Metastasis to the central nervous system (CNS) is a devastating neurological complication of cancer. CNS metastases among women with breast cancer tend to occur among those who are younger, have larger tumors, and have certain histological subtypes. Treatment involves combinations of whole brain radiation therapy, surgery, stereotactic radiosurgery, and chemotherapy.
Keywords: CNS metastases, Leptomeningeal disease, Breast cancer, Biology, Treatment
Abstract
Metastasis to the central nervous system (CNS) is a devastating neurological complication of systemic cancer. Brain metastases from breast cancer have been documented to occur in approximately 10%–16% of cases over the natural course of the disease with leptomeningeal metastases occurring in approximately 2%–5% of cases of breast cancer. CNS metastases among women with breast cancer tend to occur among those who are younger, have larger tumors, and have a more aggressive histological subtype such as the triple negative and HER2-positive subtypes. Treatment of CNS metastases involves various combinations of whole brain radiation therapy, surgery, stereotactic radiosurgery, and chemotherapy. We will discuss the progress made in the treatment and prevention of breast cancer-associated CNS metastases and will delve into the biological underpinnings of CNS metastases including evaluating the role of breast tumor subtype on the incidence, natural history, prognostic outcome, and impact of therapeutic efficacy.
Implications for Practice:
With progress in more effective treatment strategies that control systemic disease and advanced diagnostic imaging modalities that allow for more accurate and earlier diagnosis, the incidence of central nervous system (CNS) metastases is currently on the rise. This article focuses on current management strategies available for breast cancer-associated CNS metastases. We also summarize our current understanding about the biology of breast cancer CNS metastases and how that knowledge is being used to improve therapeutic strategies. Whole brain radiation therapy (WBRT), surgery, and sterotactic surgery remain the standard of care for CNS metastases, however, it is increasingly being recognized that systemic therapies could be effective. Accumulating data indicate that breast cancer subtype influences incidence and survival following a diagnosis of CNS metastases. Recent trials have also focused on using agents geared to specific subtypes in the hope of improving prognostic outcome following a diagnosis of CNS metastases.
Introduction
Metastasis to the central nervous system (CNS) is a devastating neurological complication of systemic cancer. The CNS is composed of the brain, spinal cord, leptomeninges, and eyes with each component being at risk of developing metastases from a variety of solid tumors. With progress in more effective treatment strategies that control systemic disease and advanced diagnostic imaging modalities that allow for more accurate and earlier diagnosis, the incidence of CNS metastases is currently on the rise [1]. In the United States, the annual incidence of CNS metastases is estimated to be approximately 170,000 cases [2, 3] that are associated with a poor median survival of approximately 7 months even among patients with a good performance status and controlled extracranial disease [4]. Brain metastases from breast cancer have been documented to occur in approximately 10%–16% of cases over the natural course of the disease [5], making breast cancer the second most common source of brain metastases lagging behind lung cancer. The total incidence of CNS metastases associated with breast cancer, however, may be much higher as currently the majority of CNS metastases are diagnosed in response to symptoms. When screening for asymptomatic CNS metastases, data indicates that 14.8% of patients have occult lesions [6], whereas autopsy data indicate that the percentage of breast cancer-afflicted cases may be closer to 30% [7]. Several studies have indicated that brain metastases among women with breast cancer tend to occur among those who are younger, have larger tumors, and have a more aggressive histological subtype such as the triple negative and HER2-positive subtypes [8–10]. Prognostic outcome among patients with breast cancer and brain metastases is poor, with 1- and 2- year survival rates estimated at 20% and 2%, respectively [11, 12]. The rate of breast cancer-associated leptomeningeal metastases has been documented to range from 2% to 5% with autopsy studies documenting a range of approximately 3% to 6% [13].
Over the decades, various strategies have been evaluated for the treatment of breast cancer-associated CNS metastases. Strategies have been developed aimed at improving locoregional control and include various combinations of surgery, whole brain radiation therapy (WBRT), stereotactic radiosurgery (SRS), and chemotherapy in combination with strategies aimed at reducing associated symptoms that arise because of the metastatic lesions including the appropriate use of corticosteroids to reduce peritumoral edema and anticonvulsants to prevent recurrent seizures. In this review, progress in the different strategies used to manage CNS metastases will be discussed. The last decade has also seen significant advances in our understanding of the biology of breast cancer-associated CNS metastases including the role of the blood–brain barrier (BBB), pericytes, astrocytes, and glial cells that in combination create an environment that protects the brain from systemic therapy used to treat the primary tumor while allowing for colonization of metastatic disease. Our understanding of the effect of these factors on the therapeutic response to developed treatment strategies will be explored in this review. Despite advances made, the prognostic outcome still remains poor once CNS metastases develop and as such there has been a keen interest in exploring possible prevention strategies that can be implemented, especially among certain subtypes of breast cancers such as those associated with HER2-positive disease. We will thus also review existing data and research strategies currently being evaluated with regard to both primary and secondary prevention strategies.
Biology of Breast Cancer-Associated CNS Metastases
The Blood–Brain Barrier
The BBB functions to regulate the passage of regulatory proteins, nutrients, neurotoxins, and electrolytes [14], as well as to prevent CNS entry of a number of chemotherapeutic and molecular targeted agents. Furthermore, it contains a number of efflux pumps (e.g., P-glycoprotein) that can actively remove chemotherapeutic agents [15]. Collectively, the functions of the BBB allow the CNS to function as a sanctuary site for the seeding of metastases. As such, there has been a keen interest in looking at ways to disrupt the BBB to allow for agent entry to subsequently enhance therapeutic efficacy.
Several interesting observations about the BBB have been made. First, as metastatic lesions within the brain grow beyond 1 mm, the BBB becomes functionally comprised [16], and this may be more so within certain subtypes of breast cancers. Yonemori and colleagues [17] evaluated the BBB on a cohort of 29 patients, with breast cancer whose brain metastases was resected, by performing immunohistochemical staining for glucose transporter 1 (GLUT1) and breast cancer-resistance protein (BCRP). The investigators noted that disruption of the BBB was more often observed among patients with triple receptor negative breast cancer and more often preserved among patients with HER2-positive breast cancer. Second, evidence is accumulating that radiotherapy can affect the permeability of the BBB [18]. In a pilot study, Stemmler and colleagues [19] measured serum and cerebrospinal fluid (CSF) trastuzumab (a monoclonal antibody that is too large to cross the BBB) before and after radiotherapy among six patients with breast cancer and brain metastases. The authors reported a serum to CSF trastuzumab level ratio of 420:1 preradiotherapy and 76:1 postradiotherapy. Furthermore, the ratio was reported as 49:1 postradiotherapy if there was evidence of concomitant leptomeningeal disease. Collectively, the results indicate that increased penetration of monoclonal antibodies such as trastuzumab may be observed when BBB is disrupted by either radiotherapy or by the cancer itself. Third, although large molecules such trastuzumab are unable to cross an intact BBB, there is preclinical as well as limited clinical evidence that smaller tyrosine kinase inhibitor molecules such as lapatinib can cross the BBB [20–22].
Drawing on the above observations, one could hypothesize that when treatment of CNS metastases with systemic agents, timing of administration of the agent (e.g., following radiation therapy) and the subtype of breast cancer, both of which dictate BBB permeability, are important. However, despite all the preclinical and clinical evidence to suggest increased penetration of therapeutic agents upon disruption of the BBB, this may not always translate into increased therapeutic efficacy toward CNS metastases. For example, one of the lowest CNS to blood ratios is observed for temozolamide [23], which is considered one of the most effective agents for the treatment of primary CNS tumors, indicating that therapeutic efficacy may depend on not only penetration of the BBB but also properties of the agent under investigation as well as sensitivity of the tumor to that agent.
Genes Mediating CNS Metastases
Gene expression analysis of cells that preferentially infiltrate the brain isolated from patients with advanced disease has identified several mediators of cancer cell passage through BBB [24]. These include cyclooxygenase (COX)-2 and epidermal growth factor receptor (EGFR) ligand HBEGF that have also been linked to the development of pulmonary metastases. ST6GALNAC5 (a sialyltransferase) has also been identified that specifically mediates brain metastases through promotion of breast cancer cells through the BBB and allowing for enhanced adhesion to brain endothelial cells.
