This retrospective study confirms that discordance in estrogen receptor (ER) and progesterone receptor expression between the primary breast tumor and the corresponding metastatic lesions is high, whereas HER2 status remains relatively constant. Chemotherapy, and specifically anthracycline-based chemotherapy, was associated with switch in ER status.
Keywords: Breast cancer, Estrogen receptor, Progesterone receptor, Human epidermal growth factor receptor 2
Abstract
Background.
The primary aim of this retrospective study was to investigate intraindividual correlation of estrogen receptor (ER) status, progesterone receptor (PR) status, and HER2 status between primary breast cancer and metastatic breast cancer (mBC). Secondary aims included patients' characteristics, overall survival, feasibility of histopathological evaluation in the metastatic setting, and predictive factors associated with receptors status discordance.
Methods.
Patients with either biopsy of metastatic relapse or surgery of metastasis were identified. Demographics, tumor characteristics, treatment characteristics, and ER, PR, and HER2 statuses were retrospectively obtained in patients' reports. Receptors statuses were assessed by immunohistochemistry with a positivity cutoff of more than 10% for ER and PR. HER2 was considered as positive if overexpression was scored at 3+ in immunohistochemistry or if amplification ratio was >2 in fluorescent in situ hybridization.
Results.
From a cohort of 489 patients with mBC, 269 patients had histopathological samples of metastatic relapse. Histopathological analysis of the specimen confirmed the diagnosis of mBC in 235 patients. Discordance in one or more receptors between primary breast cancer and mBC was found in 99 patients (42%). A switch in receptor status was identified for ER in 17% of tumors (p = 4 × 10−3), PR in 29% of cancers (p < 4 × 10−4), and HER2 in 4% of lesions (p = .16). Exposure to chemotherapy and to anthracycline-based chemotherapy was statistically associated with switches in ER status. Seven (2%) second malignancies and three benign diseases (1%) were diagnosed.
Conclusions.
This study confirms that discordance in ER and PR receptor expression between the primary breast tumor and the corresponding metastatic lesions is high, whereas HER2 status remains relatively constant. Chemotherapy, and specifically anthracycline-based chemotherapy, was associated with switch in ER status. These results were obtained in a selected population of patients; further studies are warranted to confirm these data and to determine the interest of systematic rebiopsy in the metastatic setting.
Implications for Practice:
Discordance in estrogen receptor and progesterone receptor expression between the primary breast tumor and the corresponding metastatic lesion was high (17% and 29%, respectively), whereas HER2 status remained stable (4% of discordance). Previous chemotherapy, and specifically anthracycline-based chemotherapy, was associated with a switch in estrogen receptor status. Further studies are warranted to confirm these data and to determine the interest of systematic rebiopsy in the metastatic setting. In the era of a more personalized approach to medicine and of genomic investigations of tumors, reliable and recent evaluation of tumor prognostic and predictive factors remains a challenge.
Introduction
The treatment of metastatic breast cancer (mBC) is complex with few recognized therapeutic standards, particularly after the first line of treatment. Multiple factors, such as human epidermal growth factor receptor 2 (HER2) status, hormonal receptors status, disease-free interval, number and sites of metastases, symptoms of the disease, physiological age, performance status, and previous therapies, must be taken into account to determine the best therapeutic management of patients diagnosed with mBC [1]. Estrogen receptor (ER) status, progesterone receptor (PR) status, and HER2 status are essential to classify breast cancer into three major subgroups requiring different managements: HER2-positive cancers, hormone receptor-positive cancers, and triple-negative breast cancers. Metastatic relapse of breast cancer is usually diagnosed on clinical, biological, and radiologic findings [2]. Most of the time, histopathological proof of metastatic relapse is not required and the choice of metastatic therapy is based on the immunophenotype of the primary tumor. However, a biopsy of metastatic disease can be performed with three aims: to confirm the diagnosis of cancer, to confirm that the lesion is a metastasis of the primary known cancer, and to reassess tumor's characteristics. Changes in hormonal receptor status between primary and metastatic breast cancer have been described for over 30 years [3, 4], with discordance rates reaching up to 40%. HER2 discordance has also been recognized but seems less common [5]. Modifications in hormonal receptors status and HER2 status have clinical consequences and can result in therapeutic changes [6].
The primary aim of this retrospective study was to investigate the intraindividual correlation of ER status, PR status, and HER2 status between primary breast cancer and metastatic relapse. Secondary aims were to describe the patients' characteristics, assess the feasibility of histopathological evaluation at the metastatic setting, search for predictive factors associated with receptor status discordance, and determine the effect of discordance on disease prognostic.
