to 4,893 ng/mL.
Efficacy
Among all 63 patients, there were three CRs and 44 PRs, yielding an ORR of 75% (47 out of 63 patients; 95% CI: 62.1–84.7) (Table 4). Among the cohorts, response rates were 79% (23 out of 29 patients; 95% CI: 60.3–92.0) in cohort 1; 71% (10 out of 14 patients; 95% CI: 41.9–91.6) in cohort 2; and 70% (14 out of 20 patients; 95% CI: 45.7–88.1) in cohort 3. As more than 50% of patients were censored without an event of progression or death, an estimation of PFS and time to progression are not reported.
Table 4.
Investigator-assessed best response by treatment cohort
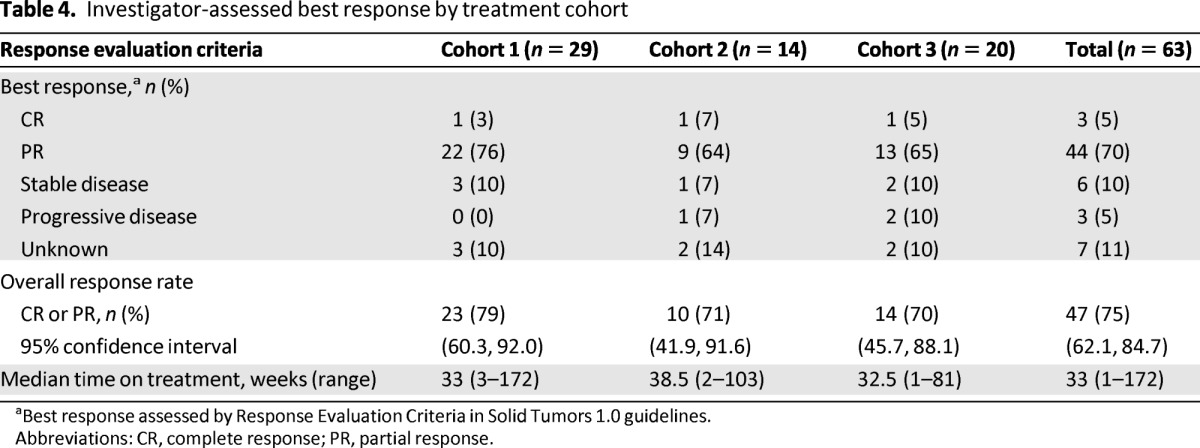
aBest response assessed by Response Evaluation Criteria in Solid Tumors 1.0 guidelines.
Abbreviations: CR, complete response; PR, partial response.
Discussion
The safety results presented in this study showed a high incidence of grade 3 diarrhea in cohorts 1 and 2 (62% and 50%, respectively) when lapatinib was given at a dose of 1,000 mg/day concurrently with paclitaxel and trastuzumab. This incidence (25%) was greatly reduced in cohort 3 with a lapatinib dose of 750 mg/day in combination with paclitaxel and trastuzumab when given with prophylactic loperamide. The diarrhea reported by patients receiving 750 mg/day of lapatinib with loperamide was manageable as indicated by the fact that only two patients withdrew from this lower dose of lapatinib because of diarrhea. However, even grades 1 and 2 diarrhea can have an important impact on quality of life. For that reason, it is important to manage diarrhea proactively before it becomes severe.
Studies using trastuzumab plus paclitaxel have reported response rates ranging from 50% to 81% in HER2-overexpressing first-line MBC patients [9, 11, 20]. In addition, a recently completed randomized phase III study demonstrated that the addition of lapatinib to paclitaxel improves response rates over paclitaxel alone (69% vs. 50%, respectively, p < .0001) [14]. In the present study, the triplet combination of lapatinib, trastuzumab, and paclitaxel yielded an across-cohort ORR of 75% (cohort 1, 79%; cohort 2, 71%; cohort 3, 70%). Although this response rate is encouraging, a large, randomized, double-blind comparative clinical study is required to determine the clinical benefit of this combination in the treatment of MBC.