The Influence of Breast Cancer Subtype
One of the most important advances in breast cancer over the past 2 decades has been the understanding that breast cancer is not a homogenous disease but a heterogeneous one made up of at least four different subtypes. These include luminal subtypes that are predominantly hormone receptor-positive, HER2-enriched subtype, and the basal-like subtype that is largely devoid of expression of hormone receptor and HER2 receptor (triple negative), each having a unique natural history and prognostic outcome [25]. Accumulating data from retrospective studies indicate that these molecular subtypes also influence incidence of and survival following development of brain metastases. Kennecke and colleagues [26] reported on 3,726 women with early breast cancer diagnosed between 1986 and 1992 that had been referred to the British Columbia Cancer Agency. The authors reported lower 15-year cumulative incidence rates of brain metastases among patients with luminal subtype breast cancer, whereas those with HER2 positive/hormone receptor negative disease and those with basal-like tumors had higher rates of 14.3% and 10.9%, respectively. Berghoff and colleagues [27] recently reported on the interval from the diagnosis of extracranial metastases to the development of brain metastases stratified by breast tumor subtype in a cohort of 213 women with metastatic breast cancer between 1996 and 2010. The authors reported a brain metastases-free survival of 14, 18, and 34 months among patients with triple negative, HER2-positive, and luminal tumors, respectively. The introduction of trastuzumab has also impacted the incidence of brain metastases among women with HER2-positive breast cancer as a result of better control of extracranial disease and the fact that the large molecular size of trastuzumab inhibits its penetration through the BBB, allowing making the CNS a sanctuary site for seeding of disease. Using data derived from the Parma Province Cancer Registry in Italy, Musolino and colleagues [28] reported that, among women with HER2-positive early-stage breast cancer who did not receive trastuzumab, 1.6% developed brain metastases, whereas 10.5% of those with HER2-positive breast cancer who received trastuzumab developed brain metastases. A meta-analysis of the three large randomized clinical trials (NSABP B-31, NCCTG N9831, and HERA) investigating adjuvant trastuzumab among women with HER2-positive early stage breast cancer has also shown a significantly increased incidence of brain metastases in the trastuzumab arm of the studies (relative risk 1.57, 95% confidence interval 1.03–2.37; p = .033), without significant heterogeneity (p = .27) and a reported absolute difference of .62 [29]. This translated into having to treat over 160 patients with HER2-positive disease to observe one CNS event.
Survival following development of brain metastases is also influenced by breast tumor subtype with cause of death among women with triple negative breast cancer and brain metastases attributed primarily to progression of extracranial metastases, whereas up to 50% of patients with HER2-positive breast cancer and brain metastases die of progressive disease in the CNS [30]. In a recent study, Vern-Gross and colleagues [31] reported on patterns of failure and survival following gamma-knife radiosurgery of breast cancer-associated brain metastases. At a median follow-up of 54 months, the authors reported a median overall survival of 7, 9, 11, and 22 months among patients with basal, luminal, HER2, and luminal/HER2 breast tumor subtypes, respectively (p = .001). Furthermore, the authors observed that breast cancer subtype did not predict for local failure (i.e., at the site of gamma-knife radiosurgery), but did significantly predict for distant brain failure (i.e., at a site in the brain away from the original site of radiosurgery). In a recent study by Sperduto and colleagues [32], the investigators put forth the breast-graded prognostic assessment tool (Breast-GPA) that is able to predict prognostic outcome following a diagnosis of brain metastases based on factors such as age, KPS, hormone receptor status, and HER2 status. The investigators were able to show a clear separation in prognostic outcome between different subgroups of patients with breast cancer and brain metastases indicating that heterogeneity exists in the natural history following the development of brain metastases. When taken together, the data indicate that, in the setting of brain metastases, it may be prudent to design clinical trials that will explore individualized treatment options with the goal of attaining the best prognostic outcome.
Symptomatic Management of CNS Metastases
Several symptoms are associated with the presence of CNS metastases, which include those caused by the development of peritumoral edema (headache, nausea, vomiting, and mental status changes) and seizures. Reduction of the pressure effects caused by peritumoral edema is achieved with the use of corticosteroids. Ryken and colleagues conducted a systemic review of available evidence on how best to use corticosteroids in this setting [33]. Among patients with symptomatic brain metastases, a starting dose of 4–8 mg/day of dexamethasone should be considered. Higher doses such as 16 mg/day or more can be considered among those exhibiting severe symptoms consistent with increased intracranial pressure. Duration of corticosteroid administration was also addressed with the consensus that the duration of treatment should be individualized based on symptoms, and that tapering of dose should be conducted slowly over a minimum 2-week time period. Concurrent administration of trimethoprim–sulphamethaxozole should also be considered during prolonged administration of corticosteroids for the prevention of pneumocystis carinii pneumonitis.
Patients presenting with seizures should be treated with anticonvulsants such as phenytoin, carbamezapine, and sodium valproate [34]. Currently, there is no data to indicate clear and robust benefits from the routine prophylactic use of anticonvulsants. Therefore, routine use of anticonvulsants among patients with brain metastases who do not have seizures is not recommended [35].
Local Therapeutic Strategies
There are currently no published guidelines to specifically guide the treatment of CNS metastases that arise in the setting of breast cancer. Published treatment algorithms are targeted to patients with CNS metastases from a variety of solid tumors and are derived from studies in which the majority of patients enrolled had non-small cell lung cancer (currently the most common cause of brain metastases). From the current available evidence, it is not entirely clear whether the standard available treatments of whole brain radiation therapy (WBRT), surgery, and stereotactic radiosurgery (SRS) are equally efficacious in breast cancer compared with other solid tumors, and whether efficacy is the same within the different subtypes known to exist for breast cancer. Guidelines from the National Comprehensive Cancer Network (NCCN) are published and updated yearly that provide algorithms to guide treatments of patients with CNS metastases based on factors such as presenting performance status, number of brain metastases, and status of control of extracranial disease. Table 1 summarizes the most current NCCN recommendations [36].
Table 1.
Summary of NCCN recommendations for treatment of brain metastases
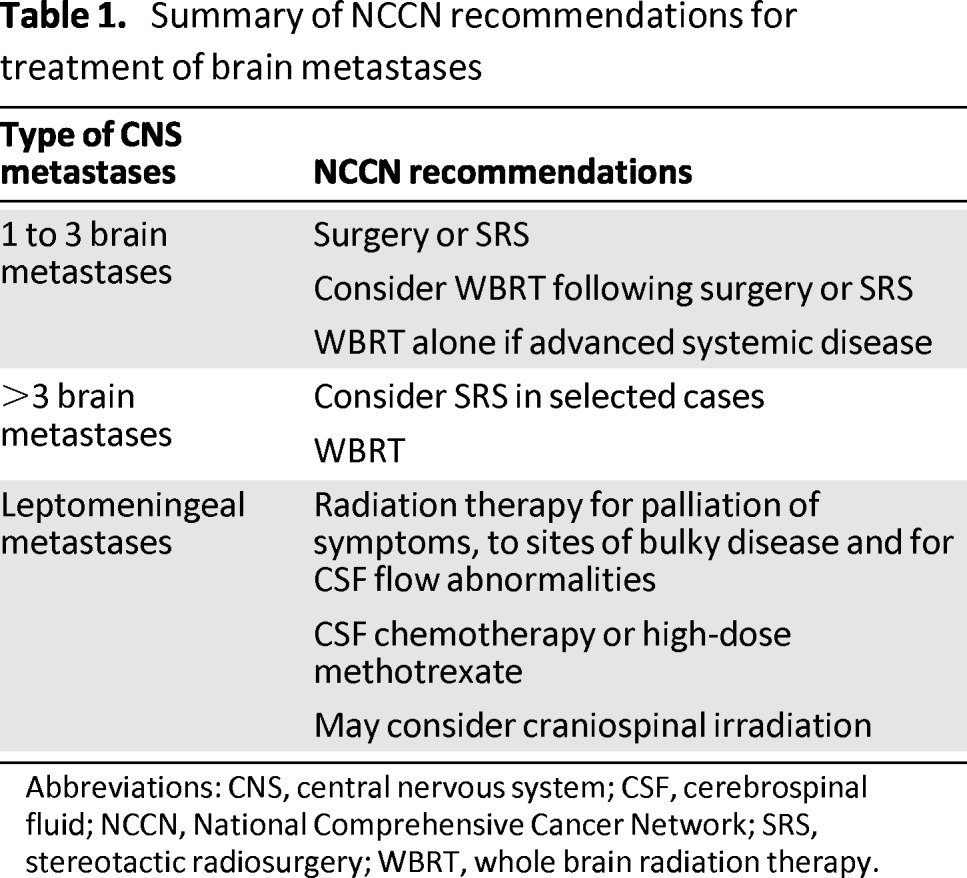
Abbreviations: CNS, central nervous system; CSF, cerebrospinal fluid; NCCN, National Comprehensive Cancer Network; SRS, stereotactic radiosurgery; WBRT, whole brain radiation therapy.