Patients and Methods
We selected retrospectively from our institutional database women who received a therapy for mBC between January 1, 1998 and December 31, 2010. Three different populations of patients were defined. The “total population” refers to all patients with mBC. Patients with either biopsy of metastatic relapse or surgery of metastasis were identified and constituted the “population with specimen collection.” Patients whose histopathological evaluation of the specimen confirmed the diagnosis of mBC are the “population with histologically proven mBC.” Correlations of ER status, PR status, and HER2 status between primary breast cancer and metastatic relapse were assessed in the last population. The metastatic histological assessment could be done at presentation of metastatic disease or later after the completion of one or several lines of treatment. Patients with only cytology of metastasis, with carcinoma in situ as initial diagnosis and patients for whom ER status, PR status, and/or HER2 status of primary breast cancer and mBC were not available were excluded from the study. Patients' charts were reviewed to collect demographic, tumor, and treatment characteristics. Sites of surgery or biopsy and grade 3–5 complications were recorded.
Assessment of ER, PR, and HER2 Statuses
ER, PR, and HER2 statuses were retrospectively obtained in pathology reports. Receptor statuses were assessed by immunohistochemistry. For patients with samples obtained before 2002, a determination of HER2 had been performed retrospectively both on primary tumor and on metastatic sample. Three laboratories performed 98.3% of the immunohistochemical analyses. Different methods were used and had common points: (a) Immunostaining was performed on 4- to 5-μm tissue sections prepared from a representative sample of the tumor. (b) Fixation was performed using 4% buffered formaldehyde solution. Biopsies were fixed for 12–48 hours and surgery specimens were fixed for 24–48 hours. (c) If needed, a decalcification procedure was performed. Hybridization techniques were not used after a decalcification procedure. (d) External and internal controls were performed. For ER, PR, and HER2 assessments, positive, intermediate, and negative controls were included in each slide. (e) Two different ER (Novocastra, clone 6F11 [Leica Microsystems, Wetzlar, Germany, http://www.leica-microsystems.com], or Ventana, clone SP1 [Ventana Medical Systems, Inc., Tucson, AZ, http://www.ventana.com]) and HER2 (Ventana clone 4B5 or Dako clone CB11 [Dako, Glostrup, Denmark, http://www.dako.com]) antibodies were used and three different PR antibodies (Dako clone PGR636 or Novocastra clone 16 or Ventana clone 1E2) were used.
For ER and PR status, results were expressed in terms of percentage of stained tumor cells with a cutoff of positivity commonly used in France higher than 10% [7]. HER2 assessment was scored from 0 to 3+. In case of overexpression scored at 2+, fluorescent in situ hybridization (FISH) was performed. HER2 was considered positive if overexpression was scored at 3+ in immunohistochemistry or if amplification ratio HER2/C-17 was >2 in FISH [8].
Statistical Analyses
The primary endpoint was to assess discordance in ER status, PR status, and HER2 status between primary breast cancer and metastatic relapse. Histopathological evaluation of the metastatic lesion could be performed either from a diagnostic biopsy or from a surgical specimen. Secondary aim points included patients' characteristics, overall survival, feasibility of histopathological evaluation in the metastatic setting, and predictive factors associated with receptors status discordance. Overall survival, defined as the time from the diagnosis of mBC to death by any cause, was analyzed using the Kaplan-Meier method for each group. The difference between groups was compared with the use of the log-rank test, with the hazard ratio and its 95% confidence interval (CI) calculated from a Cox regression model with a single covariate.
Continuous variables were described by the median with range, and qualitative variables by the size and percentage rate. Between the groups, qualitative and quantitative variables were respectively compared by the Fisher exact test or the χ2 test and nonparametric Mann-Whitney test. All tests were two-tailed and significant at an α threshold of 5% (p value).
The analysis of factors associated with discordance was performed in a three-step approach. Firstly, the association of potential factors with observed discordance (yes/no) was examined by univariate analysis. Then, quantitative and qualitative variables were transformed, whenever possible, into dichotomic variables, using different successive cutoff points. Finally, all variables with a p value <.15 in univariate analysis were entered in a stepwise logistic-regression model. The results of multivariate analyses are presented with adjusted odds ratio, 95% confidence interval (CI), and p value. Statistical analysis was performed with SAS software version 9.3 (SAS Institute, Inc., Cary, NC, http://www.sas.com).
Results
Patients and Tumor Characteristics
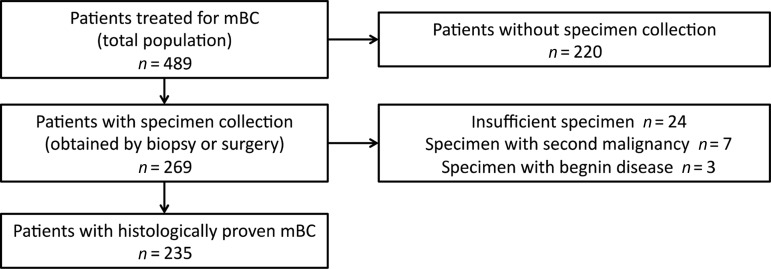
Between January 1, 1998 and December 31, 2010, a total of 489 patients with mBC were treated in our university hospital (total population). In all, 269 patients (55%) underwent either biopsy or surgery of metastatic relapse (patients with specimen collection). Among these patients, histopathological analysis of the specimen confirmed the diagnosis of mBC in 235 (48%) patients (patients with histologically proven mBC). In 34 patients (7%), histopathological analysis of the specimen did not conclude in mBC (Fig. 1). Patient demographic characteristics and disease characteristics are representative of a population with metastatic breast cancer (Table 1). The median age at mBC diagnosis was 58 years (range: 29–93 years). In all, 19% of the patients had HER2-positive (3+ overexpression or amplification) breast cancer. A total of 42% of patients had liver metastases, 36% had lung metastases, and 61% had bone metastases. The median disease free interval was 2.9 years (range: 0–23.1 years). Median overall survival was 49 months (95% CI: 43–58 months). Patients who underwent biopsy or surgery (n = 235; Table 1) were significantly younger (p = 10−2), with a longer disease-free interval (p = 10−4), were less frequently metastatic at presentation (p = 10−4), and had a significantly longer overall survival (p = 10−4). These patients were more likely to receive adjuvant chemotherapy (p = 10−4), adjuvant anthracyclines (p = 10−3), and adjuvant taxanes (p = 10−3); they also had more soft tissues metastases.