Novel combinations of anti-HER2 agents and chemotherapies are needed because many women with HER2-positive MBC do not respond to conventional anti-HER2-directed regimens [21, 22]. In addition, of those patients who experience impressive tumor responses, many develop progressive disease in less than a year [9, 23, 24]. Thus, several studies have analyzed the combination of targeted therapies as a way to optimize breast cancer treatment [25]. Dual combination therapy of lapatinib plus trastuzumab has demonstrated improved activity compared with either lapatinib or trastuzumab alone in patients with breast cancer [19, 26].These data from the present study indicate that the triplet combination of lapatinib, trastuzumab, and paclitaxel is clinically feasible and potentially effective in first-line HER2-positive MBC. However, given the high frequency of diarrhea as noted in this study, risk/benefit analysis of efficacy versus quality of life is an important consideration. The activity of both lapatinib and trastuzumab in combination with paclitaxel in the neoadjuvant and adjuvant setting is ongoing in patients with HER2-positive stage II or stage III breast cancer (CALGB 40601 [NCT00770809] and ALTTO [NCT00490139]). The doses of this triple combination treatment used in the ALTTO and other studies were determined partially as a result of interim safety data generated from the EGF104383 study.
Conclusions
The analysis of the safety and tolerability of lapatinib, trastuzumab, and paclitaxel show that the combination may be well-tolerated with a 750 mg/day dose of lapatinib. Doses of lapatinib above 750 mg/day in combination with trastuzumab and paclitaxel are not recommended given the greater incidence of severe diarrhea reported in this study. Although plasma concentrations of lapatinib were measured, the small sample size and intrinsically high variability in lapatinib pharmacokinetics preclude any meaningful analysis. Overall, these data show that the triplet combination can be administered with a manageable safety profile and offer evidence of high antitumor activity in patients with HER2-positive MBC.
Acknowledgments
This work is supported by GlaxoSmithKline (NCT00272987). We thank the patients for their participation. Kevin Koch from GlaxoSmithKline contributed to data interpretation and intellectual content for the manuscript. Editorial support in the form of editorial suggestions to draft versions of this paper, assembling tables and figures, collating author comments, copyediting, fact checking, and referencing services was provided by Brad Imwalle, Ph.D., and Antoinette Campo at SCI Scientific Communications and Information.
This manuscript was presented previously, in part, at the 2010 American Society of Clinical Oncology Annual Meeting; June 4–8, 2010; Chicago, IL.
Author Contributions
Conception and design: Francisco J. Esteva, Simon Turner, Steven Stein, Alejandra Perez
Provision of study materials or patients: Francisco J. Esteva, Sandra X. Franco, Maura K. Hagan, Abenaa M. Brewster, Robert A. Somer, Steven Stein, Alejandra Perez
Collection and/or assembly of data: Francisco J. Esteva, Sandra X. Franco, Robert A. Somer, Will Williams, Steven Stein, Alejandra Perez
Data analysis and interpretation: Francisco J. Esteva, Allison M. Florence, Simon Turner, Steven Stein, Alejandra Perez
Manuscript writing: Francisco J. Esteva, Allison M. Florance, Simon Turner, Steven Stein, Alejandra Perez
Final approval of manuscript: Francisco J. Esteva, Sandra X. Franco, Maura K. Hagan, Abenaa M. Brewster, Robert A. Somer, Will Williams, Allison M. Florance, Simon Turner, Steven Stein, Alejandra Perez
Disclosures
Francisco J. Esteva: GlaxoSmithKline (RF); Robert A. Somer: Esai, GlaxoSmithKline, Sanofi-Aventis (H); Allison M. Florance, Simon Turner, Steven Stein: GlaxoSmithKline (E, OI). The other authors indicated no financial relationships.
C/A: Consulting/advisory relationship; RF: Research funding; E: Employment; H: Honoraria received; OI: Ownership interests; IP: Intellectual property rights/inventor/patent holder; SAB: scientific advisory board
Reference
- 1.Moy B, Goss PE. Lapatinib: Current status and future directions in breast cancer. The Oncologist. 2006;11:1047–1057. doi: 10.1634/theoncologist.11-10-1047. [DOI] [PubMed] [Google Scholar]
- 2.Revillion F, Bonneterre J, Peyrat JP. ERBB2 oncogene in human breast cancer and its clinical significance. Eur J Cancer. 1998;34:791–808. doi: 10.1016/s0959-8049(97)10157-5. [DOI] [PubMed] [Google Scholar]
- 3.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 5.Esteva FJ, Hortobagyi GN, Sahin AA, et al. Expression of erbB/HER receptors, heregulin and P38 in primary breast cancer using quantitative immunohistochemistry. Pathol Oncol Res. 2001;7:171–177. doi: 10.1007/BF03032345. [DOI] [PubMed] [Google Scholar]