The treatment goals of patients with CNS metastases include palliation of symptoms, control of neurological disease, good quality of survival, and if possible, prolongation of survival. The number of brain metastases at presentation also heavily influences treatment strategies. WBRT has traditionally been the gold standard of treatment for brain metastases with its ability to cytoreduce metastatic deposits visible radiologically as well as to protect the rest of the brain from possible nonvisible micrometastatic disease. The landmark trial by Patchell and colleagues [37] randomized 48 patients (3 of whom had breast cancer) with single brain metastases to either surgery of the metastases and WBRT or WBRT alone. The investigators showed that local control (80% vs. 48%, p < .02) and median overall survival (40 vs. 15 weeks, p < .01) was better in the combination arm. The impact of combining SRS to WBRT has also been investigated in prospective clinical trials [38, 39]. In the RTOG, 9,508 trial patients with newly diagnosed one to three brain metastases were randomly assigned to SRS and WBRT or WBRT alone [36]. The authors reported improved functional autonomy for all patients in the combination arm and a survival benefit for patients with single unresectable brain metastasis who received SRS and WBRT. However, several questions still remain about the optimal strategy for treating brain metastases, which are addressed below.
Does the Addition of WBRT to Local Therapy Improve Outcome?
Table 2 summarizes the key clinical trials that have addressed the question of whether WBRT improves outcomes among patients with brain metastases undergoing local therapy with either surgery or SRS [40–44]. In the study by Patchell and colleagues [40], 95 patients (9 of whom had breast cancer) with solitary brain metastases were randomized to either surgery alone or surgery and WBRT. The investigators reported a significant decrease in locoregional recurrence (18% vs. 70%, p < .001) and decrease in rate of death from neurological disease (14% vs. 44%, p < .003) among patients who received combination treatment; there was no significant difference in overall survival or length of time that patients remained functionally independent observed between the two groups. Similarly, in a more recent study, Kocher and colleagues [44] randomized 359 patients with one to three brain metastases (42 of whom had breast cancer) who had either undergone surgery or SRS to either WBRT or observation. The investigators reported a significant decrease in locoregional recurrence (48% vs. 78%, p < .001) and death rate from neurological disease (28% vs. 44%, p = .002). However, the percentage of patients remaining alive and functionally independent at 2 years and median overall survival was not significantly different between the two groups. Taken collectively, data from prospective clinical trials indicate that the addition of WBRT to either surgery or SRS reduces intracranial failure without significantly impacting the overall survival. The question that arises then is whether everyone requires WBRT following local therapy (surgery or SRS)? In the realm of breast cancer, there is no clinical trial to address this question. One could hypothesize that subtypes of breast cancer that are more aggressive, such as triple receptor negative breast cancer, would have a higher rate of intracranial relapse that could impact overall survival, whereas patients with the HER2-positive subtype who, in the era of anti-HER2 therapy, have good systemic control of disease, live a lot longer, and could be comfortably managed should intracranial relapse occur (after close surveillance) with salvage therapy. Similarly possible long-term neurocognitive toxicities from WBRT may be more relevant among patients with subtypes of breast cancer who live longer than the more aggressive triple receptor negative subtype. It would be important for future clinical trials to address these issues specific to breast cancer-associated CNS metastases.
Table 2.
Randomized clinical trials of local therapy (surgery or stereotactic radiosurgery) with and without whole brain radiation therapy
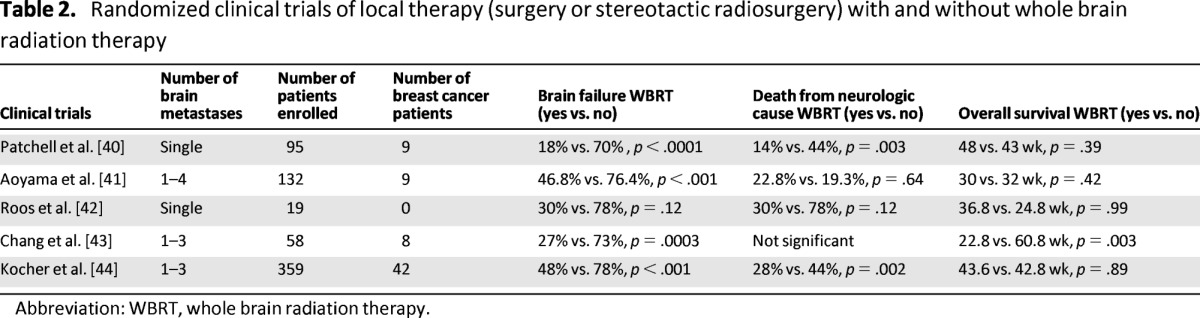
Abbreviation: WBRT, whole brain radiation therapy.
Can We Improve the Efficacy of WBRT?
A significant proportion of patients will be candidates only for WBRT, making it attractive to look at ways to improve its efficacy. Several clinical trials have looked at the question of different total doses and fractionation schedules to improve the outcome of WBRT. To date, none of the trials have demonstrated a meaningful improvement of survival or palliative effect [45]. More recent trials have explored the efficacy of an accelerated fractionation schedule and have reported some advantages in terms of intracranial control [46, 47]. The combination of WBRT and systemic therapy has also been investigated. Suh and colleagues [48] looked at the addition of efaproxiral (which enhances tumor oxygenation and radiation sensitivity) to WBRT. In the overall cohort, no difference was observed with the addition of efaproxiral in terms of survival and death by neurological progression. However, the authors noted a significant benefit with addition of efaproxiral in terms of response rate and survival among patients with breast cancer. In a phase III randomized clinical trial, WBRT was compared with WBRT plus concomitant temozolamide among 134 patients with brain metastases from a variety of primary tumors [49]. The authors reported an improved response rate (53.4% vs. 33.3%), time to progression (7.4 vs. 5.9 months, p = .0007), and median survival (7.9 vs. 4.3 months) among patients who underwent WBRT and temozolamide compared with those who received only WBRT. However, it should be noted that this trial has only been presented in abstract form and that 82% of patients had non-small cell lung cancer, and thus the precise efficacy of this regimen among patients with brain metastases and breast cancer is unknown. Other agents such as carboplatin [50], topotecan [51], thalidomide [52], and capecitabine [53] have also been investigated in randomized clinical trials in combination with WBRT with minimal, if any, benefit observed.
What Is the Best Strategy for Five or More Brain Metastases?
There is currently no level-one evidence for the use of SRS among patients with multiple brain metastases. Most of the available data are retrospective in nature. The available data so far indicate two things. First, retrospective data indicate that among patients with multiple brain metastases those treated with SRS alone (compared with those treated with WBRT alone) had longer overall survival, low morbidity, and good quality of life [54–57]. Second, accumulating data indicate that among patients treated with SRS the number of brain metastases may not necessarily predict survival [58–60]. Interestingly, despite lack of any prospective data, results of a survey conducted among radiosurgeons in 2010 indicated that more than half of the participants in the survey considered it reasonable to use SRS as the initial treatment of choice among patients with five or more brain metastases [61]. The best strategy would probably be to individualize a treatment plan based on factors such as tumor type, performance status, and extent of extracranial disease.
… retrospective data indicate that among patients with multiple brain metastases those treated with SRS alone (compared with those treated with WBRT alone) had longer overall survival, low morbidity, and good quality of life. Second, accumulating data indicate that among patients treated with SRS the number of brain metastases may not necessarily predict survival.
Does Breast Tumor Subtype Influence Efficacy of WBRT and Local Therapy?
Currently, there are no prospective data to suggest a differential impact of breast tumor subtype on the efficacy of WBRT and local therapy. Two prospective studies among patients with HER2-positive breast cancer and brain metastases who received WBRT concurrently with an anti-HER2 agent (lapatinib and trastuzumab) have reported an overall response rate of approximately 70% [62, 63]. Retrospective studies have evaluated the impact of breast tumor subtypes on outcome following SRS [64, 65]. O'Meara and colleagues [65] reported that the development of brain metastases following SRS was significantly lower among patients with HER2-positive disease compared with those with HER2-negative disease (1-year rate 56% vs. 85%, p = .03). However, whether this is more a function of patients with HER2-positive disease living longer because of better control of extracranial disease or whether it is a true impact of breast tumor subtype on SRS efficacy is unknown.