Figure 1.
Patient flow chart.
Abbreviation: mBC, metastatic breast cancer.
Table 1.
Patient demographic and clinical characteristics

aStatistically significant difference (p < .05).
Abbreviations: BC, breast cancer; ER, estrogen receptor; PR, progesterone receptor.
Specimen Collection
Sixty-three patients included in the population with histologically proven mBC (n = 235) had two tissue specimens obtained through two different processes (two biopsies or two surgeries or one biopsy and one surgery). Excluding primary tumor specimens, a total of 332 tissues from biopsy or surgery specimens obtained in the metastatic setting were available. Two thirds of specimens were collected after Janaury 1, 2006. A total of 210 specimens (63%) were obtained from biopsies and 122 (37%) were obtained from surgery. In all, 51% of specimens were obtained at the diagnosis of mBC; others were collected at any time during the evolution of the metastatic disease; the interval between specimen collection and mBC diagnosis ranged between 0 and 12.7 years.
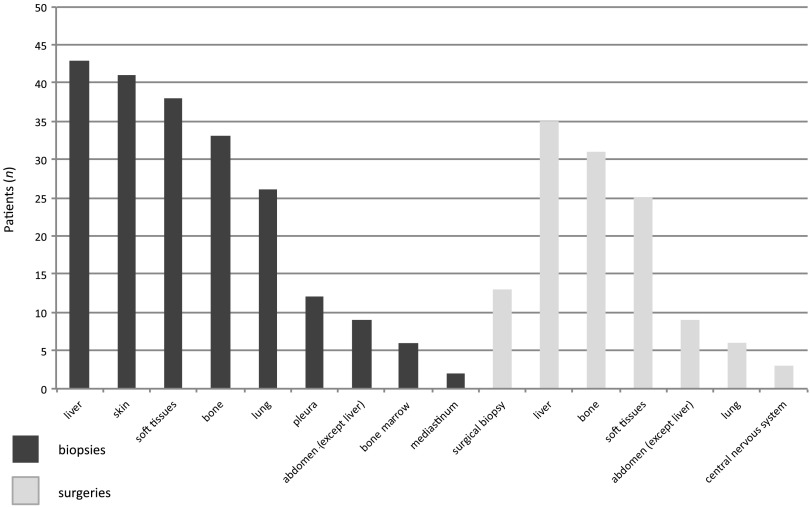
The most common site of biopsy was liver followed by skin (Fig. 2). If two metastatic specimens were available, the most recent specimen was used. Specimens collected by surgery were sampled at the time of a surgical intervention performed as part of cancer treatment (surgery of metastases) or as part of symptomatic treatment (pathological fractures surgery, for example). Of interest, histopathological analysis of 52 tissue specimens (16%) did not diagnose mBC: in 3 cases (1%), specimens showed benign disease; in 7 cases (2%), a second malignancy was discovered (2 angiosarcoma, 1 lung cancer, 1 anal cancer, 1 neuroendocrine tumor, 1 pancreatic tumor, and 1 lymphoma); in 38 cases (12%), histopathological analysis could not conclude because the specimen was insufficient; in 4 cases (1%), analyses concluded a pathological complete response caused by preoperative therapy. Patients with second malignancies were no longer treated as patients with metastatic breast cancer, with the exception of one patient. In all, 2% of specimen collection procedures result in complications grade 3 or 4. One biopsy (0.3%) led to patient's death caused by hemorrhage.
Figure 2.
Sites of specimen collection.