- 6.Esteva FJ. Monoclonal antibodies, small molecules, and vaccines in the treatment of breast cancer. The Oncologist. 2004;9(suppl 3):4–9. doi: 10.1634/theoncologist.9-suppl_3-4. [DOI] [PubMed] [Google Scholar]
- 7.Vogel CL, Cobleigh MA, Tripathy D, et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:719–726. doi: 10.1200/JCO.2002.20.3.719. [DOI] [PubMed] [Google Scholar]
- 8.Gomez HL, Doval DC, Chavez MA, et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol. 2008;26:2999–3005. doi: 10.1200/JCO.2007.14.0590. [DOI] [PubMed] [Google Scholar]
- 9.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 10.Esteva FJ, Valero V, Booser D, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002;20:1800–1808. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 11.Gasparini G, Gion M, Mariani L, et al. Randomized phase II trial of weekly paclitaxel alone versus trastuzumab plus weekly paclitaxel as first-line therapy of patients with Her-2 positive advanced breast cancer. Breast Cancer Res Treat. 2007;101:355–365. doi: 10.1007/s10549-006-9306-9. [DOI] [PubMed] [Google Scholar]
- 12.Di Leo A, Gomez HL, Aziz Z, et al. Phase III, double-blind, randomized study comparing lapatinib plus paclitaxel with placebo plus paclitaxel as first-line treatment for metastatic breast cancer. J Clin Oncol. 2008;26:5544–5552. doi: 10.1200/JCO.2008.16.2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagiello-Gruszfeld A, Tjulandin S, Dobrovolskaya N, et al. A single-arm phase II trial of first-line paclitaxel in combination with lapatinib in HER2-overexpressing metastatic breast cancer. Oncology. 2010;79:129–135. doi: 10.1159/000318043. [DOI] [PubMed] [Google Scholar]
- 14.Guan ZZ, Xu BH, Tong ZS, et al. Overall survival benefit observed with lapatinib (L) plus paclitaxel (P) as first-line therapy in patients with HER2-overexpressing metastatic breast cancer (MBC). Abstract presented at: San Antonio Breast Cancer Symposium; December 8–12, 2010; San Antonio, TX. [Google Scholar]
- 15.Scaltriti M, Verma C, Guzman M, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2008;28:803–814. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 16.Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–1639. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 17.Nahta R, Yuan LX, Du Y, et al. Lapatinib induces apoptosis in trastuzumab-resistant breast cancer cells: Effects on insulin-like growth factor I signaling. Mol Cancer Ther. 2007;6:667–674. doi: 10.1158/1535-7163.MCT-06-0423. [DOI] [PubMed] [Google Scholar]
- 18.Esteva FJ, Yu D, Hung MC, et al. Molecular predictors of response to trastuzumab and lapatinib in breast cancer. Nat Rev Clin Oncol. 2010;7:98–107. doi: 10.1038/nrclinonc.2009.216. [DOI] [PubMed] [Google Scholar]
- 19.Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: Final results from the EGF104900 Study. J Clin Oncol. 2012;30:2585–2592. doi: 10.1200/JCO.2011.35.6725. [DOI] [PubMed] [Google Scholar]
- 20.Seidman AD, Fornier MN, Esteva FJ, et al. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol. 2001;19:2587–2595. doi: 10.1200/JCO.2001.19.10.2587. [DOI] [PubMed] [Google Scholar]
- 21.Esteva FJ, Guo H, Zhang S, et al. PTEN, PIK3CA, p-AKT, and p-p70S6K status. Association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am J Pathol. 2010;177:1647–1656. doi: 10.2353/ajpath.2010.090885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mittendorf EA, Wu Y, Scaltriti M, et al. Loss of HER2 amplification following trastuzumab-based neoadjuvant systemic therapy and survival outcomes. Clin Cancer Res. 2009;15:7381–7388. doi: 10.1158/1078-0432.CCR-09-1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonard DS, Hill AD, Kelly L, et al. Anti-human epidermal growth factor receptor 2 monoclonal antibody therapy for breast cancer. Br J Surg. 2002;89:262–271. doi: 10.1046/j.0007-1323.2001.02022.x. [DOI] [PubMed] [Google Scholar]
- 24.Montemurro F, Donadio M, Clavarezza M, et al. Outcome of patients with HER2-positive advanced breast cancer progressing during trastuzumab-based therapy. The Oncologist. 2006;11:318–324. doi: 10.1634/theoncologist.11-4-318. [DOI] [PubMed] [Google Scholar]
- 25.Gluck S, Arteaga CL, Osborne CK. Optimizing chemotherapy-free survival for the ER/HER2-positive metastatic breast cancer patient. Clin Cancer Res. 2011;17:5559–5561. doi: 10.1158/1078-0432.CCR-10-2051. [DOI] [PubMed] [Google Scholar]
- 26.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): A randomised, open-label, multicentre, phase 3 trial. Lancet. 2012;379:633–640. doi: 10.1016/S0140-6736(11)61847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]