Systemic Therapies for CNS Metastases
There are currently no systemic agents approved by the Food and Drug Administration (FDA) for the treatment of brain metastases arising from breast cancer. Traditionally, chemotherapy has played a limited role in the treatment of brain metastases among patients with breast cancer because of the fact that it was believed that most agents could not cross the BBB. With a better understanding that the BBB is at least partially disrupted in brain metastases, the fact that responses of brain metastases to chemotherapy have been reported in breast cancer and the inclination to avoid the development of long-term neurotoxicities associated with WBRT among patients with certain breast tumor subtypes who live longer, there is now a revived interest in investigating the role of chemotherapy as the initial treatment of brain metastases among patients with breast cancer [66–69]. Rosner and colleagues [66] reported one of the earlier studies looking at the efficacy of systemic chemotherapy on brain metastases. The investigators reported on 100 consecutive patients with breast cancer and symptomatic brain metastases treated with systemic chemotherapy (CFP: cyclosphosphamide [C], 5-fluoruracil [F], prednisone [P]; CFP-MV: methotrexate [M], vincristine [V]; MVP and CA: Adriamycin [A]) among whom an objective response was demonstrated in approximately 50% of cases. Median duration of response was reported to be more than 10 months among patients exhibiting a complete response and 7 months among patients exhibiting a partial response. Boogerd and colleagues [69] recently reported on a retrospective study of 115 breast cancer patients who underwent surgery, WBRT, WBRT and systemic chemotherapy, or systemic chemotherapy alone as initial treatment for their brain metastases. Systemic therapy used included CMF, FAC, taxanes, capecitabine, and hormone therapy. The authors reported a response rate of 100%, 85%, 87%, 70%, and 14% among patients who had undergone surgery, WBRT, WBRT and systemic therapy, chemotherapy, or hormone therapy, respectively. Time to neurological progression among patients who had received chemotherapy alone was approximately 8 months. Currently, there are no large prospective clinical trials that have looked at systemic therapy as the initial treatment of brain metastases among patients with brain metastases. Several trials have included smaller breast cohorts who may have received prior WBRT. These studies have reported CNS response rates of up to 40% [70]. A study that evaluated the use of high dose methotrexate in a cohort largely composed of breast cancer patients noted a disease control rate of 56% [71]. Temozolomide, an alkylating agent known to be active in primary brain tumors, has also been investigated prospectively as either a single agent or in combination with other chemotherapeutic agents [72, 73]. In a multicenter phase II study, Siena and colleagues [72] reported on the efficacy of temozolamide among patients with a variety of solid tumors (including breast cancer) who had brain metastases. Among the 157 patients evaluated, the authors reported a 6% and 20% partial response and stable disease, respectively, in the brain. Among patients with breast cancer, the authors reported a median progression-free survival of 58 days. Christodoulou and colleagues [73] evaluated the efficacy of the combination of temozolamide and cisplatin among 32 patients with a variety of solid tumors (including breast cancer) and brain metastases. The authors reported that the combination was well-tolerated and with a partial response and stable disease reported in 28.1% and 16% of cases, respectively. Newer agents such as epothilones (e.g., patupilone and sogapilone) have also been investigated for the treatment of brain metastases arising from breast cancer with CNS response rates of 13%–19% and time to progression of CNS disease of 1.4–2.8 months reported [74, 75].
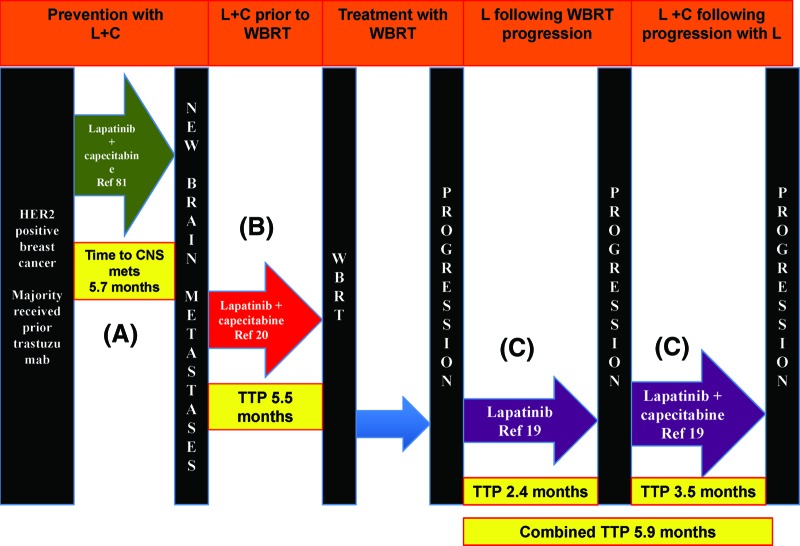
Patients with HER2-positive breast cancer with brain metastases constitute a unique cohort where the introduction of the monoclonal antibody trastuzumab has changed the natural history of this disease. Several retrospective studies indicate that use of trastuzumab not only increased the time to development of brain metastases but also has improved survival following the development of brain metastases [64, 76, 77]. Trastuzumab is a large monoclonal antibody that is known to be too large to cross the BBB. Its exhibited efficacy among patients with brain metastases is likely a result of a combination of increased control of extracranial disease as well-limited penetration through the BBB secondary to disruption of the BBB by the metastases and WBRT. Smaller molecules such as the tyrosine kinase inhibitor lapatinib can cross the BBB and has been shown in preclinical mouse models to reduce the size of HER2-positive brain metastases. Two prospective phase II clinical trials among patients with HER2-positive breast cancer and brain metastases who had progressed after WBRT evaluated the use of single agent lapatinib where objective response rates of 2.6%–6% and a progression-free survival of 2.6–3 months was reported [21, 78]. The combination of lapatinib and capecitabine has also been evaluated in several prospective, retrospective, and observational studies where overall CNS response rates (using different response criteria) ranged from 18% to 51.9% (Table 3) [79–83]. In a recent study, Bachelot and colleagues [22] reported on a phase II study of patients with HER2-positive breast cancer and newly diagnosed brain metastases who were given a combination of lapatinib and capecitabine. The authors reported an impressive overall CNS response rate of 65.9% and a median time to CNS progression of 5.5 months (Fig. 1).
Table 3.
Summary of key studies looking at the combination of lapatinib and capecitabine for the treatment of brain metastases among patients with HER2-positive breast cancer who had progressed following WBRT
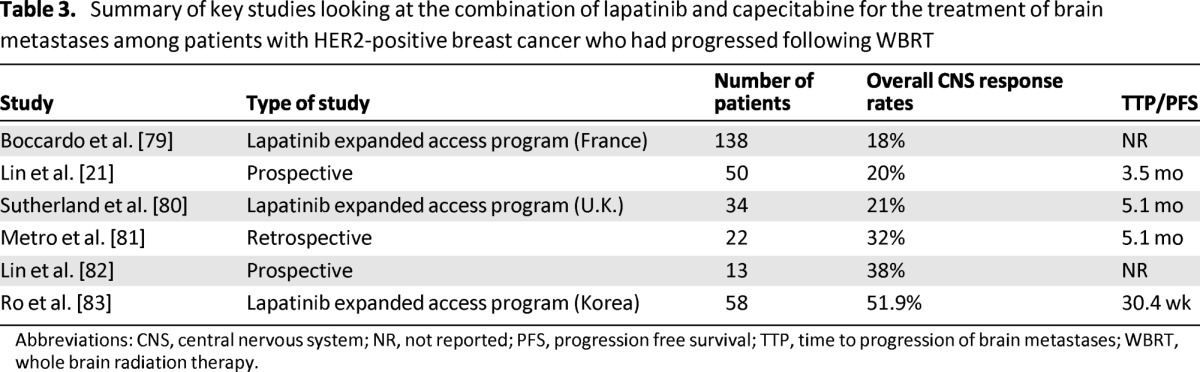
Abbreviations: CNS, central nervous system; NR, not reported; PFS, progression free survival; TTP, time to progression of brain metastases; WBRT, whole brain radiation therapy.
Figure 1.