Comparison of Receptor Status Between Primary Tumor and Metastatic Relapse
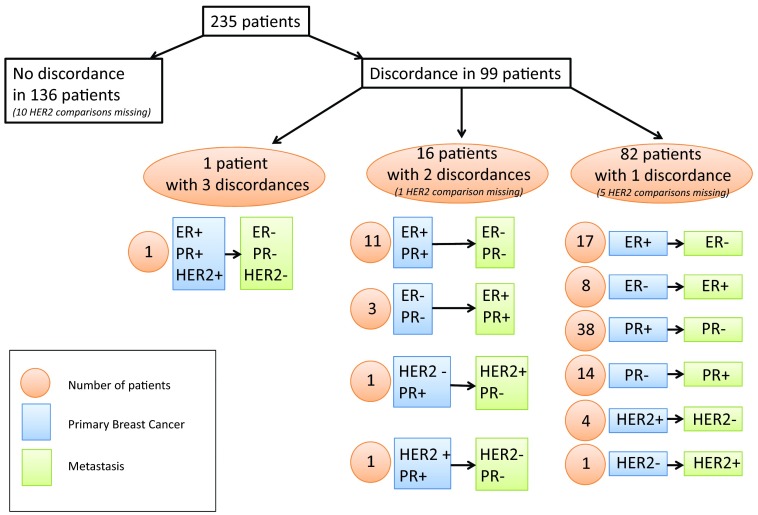
Comparison of receptor status between primary tumor and metastatic relapse was assessed in patients with histologically proven mBC (n = 235). Cases available for concordance's analysis were 235 for ER and PR status and 219 for HER2 status. Table 2 summarized concordance and discordance in receptors' expression. Discordance in one or more receptors (Fig. 3) between primary breast cancer and metastatic lesion was found in 99 patients (42%). Conversion was mainly from positive to negative. A switch in receptor status was identified for ER in 40 tumors (17%; κ = 0.50, 95% CI: 0.37–0.63; p = 4 × 10−3): 29 (12%) conversions from ER-positive to ER-negative lesions and 11 (5%) conversions from ER-negative to ER-positive tumors. A change in PR was noted in 69 cancer cases (29%; κ = 0.42, 95% CI: 0.31–0.53; p < 4 × 10−4). Two cancers (1%) became HER2-positive and six tumors (3%) went from HER2-positive to HER2-negative: concordance rate for HER2 status was 96% (κ = 0.82, 95% CI: 0.79–0.96; p =.16).
Table 2.
Concordance and discordance in receptor expression status between primary tumor and metastasis

ap < .05.
Abbreviations: ER, estrogen receptor; PR, progesterone receptor.
Figure 3.
Consolidated Standards of Reporting Trials diagram.
Abbreviations: ER, estrogen receptor; PR, progesterone receptor.
Factors Associated With Receptors Expression Discordance
In univariate analysis, various factors were tested to search for correlation with ER, PR, HR, and HER2 discordance (Table 3). Chemotherapy and anthracycline-based treatment received before the metastatic specimen collection (either as adjuvant therapy or as therapy in the metastatic setting) was significantly correlated with ER discordance (p = .0038 and p = .008, respectively). Regarding HER2 status modifications, eight tumors had a modification in HER2 expression (two gains, six losses); five of them (62.5%) were exposed to trastuzumab between primary sample collection and mBC sample collection. Among the 211 cancers with no change in their HER2 status, only 17 tumors (8%) received prior trastuzumab therapy. Therefore, previous trastuzumab therapy was statistically associated with changes in HER2 status (p < 10−4).
Table 3.
Univariate analyses of factors correlated with receptor expression discordance

Bold values indicate statistical significance.
Abbreviations: ER, estrogen receptor; mBC, metastatic breast cancer; PR, progesterone receptor.
The influence of previous adjuvant therapies on receptors' expression discordance was also assessed separately. Among the 235 patients with histologically proven mBC, 136 (58%) had received adjuvant chemotherapy (79 patients had received anthracycline-based chemotherapies, and 49 patients had received taxane plus anthracycline-based chemotherapies) and 125 (53%) had received adjuvant endocrine therapies. Adjuvant chemotherapy and adjuvant anthracycline-based chemotherapy were significantly correlated with ER discordance (p = .006 and .0039, respectively). Adjuvant trastuzumab is significantly correlated with HER2 discordance (p < 10−4). Metastatic sample obtained from liver surgery/biopsy were more likely to have a change in PR expression (p = .004). A nonsignificant trend to PR status modification was observed when PR expression was assessed on bone samples (p = .07). There was no relationship between interval of time between primary tumor diagnosis and metastatic specimen collection and receptors expression discordances (p > .05). Discordance rates were not statistically different between patients with metastatic disease at presentation and patients with a disease-free interval between primary tumor and metastatic disease (p > .05).
In a multivariate model, ER discordance was significantly correlated with previous exposure to anthracyclines (odds ratio [OR]: 0.331, 0.137–0.719; p < 8 × 10−3), with a nonsignificant trend for samples obtained from liver metastases (OR: 0.474, 0.225–1.023; p = .052). For PR, discordance was significantly correlated with samples obtained from liver metastases (OR: 0.409, 0.217–0.771; p = 6 × 10−3).