Flow diagram representing prospective trials investigating lapatinib among patients with metastatic breast cancer (A) prior to development of brain metastases [81], (B) immediately following the diagnosis of newly diagnosed brain metastases [20], or (C) following progression of disease following WBRT [21].
Abbreviations: C, capecitabine; CNS, central nervous system; L, lapatinib; TTP, time to progression of brain metastases; WBRT, whole brain radiation therapy.
Patients with HER2-positive breast cancer with brain metastases constitute a unique cohort where the introduction of the monoclonal antibody trastuzumab has changed the natural history of this disease. Several retrospective studies indicate that use of trastuzumab not only increased the time to development of brain metastases but also has improved survival following the development of brain metastases.
Several novel agents are currently being investigated among patients with breast cancer and brain metastases. Bevacizumab, a monoclonal antibody known to be effective in the treatment of glioblastoma multiforme, is being investigated in combination with carboplatin in a phase II clinical trial (ClinicalTrials.gov identifier: NCT01004172). Similarly, a phase II clinical trial of the combination trastuzumab, vinorelbine, and the mTOR inhibitor everolimus is currently under way among patients with HER2-positive breast cancer and brain metastases (ClinicalTrials.gov identifier: NCT01305941). The PARP inhibitors ABT-888 (veliparib) and iniparib are also being evaluated. GRN1005 (a peptide-drug conjugate consisting of three molecules of paclitaxel conjugated to a 19-amino acid peptide) is known to penetrate the BBB by targeting low-density lipoprotein receptor-related protein 1 that is expressed on the surface of the BBB. Preclinical evidence indicated that the uptake of this agent in the brain was 54 times that of paclitaxel [84] and a phase I trial of 48 patients with heavily pretreated brain metastases showed an overall objective response rate of 71% [85]. Unfortunately, preliminary results of the first 30 patients enrolled in an open-label phase II trial exploring the efficacy of this agent among patients with breast cancer and brain metastases did not reveal any confirmed intracranial responses [86]. Development of this drug in the setting of brain metastases has been halted. Other novel agents that are designed specifically to penetrate the BBB that are currently being investigated in the treatment of patients with brain metastases include 2B3-101, a glutathione-pegylated liposomal doxorubicin that is being investigated in a phase I setting (ClinicalTrials.gov identifier: NCT01386580) and TPI-287, a third-generation taxane designed to avoid the multidrug resistance (MDR)-1 protein drug efflux mechanism, is currently being evaluated in a phase II trial of patients with breast cancer and brain metastases (ClinicalTrials.gov identifier: NCT01332630).
Prevention Strategies: Are We There Yet?
Despite all the strategies described so far for the treatment of patients with breast cancer and brain metastases, the prognostic outcome in this cohort still remains poor. There is thus a keen interest to explore strategies for the prevention of brain metastases especially in groups who are at particularly high risk of developing brain metastases such as those with triple negative breast cancer and HER2-positive disease. Prophylactic WBRT may be one strategy to consider. Niwinska and colleagues [87] reported on a cohort of 80 patients with HER2-positive disease in which WBRT was delivered to patients who had either occult (asymptomatic) CNS metastases or symptomatic CNS. Interestingly, the authors reported a significant reduction in rate of cerebral death in the cohort with occult CNS metastases (16% vs. 48%, p = .009) with no difference in overall survival between the two groups. However, prophylactic WBRT should be viewed with caution, especially because more patients are living longer with metastatic disease. Huang and colleagues [88] reported on a prospective trial that used prophylactic WBRT in patients with breast cancer. The authors reported that among 10 patients who received prophylactic WBRT, three lived long enough to develop significant cognitive decline.
Agents such as lapatinib are also being explored in the prevention strategy. The results from the trial by Bachelot and colleagues [22] on the use of lapatinib before WBRT were impressive. Currently, the ALTTO (adjuvant lapatinib and/or trastuzumab treatment optimization) trial that uses lapatinib in the adjuvant setting has included CNS recurrence as a secondary endpoint. Pivot and colleagues [89] recently reported on a phase III trial of 540 patients with HER2-positive metastatic breast cancer with no brain metastases (screened by MRI) who were randomized to either lapatinib and capecitabine or trastuzumab and capecitabine (Fig. 1). The primary endpoint was the incidence of CNS metastases as the first site of recurrence. The authors reported a similar incidence of CNS metastases as the first site of relapse between the two groups (3% vs. 5%, p = .36) and a median progression free and overall survival that favored the trastuzumab/capecitabine group.
Management of Leptomeningeal Metastases
Incidence of leptomeningeal metastases occurs in approximately 2%–5% of patients with breast cancer. Median survival of patients with untreated leptomeningeal metastases is approximately 4–6 weeks [90]. The definitive method of establishing a diagnosis of leptomeningeal metastases is through confirmation of malignant cells in the CSF obtained via a lumbar puncture. Management of patients with leptomeningeal metastases is complex with most data derived from small nonrandomized and retrospective studies. Radiotherapy used in the setting of leptomeningeal metastases is used primarily for palliation of symptoms, to decrease bulky disease, and to correct CSF flow abnormalities, with whole neural axis radiation rarely indicated having never been demonstrated to improve survival compared with chemotherapy. Surgery in the setting of leptomeningeal metastases is indicated mainly for the placement of an intraventricular catheter for drug administration, ventriculoperitoneal shunting among patients with symptomatic hydrocephalous for resection of concurrent large symptomatic brain metastases. Systemic chemotherapy such as high-dose methotrexate has been shown to have clinical activity in the setting of breast-associated leptomeningeal carcinomatosis [91]. Several systemic combination strategies are being explored in this setting. Currently, a phase II study of high-dose systemic methotrexate and intrathecal liposomal cytarabine among patients with breast cancer and leptomeningeal disease (with or without brain parenchymal disease) is ongoing (ClinicalTrials.gov identifier: NCT00992602). A phase II study exploring the combination of bevacizumab with etoposide and cisplatin is currently being investigated among patients with breast cancer with brain metastases and/or leptomeningeal disease (ClinicalTrials.gov identifier: NCT01281696).
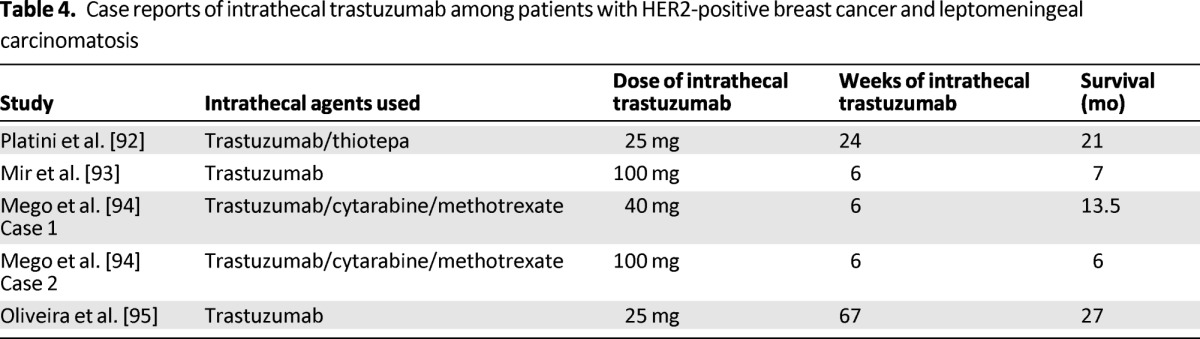
Agents that have been investigated for intrathecal chemotherapy include cytarabine, thiotepa, and methotrexate. Currently, NCCN guidelines recommend 4–6 weeks of induction intrathecal chemotherapy followed by maintenance treatment among those who respond. Over the last decade, there has been an increasing interest in exploring intrathecal administration of trastuzumab among patients with HER2-positive breast cancer and leptomeningeal metastases. Case reports of intrathecal trastuzumab have indicated good improvement in neurological symptoms with overall survival ranging from 6 to 27 months (Table 4) [92–95]. This complex and devastating disease is in urgent need of new efficacious modalities of treatment. Multiple case reports have demonstrated potential efficacy of intrathecal trastuzumab; two phase I/II clinical trials (one conducted by Institut Curie in France [ClinicalTrials.gov identifier: NCT01373710] and the second conducted by Northwestern University in the United States [ClinicalTrials.gov identifier: NCT01325207]) are currently actively recruiting patients and the results are eagerly awaited.
Table 4.
Case reports of intrathecal trastuzumab among patients with HER2-positive breast cancer and leptomeningeal carcinomatosis
Conclusion
The CNS remains a sanctuary site for metastases among patients with breast cancer. Improvements in the management of women with breast cancer and enhanced diagnostic imaging has led not only to an increasing incidence of CNS metastases in this cohort but also to the recognition that CNS metastases is developing because women with breast cancer are living longer. We now have a better understanding of the biology of breast cancer-associated CNS metastases with its natural course in all probability being strongly influenced by the subtype of breast cancer. Although WBRT, surgery, and SRS remain the standard of care for these patients, it is increasingly being recognized that systemic therapies could be effective. In particular, results from phase II trials have shown anti-HER2-directed combination therapies prolong time to progression when administered either before or after WBRT. However, several important questions remain to be answered. Will CNS screening programs targeted at patients with certain subtypes of breast cancer, known to have a high prediction for CNS metastases, have an overall impact on long-term outcome? What is the best way to manage patients with occult (i.e., asymptomatic CNS metastases)? As and when the results of trials exploring novel agents become available, how will we best incorporate them with local treatment strategies? There is absolutely no doubt that we have come a long way in our understanding of the biology of this disease. As the overall survival of patients with breast cancer is improving with the introduction of several efficacious chemotherapeutic and biological agents, we are becoming more aggressive in our management of CNS metastases in the hope of improving prognostic outcome. Future clinical trials should be designed taking into account breast tumor subtype that clearly influences both incidence and outcome. Strategies aimed at prevention and treatment should be developed. Furthermore, although the incidence of CNS metastases among patients with breast cancer appears to be on the rise, it is still low, and meaningful results can only be derived from international multicenter clinical trials; thus, collaborative efforts in the study of this disease will be vital. In addition, it will be imperative not only to explore CNS efficacy of existing agents but also to develop novel agents specific to the treatment and prevention of CNS metastases. It has taken us almost 2 decades to realize that breast cancer is not one disease but a conglomerate of diseases, each with a unique natural history. The way to move forward now is not only to realize that CNS metastases arising from breast cancer is a separate distinct entity from that arising from other solid tumors and thus deserves to be studied separately but also to recognize that CNS metastases arising from each subtype of breast cancer has its unique natural history. In the meantime, treatment of patients with breast cancer and CNS metastases should be individualized, taking into consideration several factors including performance status, burden of systemic disease, breast tumor subtype, and associated CNS symptoms.
Author Contributions
Conception/design: Shaheenah Dawood, Ana M. Gonzalez-Angulo
Manuscript writing: Shaheenah Dawood, Ana M. Gonzalez-Angulo
Final approval of manuscript: Shaheenah Dawood, Ana M. Gonzalez-Angulo
Disclosures
Shaheenah Dawood: GlaxoSmithKline (H). The other author indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Reference
- 1.Patel RR, Mehta MP. Targeted therapy for brain metastases: Improving the therapeutic ratio. Clin Cancer Res. 2007;13:1675–1683. doi: 10.1158/1078-0432.CCR-06-2489. [DOI] [PubMed] [Google Scholar]
- 2.Vogelbaum MA, Suh JH. Resectable brain metastases. J Clin Oncol. 2006;24:1289–1294. doi: 10.1200/JCO.2005.04.6235. [DOI] [PubMed] [Google Scholar]
- 3.Chang JE, Robins HI, Mehta MP. Therapeutic advances in the treatment of brain metastases. Clin Adv Hematol Oncol. 2007;5:54–64. [PubMed] [Google Scholar]
- 4.Gaspar L, Scott C, Murray K, et al. Validation of the RTOG recursive partitioning analysis (RPA) classification for the brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:1001–1006. doi: 10.1016/s0360-3016(00)00547-2. [DOI] [PubMed] [Google Scholar]
- 5.Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22:3608–3617. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 6.Miller KD, Weathers T, Haney LG, et al. Occult central nervous system involvement in patients with metastatic breast cancer: Prevalence, predictive factors and impact on overall survival. Ann Oncol. 2003;14:1072–1077. doi: 10.1093/annonc/mdg300. [DOI] [PubMed] [Google Scholar]
- 7.Tsukada Y, Fouad A, Pickren JW, et al. Central nervous system metastasis from breast carcinoma. Autopsy study. Cancer. 1983;52:2349–2354. doi: 10.1002/1097-0142(19831215)52:12<2349::aid-cncr2820521231>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Stark AM, Stohring C, Hedderich J, et al. Surgical treatment for brain metastases: Prognostic factors and survival in 309 patients with regard to patient age. J Clin Neurosci. 2011;18:34–38. doi: 10.1016/j.jocn.2010.03.046. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Angulo AM, Cristofanilli M, Strom EA, et al. Central nervous system metastases in patients with high-risk breast carcinoma after multimodality treatment. Cancer. 2004;101:1760–1766. doi: 10.1002/cncr.20530. [DOI] [PubMed] [Google Scholar]
- 10.Dawood S, Broglio K, Esteva FJ, et al. Survival of women with triple-receptor negative breast cancer and brain metastases. Ann Oncol. 2009;20:621–627. doi: 10.1093/annonc/mdn682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patanaphan V, Salazar OM, Risco R. Breast cancer: Metastatic pattern and their prognosis. South Med J. 1988;81:1109–1112. [PubMed] [Google Scholar]
- 12.Harputluoglu H, Dizdar O, Aksoy S, et al. Characteristics of breast cancer patients with central nervous system metastases: A single-center experience. J Natl Med Assoc. 2008;100:521–526. doi: 10.1016/s0027-9684(15)31298-0. [DOI] [PubMed] [Google Scholar]
- 13.DeAngelis L, Rogers L, Foley KM. Leptomeningeal metastasis. In: Harris JR, editor. Diseases of the breast. Philadelphia, PA: Lippincott William & Wilkins; 2000. pp. 867–874. [Google Scholar]
- 14.Hawkins BT, Davis TP. The blood–brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 15.Régina A, Demeule M, Laplante A, et al. Multidrug resistance in brain tumors: Roles of the blood–brain barrier. Cancer Metastasis Rev. 2001;20:13–25. doi: 10.1023/a:1013104423154. [DOI] [PubMed] [Google Scholar]
- 16.Zhang RD, Price JE, Fujimaki T, et al. Differential permeability of the blood–brain barrier in experimental brain metastases produced by human neoplasms implanted into nude mice. Am J Pathol. 1992;141:1115–1124. [PMC free article] [PubMed] [Google Scholar]
- 17.Yonemori K, Tsuta K, Ono M, et al. Disruption of the blood brain barrier by brain metastases of triple-negative and basal-type breast cancer but not HER2/neu-positive breast cancer. Cancer. 2010;116:302–308. doi: 10.1002/cncr.24735. [DOI] [PubMed] [Google Scholar]
- 18.van Vulpen M, Kal HB, Taphoorn MJ, et al. Changes in blood–brain barrier permeability induced by radiotherapy: Implications for timing of chemotherapy? Oncol Rep. 2002;9:683–688. [PubMed] [Google Scholar]
- 19.Stemmler HJ, Schmitt M, Willems A, et al. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood–brain barrier. Anticancer Drugs. 2007;18:23–28. doi: 10.1097/01.cad.0000236313.50833.ee. [DOI] [PubMed] [Google Scholar]
- 20.Smith QR, Rudraraju V, Taskar K, et al. Lapatinib distribution in brain metastases of breast cancer following oral dosing in mice. Paper presented at: Breast Cancer Symposium, Abstract 158; September 5–7, 2008; Washington, D.C. [Google Scholar]
- 21.Lin NU, Diéras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15:1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 22.Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol. 2013;14:64–71. doi: 10.1016/S1470-2045(12)70432-1. [DOI] [PubMed] [Google Scholar]
- 23.Pitz MW, Desai A, Grossman SA, et al. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neurooncol. 2011;104:629–638. doi: 10.1007/s11060-011-0564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bos PD, Zhang XH, Nadal C, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–1009. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kennecke H, Yerushalmi R, Woods R, et al. Metastatic behavior of breast cancer subtypes. J Clin Oncol. 2010;28:3271–3277. doi: 10.1200/JCO.2009.25.9820. [DOI] [PubMed] [Google Scholar]
- 27.Berghoff A, Bago-Horvath Z, De Vries C, et al. Brain metastases free survival differs between breast cancer subtypes. Br J Cancer. 2012;106:440–446. doi: 10.1038/bjc.2011.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musolino A, Ciccolallo L, Panebianco M, et al. Multifactorial central nervous system recurrence susceptibility in patients with HER2-positive breast cancer: Epidemiological and clinical data from a population-based cancer registry study. Cancer. 2011;117:1837–1846. doi: 10.1002/cncr.25771. [DOI] [PubMed] [Google Scholar]
- 29.Bria E, Cuppone F, Fornier M, et al. Cardiotoxicity and incidence of brain metastases after adjuvant trastuzumab for early breast cancer: The dark side of the moon? A meta-analysis of the randomized trials. Breast Cancer Res Treat. 2008;109:231–239. doi: 10.1007/s10549-007-9663-z. [DOI] [PubMed] [Google Scholar]
- 30.Lin NU, Claus E, Sohl J, et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: High incidence of central nervous system metastases. Cancer. 2008;113:2638–2645. doi: 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vern-Gross TZ, Lawrence JA, Case LD, et al. Breast cancer subtype affects patterns of failure of brain metastases after treatment with stereotactic radiosurgery. J Neurooncol. 2012;110:381–388. doi: 10.1007/s11060-012-0976-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sperduto PW, Kased N, Roberge D, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012;82:2111–2117. doi: 10.1016/j.ijrobp.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryken TC, McDermott M, Robinson PD, et al. The role of steroids in the management of brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:103–114. doi: 10.1007/s11060-009-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yung W, Kunschner L, Saway R, et al. Intracranial metastases. In: Levin V, editor. Cancer in the nervous system. 2nd ed. New York, NY: Oxford University Press; 2002. pp. 321–340. [Google Scholar]
- 35.Mikkelsen T, Paleologos NA, Robinson PD, et al. The role of prophylactic anticonvulsants in the management of brain metastases: A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:97–102. doi: 10.1007/s11060-009-0056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Comprehensive Cancer Network. [Accessed November 1, 2013]. Available at http://www.nccn.org/professionals/physician_gls/pdf/cns.pdf.