Survival Analyses
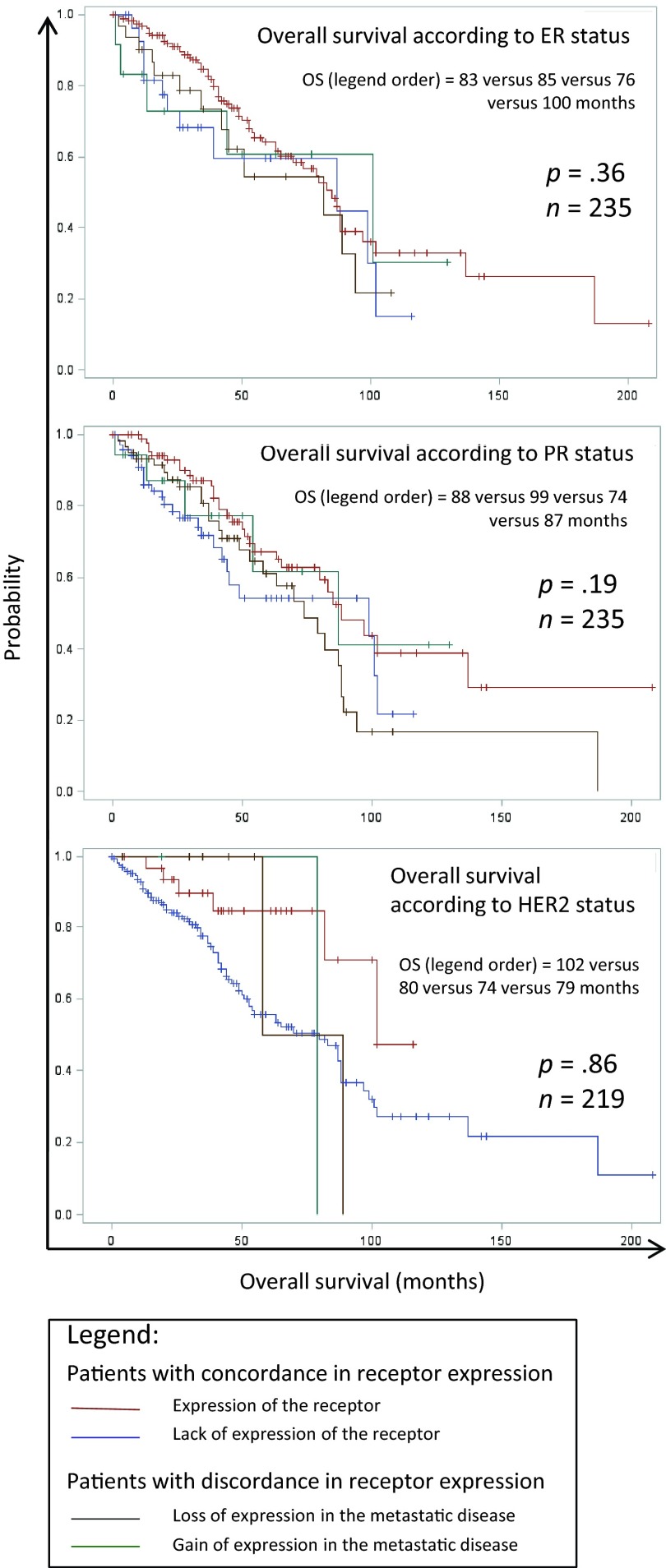
Median overall survival was 49 months (95% CI: 43–58 months). Patients with tissue specimen collection had a significantly better median overall survival compared with patients without metastatic specimen collection: 79 months (95% CI: 63–88) versus 32 months (95% CI: 28–39; p = 10−4). Discordance in ER status, PR status, and HER2 status did not significantly affect patients' overall survival (p > .05). Survival curves for the different subgroups (disease with discordance/concordance for ER, PR, and HER2) are shown in Figure 4.
Figure 4.
Kaplan-Meier curves for overall survival according to estrogen receptor, progesterone receptor, and HER2 expressions in the primary tumor and in the metastatic disease.
Abbreviations: ER, estrogen receptor; OS, overall survival; PR, progesterone receptor.
Discussion
In this retrospective study, discordance in ER status, PR status, and HER2 status between primary breast cancer and metastatic lesion respectively occurred in 17%, 29%, and 4% of cases. Conversions were mainly from positive to negative and discordances in ER status and PR status were statistically significant, whereas rate of concordance in HER2 status was high. Factors significantly associated with changes in receptors status were chemotherapy and anthracycline-based chemotherapy received before the second specimen collection or received as adjuvant therapies for ER expression, liver tissue specimen for PR status, and previous trastuzumab treatment for HER2 status. Tumors exposed to chemotherapy and more specifically to anthracycline showed a higher discrepancy rate in their ER status. These observations might suggest that exposure to systemic treatments trigger changes in the tumor profile of receptors' expression. However, association between prior trastuzumab and HER2 status change should be interpreted with caution [3] and did not involve a causal relationship. HER2 conversions were mainly from positive to negative, and patients with HER2-positive primary breast cancer diagnosed after 2005 received adjuvant trastuzumab, a humanized monoclonal antibody directed against HER2. It at least partly explains the high percentage of patients with HER2 modifications previously exposed to trastuzumab and the statistically significant association between HER2 changes and trastuzumab exposure. The level of benign disease diagnosis was low because all patients included in the database received therapy for mBC. Thus, all of the three patients with a benign lesion had mBC histologically proven by a second sample collected in another localization. The rate of second malignancy diagnosis reached 2%.