- 37.Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 38.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 39.Kondziolka D, Patel A, Lunsford LD, et al. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastases. Int J Radiat Oncol Biol Phys. 1999;45:427–434. doi: 10.1016/s0360-3016(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 40.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: A randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 41.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 42.Roos DE, Wirth A, Burmeister BH, et al. Whole brain irradiation following surgery or radiosurgery for solitary brain metastases: Mature results of a prematurely closed randomized Trans-Tasman Radiation Oncology Group trial (TROG 98.05) Radiother Oncol. 2006;80:318–322. doi: 10.1016/j.radonc.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 43.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: A randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 44.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: Results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29:134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scoccianti S, Ricardi U. Treatment of brain metastases: Review of phase III randomized controlled trials. Radiother Oncol. 2012;102:168–179. doi: 10.1016/j.radonc.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 46.Davey P, Hoegler D, Ennis M, et al. A phase III study of accelerated versus conventional hypofractionated whole brain irradiation in patients of good performance status with brain metastases not suitable for surgical excision. Radiother Oncol. 2008;88:173–176. doi: 10.1016/j.radonc.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 47.Graham PH, Bucci J, Browne L. Randomized comparison of whole brain radiotherapy, 20 Gy in four daily fractions versus 40 Gy in 20 twice-daily fractions, for brain metastases. Int J Radiat Oncol Biol Phys. 2010;77:648–654. doi: 10.1016/j.ijrobp.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 48.Suh JH, Stea B, Nabid A, et al. Phase III study of Efaproxiral as an adjunct to whole brain radiation therapy for brain metastases. J Clin Oncol. 2006;24:106–114. doi: 10.1200/JCO.2004.00.1768. [DOI] [PubMed] [Google Scholar]
- 49.Antonadou D, Coliarakis N, Paraskevaidis M, et al. Whole brain radiotherapy alone or in combination with temozolomide for brain metastases. A phase III trial. Int J Radiat Oncol Biol Phys. 2002;54:93–94. [Google Scholar]
- 50.Guerrieri M, Wong K, Ryan G, et al. A randomized phase III study of palliative radiation with concomitant carboplatin for brain metastases from nonsmall cell carcinoma of the lung. Lung Cancer. 2004;46:107–111. doi: 10.1016/j.lungcan.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 51.Neuhaus T, Ko Y, Muller RP, et al. A phase III trial of topotecan and whole brain radiation therapy for patients with CNS metastases due to lung cancer. Br J Cancer. 2009;100:291–297. doi: 10.1038/sj.bjc.6604835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Knisely JPS, Berkey B, Chakravarti A, et al. A phase III study of conventional radiation therapy plus thalidomide versus conventional radiation therapy for multiple brain metastases (RTOG 0118) Int J Radiat Oncol Biol Phys. 2008;71:79–86. doi: 10.1016/j.ijrobp.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 53.Chargari C, Kirova YM, Diéras V, et al. Concurrent capecitabine and whole-brain radiotherapy for treatment of brain metastases in breast cancer patients. J Neurooncol. 2009;93:379–384. doi: 10.1007/s11060-008-9791-2. [DOI] [PubMed] [Google Scholar]
- 54.Serizawa T, Iuchi T, Ono J, et al. Gamma knife treatment for multiple metastatic brain tumors compared with whole-brain radiation therapy. J Neurosurg. 2000;93(suppl 3):32–36. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 55.Park SH, Hwang SK, Kang DH, et al. Gamma knife radiosurgery for multiple brain metastases from lung cancer. J Clin Neurosci. 2009;16:626–629. doi: 10.1016/j.jocn.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki S, Omagari J, Nishio S, et al. Gamma knife radiosurgery for simultaneous multiple metastatic brain tumors. J Neurosurg. 2000;93(suppl 3):30–31. doi: 10.3171/jns.2000.93.supplement. [DOI] [PubMed] [Google Scholar]
- 57.Amendola BE, Wolf A, Coy S, et al. Radiosurgery as palliation for brain metastases: A retrospective review of 72 patients harboring multiple lesions at presentation. J Neurosurg. 2002;97(suppl):511–514. doi: 10.3171/jns.2002.97.supplement. [DOI] [PubMed] [Google Scholar]
- 58.Nam TK, Lee JI, Jung YJ, et al. Gamma knife surgery for brain metastases in patients harboring four or more lesions: Survival and prognostic factors. J Neurosurg. 2005;102(suppl):147–150. doi: 10.3171/jns.2005.102.s_supplement.0147. [DOI] [PubMed] [Google Scholar]
- 59.Bhatnagar AK, Flickinger JC, Kondziolka D, et al. Stereotactic radiosurgery for four or more intracranial metastases. Int J Radiat Oncol Biol Phys. 2006;64:898–903. doi: 10.1016/j.ijrobp.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 60.Karlsson B, Hanssens P, Wolff R, et al. Thirty years' experience with Gamma Knife surgery for metastases to the brain. J Neurosurg. 2009;111:449–457. doi: 10.3171/2008.10.JNS08214. [DOI] [PubMed] [Google Scholar]
- 61.Knisely JP, Yamamoto M, Gross CP, et al. Radiosurgery alone for 5 or more brain metastases: Expert opinion survey. J Neurosurg. 2010;113(suppl):84–89. doi: 10.3171/2010.8.GKS10999. [DOI] [PubMed] [Google Scholar]
- 62.Chargari C, Idrissi HR, Pierga JY, et al. Preliminary results of whole brain radiotherapy with concurrent trastuzumab for treatment of brain metastases in breast cancer patients. Int J Radiat Oncol Biol Phys. 2011;81:631–636. doi: 10.1016/j.ijrobp.2010.06.057. [DOI] [PubMed] [Google Scholar]
- 63.Lin NU, Ramakrishna N, Younger WJ, et al. Phase I study of lapatinib (L) in combination with whole-brain radiation therapy (WBRT) in patients (pts) with brain metastases from HER2-positive breast cancer. J Clin Oncol. 2010;28(suppl):1154. [Google Scholar]
- 64.Xu Z, Marko NF, Chao ST, et al. Relationship between HER2 status and prognosis in women with brain metastases from breast cancer. Int J Radiat Oncol Biol Phys. 2012;82:e739–e747. doi: 10.1016/j.ijrobp.2011.06.1968. [DOI] [PubMed] [Google Scholar]
- 65.O'Meara WP, Seidman AD, Yamada Y, et al. Impact of HER2 status in breast cancer patients receiving stereotactic radiosurgery for brain metastases. J Clin Oncol. 2005;23(suppl):637. [Google Scholar]
- 66.Rosner D, Nemoto T, Lane WW. Chemotherapy induces regression of brain metastases in breast carcinoma. Cancer. 1986;58:832–839. doi: 10.1002/1097-0142(19860815)58:4<832::aid-cncr2820580404>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 67.Boogerd W, Vos VW, Hart AA, et al. Brain metastases in breast cancer; natural history, prognostic factors and outcome. J Neurooncol. 1993;15:165–174. doi: 10.1007/BF01053937. [DOI] [PubMed] [Google Scholar]