Two major biases can be highlighted in this study: a methodological bias due to the retrospective assessment of ER, PR, and HER2 expression and a selection bias. With respect to the first one, bioassays were not done simultaneously but rather when the samples of primary tumor and metastatic disease were collected; also, three different laboratories did 98.3% of the analyses. Different antibodies were used over time. However, all laboratories used external and internal controls, and internal controls were included in each slide with immunohistochemistry staining. Of note, when the same tumor sample is tested twice in different laboratories or even in the same unit with the same methods, ER and PR expression show important discordance rates, whereas HER2 staining is more reproducible [9]. Therefore, an unknown but significant portion of the discordance rate is linked to methodological and technical biases.
With respect to the second bias, the selection bias is inherent to all retrospective studies. Patients included in the study underwent a rebiopsy in the metastatic setting or a surgery of metastasis. The decision for rebiopsy and/or surgery was taken by the physician in agreement with the patient. To assess the selection bias, we compared the characteristics of the total population of patients with mBC to the population of patients with specimen collection in the metastatic setting. The two populations were different and patients with tissue specimen collection had different clinical and demographic characteristics, which confirms that the selection bias is important. Physicians were more likely to propose rebiopsy and/or surgery to patients with good prognosis factors (young age, long disease-free interval) who had received adjuvant chemotherapies and with soft-tissue metastases. They selected a population of patients statistically different from the general population of patients with mBC, characterized by a long overall survival. Three parameters can explain the prolonged overall survival. First, it is a population with favorable prognostic factors. Second, 37% of patients benefited from surgery for their metastases. According to our local recommendations, patients who underwent surgery of metastases were more likely to have a lower tumor burden, which is a factor associated with long-term complete response [10] and better overall survival. Moreover, surgery of metastases might give favorable results in a small subset of patients [11]. Third, two thirds of metastatic specimens were collected after January 1, 2006, so patients with metastatic specimen collection were more likely to benefit from new treatments such as trastuzumab or taxanes or bevacizumab during their metastatic disease than patients without metastatic specimens.
This study also presented several major strengths: It is a large collection of samples with paired primary and metastatic samples; all specimen collections were recorded including insufficient samples, samples with benign disease or second malignancy; factors were correlated with discordance in receptors expression in univariate and multivariate analysis; modalities and complications of specimen collections were collected. Moreover, we report here for the first time a statistically significant correlation between previous systemic treatments and modifications in receptors' expression.
Several studies have addressed the issue of concordance/discordance in receptors expression between primary tumor and metastases [9, 12–24]. Published studies have found discordance rates for ER status ranging from 10.2% to 56%, PR status ranging from 24.8% to 48.6%, and HER2 status ranging from 2.9% to 16%. The discrepancies rates reported in our study (17%, 29%, and 4% for ER, PR, and HER2 status, respectively) are consistent with those reported in literature. A recent large prospective trial [24] confirmed the possibility of changes in receptors expression throughout tumor progression with relatively high discordance rates (16%, 40%, and 10% for ER, PR, and HER2 status, respectively).
The prognostic impact of discordance between receptors expression in primary and metastatic breast cancer is doubtful [9, 22–24]. A large retrospective study concluded that discordant cases have poor survival rates [23], but a prospective trial did not highlight detrimental effects of discordance on outcome [24]. Our study failed to demonstrate an impact of discordance on survival. Some published data confirmed that a confirmatory biopsy alters management in 15%–30% of patients [19–21]. Changes in HER2 status and in hormone receptor ER status can alter patients' treatments modifying the indications of HER2 targeted therapies or endocrine therapies. In our study, we had no information about whether there was a change in management based on the results of biopsy. We could only suppose that changes in ER status (17% of patients) and in HER2 status (4% of patients) perhaps affected patients' treatments.
Changes in ER, PR, and HER2 expression should be construed carefully [2]. Discordances can result from at least three factors: false-positive and false-negative results in receptors expression assessment, heterogeneity of receptors expression in the same tumor and eventually modifications in the biology of the cancer [25, 26]. Relative contribution of these three parameters to changes in receptors expression is unknown. Technical variability in receptors expression measurements reflects the fact that any method is 100% accurate and reproducible. Inadequate fixation, bone decalcification, staining methods, and subjective scoring can explain some changes in receptors expression. Inadequate sampling of a heterogeneous cancer can also give erroneous results.
Evolution in the biology of the tumor during the disease can also be responsible for a real switch in receptor expression. There is emerging evidence that tumor characteristics and molecular profile may change during the natural history of the disease. Modifications can occur either spontaneously or as a consequence of mechanisms of resistance to systemic therapies. Two main hypotheses can explain the modifications of receptors expression during the evolution of the disease: the selection of a small aggressive clone of the primary tumor or the development of a new clone derived from cells of the primary tumor. Massive DNA sequencing technologies permit the screening of entire genomes to identify genetic changes associated with tumor progression. A recent study [27] established the genomic profile of a triple-negative primary breast tumor and its paired brain metastasis. The differential mutation frequencies and structural variation patterns in metastasis compared with the primary tumor indicate that secondary tumors may arise from a minority of cells within the primary tumor. Changes in protein expression may be the consequences of transcriptional (genetic or epigenetic alterations) or post-transcriptional modifications. Different mechanisms of resistance to endocrine treatment [28] and HER2-targeted therapy [29, 30] are described, including loss of receptor expression, expression of truncated isoforms, or post-translational modifications of receptors. Loss of expression of ER or HER2 assessed by immunohistochemistry can reflect one of these mechanisms of resistance.