- 68.Ekenel M, Hormigo AM, Peak S, et al. Capecitabine therapy of central nervous system metastases from breast cancer. J Neurooncol. 2007;85:223–227. doi: 10.1007/s11060-007-9409-0. [DOI] [PubMed] [Google Scholar]
- 69.Boogerd W, Groenveld F, Linn S, et al. Chemotherapy as primary treatment for brain metastases from breast cancer: Analysis of 115 one-year survivors. J Cancer Res Clin Oncol. 2012;138:1395–1403. doi: 10.1007/s00432-012-1218-y. [DOI] [PubMed] [Google Scholar]
- 70.Lim E, Lin NU. New insights and emerging therapies for breast cancer brain metastases. Oncology (Williston Park) 2012;26:652–659. 663. [PubMed] [Google Scholar]
- 71.Lassman AB, Abrey LE, Shah GD, et al. Systemic high-dose intravenous methotrexate for central nervous system metastases. J Neurooncol. 2006;78:255–260. doi: 10.1007/s11060-005-9044-6. [DOI] [PubMed] [Google Scholar]
- 72.Siena S, Crinò L, Danova M, et al. Dose-dense temozolomide regimen for the treatment of brain metastases from melanoma, breast cancer, or lung cancer not amenable to surgery or radiosurgery: A multicenter phase II study. Ann Oncol. 2010;21:655–661. doi: 10.1093/annonc/mdp343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Christodoulou C, Bafaloukos D, Linardou H, et al. Temozolomide (TMZ) combined with cisplatin (CDDP) in patients with brain metastases from solid tumors: A Hellenic Cooperative Oncology Group (HeCOG) Phase II study. J Neurooncol. 2005;71:61–65. doi: 10.1007/s11060-004-9176-0. [DOI] [PubMed] [Google Scholar]
- 74.Murphy C, Nulsen B, Rump M, et al. Phase II trial of patupilone in patients (pts) with breast cancer brain metastases (BCBM) progressing or recurring after whole brain radiotherapy (WBXRT). Paper presented at ASCO Breast Cancer Symposium; 2009; Abstract no. 234. [Google Scholar]
- 75.Freedman RA, Bullitt E, Sun L, et al. A phase II study of sagopilone (ZK 219477; ZK-EPO) in patients with breast cancer and brain metastases. Clin Breast Cancer. 2011;11:376–383. doi: 10.1016/j.clbc.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dawood S, Broglio K, Esteva FJ, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol. 2008;19:1242–1248. doi: 10.1093/annonc/mdn036. [DOI] [PubMed] [Google Scholar]
- 77.Le Scodan R, Jouanneau L, Massard C, et al. Brain metastases from breast cancer: Prognostic significance of HER-2 overexpression, effect of trastuzumab and cause of death. BMC Cancer. 2011;11:395. doi: 10.1186/1471-2407-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin NU, Carey LA, Liu MC, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26:1993–1999. doi: 10.1200/JCO.2007.12.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boccardo F, Kaufman B, Baselga J, et al. Evaluation of lapatinib (Lap) plus capecitabine (Cap) in patients with brain metastases (BM) from HER2+ breast cancer (BC) enrolled in the Lapatinib Expanded Access Program (LEAP) and French Authorisation Temporaire d'Utilisation (ATU) J Clin Oncol. 2008;26(suppl):1094. [Google Scholar]
- 80.Sutherland S, Ashley S, Miles D, et al. Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access programme, including efficacy in brain metastasesthe UK experience. Br J Cancer. 2010;102:995–1002. doi: 10.1038/sj.bjc.6605586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Metro G, Foglietta J, Russillo M, et al. Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann Oncol. 2011;22:625–630. doi: 10.1093/annonc/mdq434. [DOI] [PubMed] [Google Scholar]
- 82.Lin NU, Eierman W, Greil R, et al. Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. J Neurooncol. 2011;105:613–620. doi: 10.1007/s11060-011-0629-y. [DOI] [PubMed] [Google Scholar]
- 83.Ro J, Park S, Kim S, et al. Clinical outcomes of HER2-positive metastatic breast cancer patients with brain metastasis treated with lapatinib and capecitabine: An open-label expanded access study in Korea. BMC Cancer. 2012;12:322. doi: 10.1186/1471-2407-12-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thomas FC, Taskar K, Rudraraju V, et al. Uptake of ANG1005, a novel paclitaxel derivative, through the blood–brain barrier into brain and experimental brain metastases of breast cancer. Pharm Res. 2009;26:2486–2494. doi: 10.1007/s11095-009-9964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sarantopoulos J, Gabrail NY, Moulder SL, et al. ANG1005: Results of a Phase I study in patients with advanced solid tumors and brain metastases. Paper presented at: 2010 ASCO Annual Meeting; June 4–8 2011; Chicago, IL. [Google Scholar]
- 86.Lin NU, Schwartzberg LS, Kesari S, et al. A phase 2, multi-center, open label study evaluating the efficacy and safety of GRN1005 alone or in combination with trastuzumab in patients with brain metastases from breast cancer. Paper presented at San Antonio Breast Cancer Symposium; December 4–8, 2012; San Antonio, TX, Poster 3-12-04. [Google Scholar]
- 87.Niwinska A, Tacikowska M, Murawska M. The effect of early detection of occult brain metastases in HER2-positive breast cancer patients on survival and cause of death. Int J Radiat Oncol Biol Phys. 2010;77:1134–1139. doi: 10.1016/j.ijrobp.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 88.Huang F, Alrefae M, Langleben A, et al. Prophylactic cranial irradiation in advanced breast cancer: A case for caution. Int J Radiat Oncol Biol Phys. 2009;73:752–758. doi: 10.1016/j.ijrobp.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 89.Pivot X, Żurawski B, Allerton R, et al. Cerebel (EGF111438): An open label randomized phase III study comparing the incidence of CNS metastases in patients with HER2+ metastatic breast cancer, treated with lapatinib plus capecitabine versus trastuzumab plus capecitabine. Ann Oncol. 2012;23(suppl 9):ixe1–ixe30. [Google Scholar]
- 90.Chamberlain MC. Leptomeningeal metastasis. Curr Opin Oncol. 2010;22:627–635. doi: 10.1097/CCO.0b013e32833de986. [DOI] [PubMed] [Google Scholar]
- 91.Lassman AB, Abrey LE, Shah GD, et al. Systemic high-dose intravenous methotrexate for central nervous system metastases. J Neurooncol. 2006;78:255–260. doi: 10.1007/s11060-005-9044-6. [DOI] [PubMed] [Google Scholar]
- 92.Platini C, Long J, Walter S. Meningeal carcinomatosis from breast cancer treated with intrathecal trastuzumab. Lancet Oncol. 2006;7:778–780. doi: 10.1016/S1470-2045(06)70864-6. [DOI] [PubMed] [Google Scholar]
- 93.Mir O, Ropert S, Alexandre J, et al. High-dose intrathecal trastuzumab for leptomeningeal metastases secondary to HER-2 overexpressing breast cancer. Ann Oncol. 2008;19:1978–1980. doi: 10.1093/annonc/mdn654. [DOI] [PubMed] [Google Scholar]
- 94.Mego M, Sycova-Mila Z, Obertova J, et al. Intrathecal administration of trastuzumab with cytarabine and methotrexate in breast cancer patients with leptomeningeal carcinomatosis. Breast. 2011;20:478–480. doi: 10.1016/j.breast.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 95.Oliveira M, Braga S, Passos-Coelho JL, et al. Complete response in HER2+ leptomeningeal carcinomatosis from breast cancer with intrathecal trastuzumab. Breast Cancer Res Treat. 2011;127:841–844. doi: 10.1007/s10549-011-1417-2. [DOI] [PubMed] [Google Scholar]