Conclusion
In conclusion, this study confirms that discordance in ER and PR receptor expression between the primary breast tumor and the corresponding metastatic lesions is high, whereas HER2 status remains relatively constant. Importantly, we show for the first time that previous chemotherapy, specifically anthracycline-based chemotherapy, was associated with switch in ER status. These results were obtained in a selected population of patients with good prognostic factors and long overall survival; further studies are warranted to confirm these data and to determine the interest of systematic rebiopsy in the metastatic setting. In the era of a more personalized approach [31, 32] to medicine and of genomic investigations of tumors, reliable and recent evaluation of tumor prognostic and predictive factors remains a challenge.
Author Contributions
Conception/Design: Elsa Curtit, Philippe Montcuquet, Nathalie Meneveau, Xavier Pivot
Provision of study material or patients: Loic Chaigneau, Laurent Cals, Cristian Villanueva, Fernando Bazan, Philippe Montcuquet, Nathalie Meneveau, Xavier Pivot
Collection and/or assembly of data: Elsa Curtit, Virginie Nerich, Laura Mansi, Loic Chaigneau, Laurent Cals, Cristian Villanueva, Fernando Bazan, Philippe Montcuquet, Sophie Perrin, Marie-Paule Algros, Xavier Pivot
Data analysis and interpretation: Elsa Curtit, Virginie Nerich, Laura Mansi, Loic Chaigneau, Laurent Cals, Cristian Villanueva, Fernando Bazan, Philippe Montcuquet, Nathalie Meneveau, Sophie Perrin, Marie-Paule Algros, Xavier Pivot
Manuscript writing: Elsa Curtit, Virginie Nerich, Laura Mansi, Loic Chaigneau, Laurent Cals, Cristian Villanueva, Fernando Bazan, Philippe Montcuquet, Nathalie Meneveau, Sophie Perrin, Marie-Paule Algros, Xavier Pivot
Final approval of manuscript: Elsa Curtit, Virginie Nerich, Laura Mansi, Loic Chaigneau, Laurent Cals, Cristian Villanueva, Fernando Bazan, Philippe Montcuquet, Nathalie Meneveau, Sophie Perrin, Marie-Paule Algros, Xavier Pivot
Disclosures
Xavier Pivot: Roche; Philippe Montcuquet: GSK; Elsa Curtit: Novartis. The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Reference
- 1.Cardoso F, Costa A, Norton L, et al. First international consensus guidelines for advanced breast cancer (ABC 1) Breast. 2012;21:242–252. doi: 10.1016/j.breast.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Amir E, Clemons M. Should a biopsy be recommended to confirm metastatic disease in women with breast cancer? Lancet Oncol. 2009;10:933–935. doi: 10.1016/S1470-2045(09)70295-5. [DOI] [PubMed] [Google Scholar]
- 3.Brennan MJ, Donegan WL, Appleby DE. The variability of estrogen receptors in metastatic breast cancer. Am J Surg. 1979;137:260–262. doi: 10.1016/0002-9610(79)90159-4. [DOI] [PubMed] [Google Scholar]
- 4.Brunn Rasmussen B, Kamby C. Immunohistochemical detection of estrogen receptors in paraffin sections from primary and metastatic breast cancer. Pathol Res Pract. 1989;185:856–859. doi: 10.1016/s0344-0338(89)80286-9. [DOI] [PubMed] [Google Scholar]
- 5.Wilking U, Karlsson E, Skoog L, et al. HER2 status in a population-derived breast cancer cohort: Discordances during tumor progression. Breast Cancer Res Treat. 2011;125:553–561. doi: 10.1007/s10549-010-1029-2. [DOI] [PubMed] [Google Scholar]
- 6.Simmons C, Miller N, Geddie W, et al. Does confirmatory tumor biopsy alter the management of breast cancer patients with distant metastases? Ann Oncol. 2009;20:1499–1504. doi: 10.1093/annonc/mdp028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Balaton AL, Coindre JM, Collin F, et al. Recommendations for the immunohistochemical evaluation of hormone receptors on paraffin sections of breast cancer. Ann Pathol. 1996;16:144–148. [PubMed] [Google Scholar]
- 8.Penault-Llorca F, Vincent-Salomon A, Bellocq JP, et al. Update of the GEFPICS' recommendations for HER2 status determination in breast cancers in France. Ann Pathol. 2012;30:357–373. doi: 10.1016/j.annpat.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Liedtke C, Broglio K, Moulder S, et al. Prognostic impact of discordance between triple-receptor measurements in primary and recurrent breast cancer. Ann Oncol. 2009;20:1953–1958. doi: 10.1093/annonc/mdp263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenberg P, Hortobagyi G, Smith T, et al. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J Clin Oncol. 1996;14:2197–2205. doi: 10.1200/JCO.1996.14.8.2197. [DOI] [PubMed] [Google Scholar]
- 11.Pagani O, Senkus E, Wood W, et al. International guidelines for management of metastatic breast cancer: Can metastatic breast cancer can be cured? J Natl Cancer Inst. 2010;102:456–463. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong Y, Han EY, Guo M, et al. Stability of estrogen receptor status in breast carcinoma: A comparison between primary and metastatic tumors with regard to disease course and intervening systemic therapy. Cancer. 2011;117:705–713. doi: 10.1002/cncr.25506. [DOI] [PubMed] [Google Scholar]
- 13.Amir E, Ooi WS, Simmons C, et al. Discordance between receptor status in primary and metastatic breast cancer: An exploratory study of bone and bone marrow biopsies. Clin Oncol. 2008;20:763–768. doi: 10.1016/j.clon.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Macfarlane R, Seal M, Speers C, et al. Molecular alterations between the primary breast cancer and the subsequent locoregional/metastatic tumor. The Oncologist. 2012;17:172–178. doi: 10.1634/theoncologist.2011-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broom RJ, Tang PA, Simmons C, et al. Changes in estrogen receptor, progesterone receptor and Her-2/neu status with time: Discordance rates between primary and metastatic breast cancer. Anticancer Res. 2009;29:1557–1562. [PubMed] [Google Scholar]
- 16.Hoefnagel LD, van de Vijver MJ, van Slooten HJ, et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2009;2:R75. doi: 10.1186/bcr2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson AM, Jordan LB, Quinlan P, et al. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: The Breast Recurrence In Tissues Study (BRITS) Breast Cancer Res. 2010;12:R92. doi: 10.1186/bcr2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilton JF, Amir E, Hopkins S, et al. Acquisition of metastatic tissue from patients with bone metastases from breast cancer. Breast Cancer Res Treat. 2011;129:761–765. doi: 10.1007/s10549-010-1264-6. [DOI] [PubMed] [Google Scholar]
- 19.Curigliano G, Bagnardi V, Viale G, et al. Should liver metastases of breast cancer be biopsied to improve treatment choice? Ann Oncol. 2011;22:2227–2233. doi: 10.1093/annonc/mdq751. [DOI] [PubMed] [Google Scholar]
- 20.Guarneri V, Giovannelli S, Ficarra G, et al. Comparison of HER-2 and hormone receptor expression in primary breast cancers and asynchronous paired metastases: Impact on patient management. The Oncologist. 2008;13:838–844. doi: 10.1634/theoncologist.2008-0048. [DOI] [PubMed] [Google Scholar]
- 21.Amir E, Clemons M, Purdie CA, et al. Tissue confirmation of disease recurrence in breast cancer patients: Pooled analysis of multi-centre, multi-disciplinary prospective studies. Cancer Treat Rev. 2012;38:708–714. doi: 10.1016/j.ctrv.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Botteri E, Disalvatore D, Curigliano G, et al. Biopsy of liver metastasis for women with breast cancer: Impact on survival. Breast. 2012;21:284–288. doi: 10.1016/j.breast.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 23.Lindström LS, Karlsson E, Wilking UM, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012;30:2601–2608. doi: 10.1200/JCO.2011.37.2482. [DOI] [PubMed] [Google Scholar]
- 24.Amir E, Miller N, Geddie W, et al. Prospective study evaluating the impact of tissue confirmation of metastatic disease in patients with breast cancer. J Clin Oncol. 2012;30:587–592. doi: 10.1200/JCO.2010.33.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pusztai L, Viale G, Kelly CM, et al. Estrogen and HER-2 receptor discordance between primary breast cancer and metastasis. The Oncologist. 2010;15:1164–1168. doi: 10.1634/theoncologist.2010-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khasraw M, Brogi E, Seidman AD. The need to examine metastatic tissue at the time of progression of breast cancer: Is re-biopsy a necessity or a luxury? Curr Oncol Rep. 2011;13:17–25. doi: 10.1007/s11912-010-0137-9. [DOI] [PubMed] [Google Scholar]
- 27.Ding L, Ellis MJ, Li S, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 29.Mukohara T. Mechanisms of resistance to anti-human epidermal growth factor receptor 2 agents in breast cancer. Cancer Sci. 2011;102:1–8. doi: 10.1111/j.1349-7006.2010.01711.x. [DOI] [PubMed] [Google Scholar]
- 30.Pohlmann PR, Mayer IA, Mernaugh R. Resistance to trastuzumab in breast cancer. Clin Cancer Res. 2009;15:7479–7491. doi: 10.1158/1078-0432.CCR-09-0636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hortobagyi GN. Toward individualized breast cancer therapy: Translating biological concepts to the bedside. The Oncologist. 2012;17:577–584. doi: 10.1634/theoncologist.2012-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dawood S, Gonzalez-Angulo AM. To biopsy or not to biopsy: Is that the only question? The Oncologist. 2012;17:151–153. doi: 10.1634/theoncologist.2011-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]